Abstract
The present study is based on developing a dual-step ultrasonically modified cloud point extraction (DsUm-CPE) method for enrichment of mercury (Hg) in artificial saliva extract (ASE) of the numerous snuff products (dry and moist snuff, having green, brown and black in colors). This method relies on the complexation of Hg with ammonium O, O-diethyldithiophosphate, followed by the entrapped in nonionic surfactant (Triton X-114) prior to analysis by cold vapor atomic absorption spectrometry (CVAAS). The dispersion of hydrophobic complex in micellar phase was carried out by ultrasound energy, in addition other shaking method, vortex mixer was also applied for comparison purposes, vortex shaking method was also used. Several variables of developed methods, such as complexing agent, Triton X-114, dispersion modes (sonication/vortex shaking), equilibrium temperature, centrifugation time and concentration of back extraction reagent, have been studied. The detection and quantification limit under the optimum conditions were attained as 0.004 and 0.014 µg L−1, respectively. The accuracy for the analysis of total Hg was checked by certified samples of Virginia tobacco leaves (ICHTJ-cta-VTL-2) and both biological samples. The obtained values of all three certified samples have insignificant difference (p > 0.05) among their certified and experimental values. The resulted statistics specified that dry snuff has about fourfold higher contents of Hg as compared to moist snuff, whereas the biological samples of male subject (25 to 60 years) sniffing moist and dry snuff were also analyzed to assess the Hg exposure through their consumption. The biological samples of snuff consumers have about twofold higher concentration of Hg as compared to referents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pakistan stands among the top four states and poses rapid increase in tobacco market. Tobacco is consumed in several ways, for example, cigarette smoking and chewing or sniffing. Tobacco that is used without burning is known as smokeless tobacco products (SLT). Usage of SLT is a common practice among the people of Asia including Pakistan (Middleton 2016). More than 19 carcinogens and around 30 metallic compounds including heavy metals are known to be present in tobacco. Several health disorders are associated with the exposure of heavy metals, especially mercury (Hg), which is one of these heavy metals (Dhaware et al. 2009). Plants have different tolerance mechanisms for higher levels of heavy metals (Schat et al. 2000). Tobacco plant efficiently absorbs Hg from soil (Hussein et al. 2007). It is introduced as a contaminant of lime, fertilizers, manures, poor quality urban waste composite and pesticides.
The SLT products are made of finely crushed tobacco leaves and are sold in either packed or loose form. Moist snuff is dipped between the gum and cheek, while dry snuff is sniffed through nasal or oral route to enlighten the head and used as stimulant and depressant (Bloomfield and Stephens 1996; Popova and Ling 2013; Wrangsjo et al. 2015). The snuff products are also called as dipped/spit tobacco products (Mirbod and Ahing 2000). These products comprises of several inorganic toxicants which release on continuous mastication and absorb into the soft tissues of oral cavity, while the residual portion may be absorbed in the digestive tract (Boffetta et al. 2008). Snuff is commonly used in all cultures across the globe; in Pakistan its common name is “naswar,” but we use the term snuff as its international name. The packets of snuff contain 5–10 g to be sold in markets (Kazi et al. 2013). These items are known to enhance the risk of oral cancer (Farrand et al. 2001; Gupta and Ray 2003; Mazahir et al. 2006; Subramanian et al. 2004). The habituated people may have oral submucous fibrosis, which become more severe to adolescents (Khawaja et al. 2005).
Mercury (Hg) is very poisonous and extremely bio-accumulative (Patrick 2002). It is thought to be most toxic element in the environment and one of the major global and environmental pollutants. It is released from the industrial wastewater, which may further polluted the agricultural soil (Wu and Cao 2010). It has been recently reported that anthropogenic activities are the main cause of Hg pollution. Maximum soil Hg originates from urban wastes, smelting and mining (Patra and Sharma 2000). The Hg enters in the body through food chain and causes severe health risk, especially to the nervous system (Gnamus et al. 2000). It has been reported that organic and aqueous extracts of Indian chewing tobacco, Swedish and American moist snuff originate chromosomal alterations in mammalian and microbial cell cultures (Emerging and Risks 2008). The literature-reported data indicate augmented development of micronuclei in oral epithelial cells as sign of chromosomal impairment in oral cancer patients due to the consumption of SLT products including snuff (Rodu et al. 2005; Rodu and Jansson 2004). The Hg could either be accidentally added in these products, as contaminant, or may be added during the process of manufacturing or through raw material.
The health risks associated with high exposure of Hg are well known (Bull 2011). However, risk associated with the long-term exposure of Hg at trace levels is still under debate for the researchers (Gnamus et al. 2000). However, less research is carried out on the oral exposure of toxic metals through the snuff products (Bolewska et al. 1990). The Hg is absorbed into the brain after passing from olfactory bulb, which was settle on mucous membrane in the upper nasal cavity as Hg vapors, during nose breathing. In addition, extraction rate for Hg from snuff products in saliva will differ according to each product. However, the frequent use of these products permits the accumulation of non-degradable and hazardous components in the consumer’s body (Pappas et al. 2008). Heavy metals have lethal effects, and even trace amounts of them can cause severe physiological disorders (García-Rico et al. 2007).
Recently, advance preconcentration methods for the analysis of trace levels of Hg have been observed in substantial depth (Carabias-Martınez et al. 2000). These preconcentration steps are crucial because the sensitivity or the selectivity of the methods could be affected by the presence of organic/inorganic contaminations (Coelho and Arruda 2005). Co-precipitation, liquid–liquid extraction, micellar, sorption and ion exchange system are the most commonly used techniques in various fields of analytical chemistry (Carabias-Martınez et al. 2000). The most common methodology is cloud point extraction for preconcentration and separation of metals in different matrices (Manzoori and Bavili-Tabrizi 2002a). In this method, solution becomes turbid above a specific temperature that is recognized as “cloud point temperature” (Coelho and Arruda 2005), above which the phase separation occurs as the bulk aqueous solution, comprising surfactant monomers and the surfactant-rich phase with very small volume (Manzoori and Bavili-Tabrizi 2002a). Micellar system offers several advantages, such as higher capacity to concentrate the inclusive range of analytes with elevated preconcentration factors, recoveries and low cost (Manzoori and Bavili-Tabrizi 2002b; Shemirani et al. 2005). Due to pure hydrophobic interactions between analyte complex and nonionic surfactant, metal chelate exists in surfactant phase.
After the advent of cloud point, micelle dispersion is imperative factor that has significant impact on analysis time and extraction efficacy (Guiñez et al. 2018). Dispersion of micelle in the solution could be carried out by different energy modes such as automatic agitation (mixer, colloid mill, stirrer, valve homogenizer) and ultrasound energy (Chiha et al. 2010). In recent times, ultrasound energy has attain huge acceptance to be used as a safe way for the dispersion of solution due to its greener nature and low cost (Khan et al. 2017; Miano et al. 2016). Sonication decreases the analysis time by increasing the analyte’s interaction with nonionic surfactant rapidly. Cavitation effect is produced by ultrasound energy, which improves the contact area between two phases and mass transfer (Mehrabi and Dil 2017). With the help of ultrasound energy, reduced concentration gradients and rapid mass transfer are the main advantages that eventually enhance the extraction proficiency of the method by reducing the internal/external resistance to analyte transportation (Ju et al. 2020; Khan et al. 2017).
Despite legal warning and proposal for prohibition of smoking and consuming SLT, the public still use these products, frequently. Lack of information on the levels of different toxic metals contamination of SLT products consumed in Pakistan/different parts of world should be seriously concerned and monitored by health administrative agencies. In the present study, the concentration of Hg will be determined in different brands of commercially available SLT (snuff) products and in their ASE. This study was undertaken to help well comprehend the level of Hg, to which the consumers of snuff products are exposed through risk assessment.
The purpose of present work was to develop dual-step ultrasonically modified cloud point extraction (DsUm-CPE) method to determine the total mercury (Hg) content in artificial saliva extract (ASE) of different types of snuff products (moist and dry) having different colors, green, brown and black. Ultrasound energy and vortex shaking were compared as dispersion modes for the extraction efficiencies. Several variables of developed methods including concentration of complexing agent, Triton X110, incubation time in ultrasonic bath, centrifugation time and concentration of back extraction reagent have been studied in order to achieve optimum recovery of Hg. The accuracy for the analysis of total Hg was checked by certified reference materials of tobacco, human hair and whole blood. In addition, the present study also aims to determine total Hg contents in the biological samples (scalp hair and blood) of the adult population (25–60 years) having sniffing habits. Healthy referent subjects of the same age group were also selected for comparison purpose; those don’t sniff or consume any tobacco product. Different risk factors associated with the Hg intake via consuming snuff products were also studied.
Material and method
Reagents and chemicals
For the preparation of samples/standard solutions, deionized water was utilized throughout the investigation, attained from ELGA lab water system (Bucks, UK). The complexing reagent and other chemicals such as ammonium O, O-diethyldithiophosphate (DDTP), concentrated HNO3 (65%), HCl (37%), H2O2 (30%) and α-amylase were of high purity/analytical grade. The octylphenoxypolyethoxyethanol (Triton X-114) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Stock solution of mercury (1000 mg L−1) was attained from Fluka Kamica (Bushs, Switzerland) to prepare the working standard by successive dilution. The glassware was infused in 10% HNO3; then washed by means of distilled and deionized water; and kept in close containers to avoid any contamination. For accuracy of the analytical technique, to determine the total Hg concentrations in certified reference materials, Virginia tobacco leaves (ICHTJ-cta-VTL-2) Clincheck control-lyophilized human whole blood (Recipe, Munich Germany) and human hair (BCR 397, Brussels®, Belgium) were analyzed for total Hg concentrations.
Instrumentation
To determine the total Hg contents, the certified and real samples were acid digested in close PTFE tubes using a domestic microwave oven (Pel, Osaka, Japan). pH meter (Ecosan Ion 6, Malaysia) was used to measure the pH of samples. For systematic mixing, programmable ultrasonic water bath (0–80 °C temperature range and 35 kHz intensification frequency), model no: SC-121TH (Sonicor, Deep Park, NY, USA), and MX-E vortex mixer (Dragon Lab instrument, Beijing 101, 318 China) were utilized. WIROWKA Laboratory type WE-1, nr-6933 centrifuge machine (220/50 Hz, 0–6000 rpm speed range, 0–60 min timer, Mechanika Phecyzyjna, Poland) was used to separate both phases. An Analyst 700 atomic absorption spectrometer, PerkinElmer (Norwalk, CT, USA), MHS-15 chemical vapor generation system (PerkinElmer), joined with AA spectrometer system was used to produce cold vapor of Hg. A mercury hollow cathode lamp was used as the radiation source, functioning at 5 mA. Measurements were carried out in the integrated absorbance (peak area) mode at 253.7 nm, using a spectral band width of 2.6 nm. Argon with 99.99% purity was used as the carrier gas.
Sampling and treatment
Different types of 23 snuff samples (n = 10 of each) were purchased form markets of different cities of Pakistan during January to December 2018. The snuff samples were divided into two mains categories, on the basis of texture as moist and dry snuff samples. The snuff samples were further classified on the basis of their color as green moist snuff (GMS n = 7), brown moist snuff (BMS n = 7), dry brown snuff (DBS n = 5) and dry black snuff (DBKS n = 4). Brand identity of each sample has not been declared in the text due to authority rules. All snuff samples were prepared carefully in clean environment to avoid any contamination. The snuff samples were dried at 40 °C for 12 h, then ground into fine particles and passed through the nylon sieve with 125 μm of mesh size to remove the larger granules. Then, sieved samples were stored in clean polyethylene bags till further analysis.
A survey among male adults was carried out to collect information based on structured questionnaire. Biological samples (scalp hair and blood) were collected from male subjects (n = 100) age ranged from 25 to 60 years resided in two big cities of Pakistan, consuming different types of snuff. For comparative purposes, 60 age matched male subjects, not consuming any tobacco products were selected as referents. All contributors were aware about the purpose of the study; all of them gave consent to take part in the study and signed the form. Ethical committee of Sindh University Jamshoro approved this study. A 7-mm heparinized lithium Vacutainer® tube (Becton Dickinson) was used to collect 5-ml blood samples from the selected subjects. Blood samples were stored at − 20 °C till analysis. The scalp hair samples were taken from the nape of the neck of each study subjects and kept in separate labeled plastic bags. Hair samples were cut into small pieces, around 0.2 to 0.3 cm, and thoroughly washed with diluted Triton X-100, followed by acetone and ultrapure water. After drying at 80–85 °C, the samples were stored. The details about collection and pretreatment of scalp hair samples are given in our previous work (Akhtar et al. 2017).
Procedure for total mercury analysis
To determine the total Hg contents in replicate six (0.2 g) of each certified reference materials (scalp hair, blood and tobacco) and triplicate sets of each real samples of different types of snuff, scalp hair and blood samples (0.5 mL) of referents and snuff consumers were kept into Teflon PTFE flasks. The fresh mixture of concentrated H2O2 and HNO3 in the equal ratio was prepared and added 2 mL of it to each flask and reserved for 5 min at room temperature; then, enclosed vessels were positioned in microwave heating system for 2 to 5 min (Shah et al. 2009). Volume of the final digests was made up to 25 mL with 0.1 M HCl. Resulted solutions were analyzed by CVAAS to determine total Hg contents.
Extraction of Hg by Artificial saliva
Artificial saliva was prepared by following the procedure as reported in previous work (Arain et al. 2014). Replicate six (0.2 g) of each snuff sample (dray and moist) in PTFE flasks was weighted, and 25 mL of freshly prepared artificial saliva was added. Flasks were then kept for dissimilar time intervals (5–30 min) in an ultrasonic bath. After each interval, the contents of the flasks were centrifuged for 5 min and aqueous phase was separated by means of pasture pipette; 100 µL of HCl was added (to avoid fungal growth) and kept at 4 °C till further analysis.
Procedure
A DsUm-CPE was applied to the ASE of different types of snuff samples for preconcentration of Hg. For the optimization of different variables, two set of six replicate of Hg standards (2 µg L−1) and triplicates of each ASE (10 mL) of real samples were taken in PTFE flasks. Then, 0.1–0.5% (w/v) Triton X-114 (TX-114) and complexing agent (DDTP) were added in the concentration range of 0.2–1.0% (w/v). One set was placed for 1–5 min at 20–60 °C in an ultrasonic water bath for the dispersion/shaking, whereas other set was subjected to vortex mixer for 30–120 s. The content of the tubes was than centrifuged at 4000 rpm for 5 to 10 min to separate both phases. Flasks were then positioned in an ice bath for 5 min, to upsurge the viscidity of the sediment phase. The upper aqueous phase was discarded with the help of pipette and added 1.0 to 2.0 mol L−1 of HNO3 and HCl separately to the enriched phase and subjected to the second round of cloud point extraction (CPE) (sonication/vortex mixing, then cooling and centrifugation as given above). The aqueous phase is separated after centrifugation. Triplicate of each (500 µl) of sedimented samples was taken in PTFE flasks of the MHS-15 system and added 40 µl of the antifoam agent along with 1.0 M HCl (2.5 ml). After the addition of 3% (m/v) NaBH4 in PTFE flasks for 5 s, system was sealed. Hg vapors produced in the system were carried to the quartz cell using Argon stream, and the signals were noted.
Statistical Analysis
For statistical analysis and data processing different computer programs such as Minitab13.2 (Minitab Inc., State College, PA), XL State (Addin soft, NY, USA) and Excel 2003 (Microsoft Office ®) were used. Resulted data of all samples are given as means ± std. Significant difference was evaluated between certified and experimental values of CRM using Student’s t test.
Results and discussion
The DsUm-CPE method has not been commonly used for the Hg determination and limited to few environmental and biological samples (Yu 2005). Certified standard and real samples (ASE extracts of snuff) were treated with complexing agent; formed complex was entrapped in TX-114. It was manifested that the addition of complexing agent and Triton X-114 has numerous benefits, i.e., appreciable hydrophobicity of the DDTP-Hg complex, and quite low cloud point temperature of Triton X-114 (Borges et al. 2003). The parameters affecting the CPE performance were studied to attain an optimum recovery of Hg from ASE of different snuff samples. Different variables including volume of complexing agent, surfactant and back extracting agent, equilibration temperature, dispersion mode and time were selected.
The pH is an important factor to be studied to expand the preconcentration effectiveness of the methodology. However, in the present study, pH was not optimized because the Hg-DDTP complex is stable in the acidic media, so it is directly preconcentrated in previously acidified sample (ASE of snuff samples), without using any buffer (Dressler et al. 2002). For back extraction of analyte complex (Hg-DDTP) from surfactant enriched phase, 0.5 mL of 1 to 2 mol L−1 of HCl/HNO3 was added and subjected to ultrasonic energy only, for shaking and heating at 50 °C (second round of CPE), prior to analyzed by CVAAS.
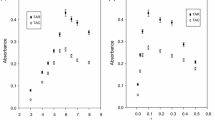
The concentration of the DDTP was studied in the range of (0.2–1.0%) to attain the optimum recovery of Hg (Fig. 1). Maximum recovery of the analyte was observed at 0.4% DDTP (w/v). Significant decrease (> 20%) was observed in the extraction efficiency as the concentration of DDTP reached up to 1% (w/v). This action may be due to competition for the complexation in sample solution. DDTP was selected as complexing agent for Hg due to its high stability in acidic media and aqueous media. It can be added to the aqueous solutions of standards and samples.
The TX-114 was chosen as the surfactant due to its less toxicity, accessibility in purified form, high density and low cost to enable the better phase separation. Moreover, it has low cloud point temperature (about 40 °C), which allows its application for the extraction of numerous hydrophobic complexes (Filik et al. 2006). The concentration of TX-114 was studied in the range of 0.05–0.3% as shown in Fig. 2. The extraction efficacy of the Hg was found to be highest at 0.2% (m/v). More upsurge concentration of surfactant leads to the lower recovery of Hg. For this purpose, back extracting agent was used to extract the analyte into acidic aqueous solution, and second round of CPE was performed.
Dispersion of the solution’s contents increases the interface between complexing agent and analyte. This actually enhances its entrapment by chelating agent in addition to mass transfer to the organic phase. It is anticipated that shaking the content of tubes after addition of chelating agent and surfactant has strong influence on the extraction efficacy of the developed method. This is because shaking enables additional protracted and close contact between extraction mixture and analyte in both phases. Two dispersion modes have been studied for the present methodology, which comprises vortex and ultrasound energy (Fig. 3). Among both dispersion modes, ultrasound was found to be best for the dispersion of extraction mixture as compared to vortex shaking, in relation to the analyte recovery, extraction efficacy, energy consumption, greener tactic, low cost and little chances of reagent loss. For the present study, sonication time was studied for the time duration of 1–5 min. The % recovery of Hg for the developed methodology increases with sonication time as shown in Fig. 4. The % recovery increases up to three min; further increase in time has no significant effect on the recovery. So, for the optimum recovery 3.0 min was selected and applied to rest of the experiments. Time for vortex mixing was studied in the range of (20–120) sec, and optimum recovery was observed at 90 s. However, in spite of lesser time period, the % recovery was around 10–20% lesser than those values obtained through sonication. Therefore, ultrasound energy was used for further experimental work, as it provides maximum extraction recoveries.
The optimal incubation time and sonication temperature are required for the ease in phase separation and preconcentration step. It is obvious that the cloud point temperature of the surfactant is responsible for the precipitation and formation of the surfactant-rich phase. The efficiency of CPE has been strongly influenced by the incubation temperature of the nonionic surfactant (TX-114). Efficient extraction of analyte and better phase separation could be enhanced at the equilibration temperature above than cloud point temperature of TX-114. Volume of surfactant-rich phase may reduce due to an increase in cloud point temperature (40 °C). At higher temperature, it causes dehydration that results hydrogen bonds disruption. Incubation temperature is therefore required at shorter time for effectual phase parting and complete analyte extraction. In the current study, the incubation temperature was studied for ultrasonic assisted dispersion at 40 °C, at room temperature, whereas vortex shaking was also carried out at room temperature.
Back extraction of the Hg in second round of CPE was carried out by means of sonication for 5 min at 40 °C. The complete phase separation was achieved after centrifugation at rate of 4000 rpm at 5 min, whereas 10 min was required for those samples obtained after vortex mixing.
For the back extraction of the Hg from the enriched phase, both nitric and hydrochloric acid in the range of 1.0 to 2.0 mol L−1 were selected. The volume was studied in the range of 0.2–1.0 mL. The optimal extraction was attained at ≤ 0.4 mL of HCl (Fig. 5), whereas 10 to 15% less value of analyte was obtained in the case HNO3. The better recoveries were achieved at volume of 0.4 mL HCl at 1.5 mol L−1, which was selected for following experimentation.
The consequence of interfering ions was studied for the recovery of Hg in ASE of snuff samples. For this purpose, various interfering analytes were added at diverse ratios to 10 mL solution of Hg (100 µg L−1), and developed methodology was applied to the solution. The results indicate that recovery of the target analyte was > 95% for all studied metals in the proposed DsUm-CPE method, as shown in Table 1.
Analytical figure of merit
The correlation coefficient of the calibration graph was found as 0.993 and 0.997 for vortex shaking and sonication, respectively. Preconcentration factor (PF) was calculated by the ratio among the volumes of the solution before and after the application of preconcentration method; however, the enhancement factor (EF) was intended as the ratio between the slope of calibration graph for with and without preconcentration step. The PF was found to be 78 and 63, with sonication and vortex shaking, respectively. The EF was found to be 97 and 84 with sonication and vortex shaking, respectively. The resulted limit of detection (LOD) was found as 0.004 µg L−1, which was adequate and low for the Hg determination at trace levels in ASE of snuff samples, using ultrasound energy as a dispersion mode. However, LOD for the developed method using vortex shaking was found as 0.25 µg L−1. Improved extraction efficiency 98 to 99.0% was observed in aqueous solutions by applying DsUm-CPE. The accuracy for the analysis of total Hg was checked by CRMs, and insignificant difference (p > 0.05) was found between certified and experimental values (Table 2). The analytical figure of merit for the proposed method is given in Table. 3. The developed method was found to be efficient when compared with the previously reported methods (Table 4) (Ali et al. 2017; Shah et al. 2009; Shirkhanloo et al. 2015; Stanisz et al. 2013; Yuan et al. 2012).
Application
The pH of all snuff samples was found to be very basic, in the range of 8.4–8.7. At this pH, tobacco-specific amines are formed making these products more toxic. The mean concentration of Hg with standard deviation for five composite samples of each type is shown in Table 5. The range of the Hg concentration in green and brown moist snuff was analyzed in the ranges of 0.13—0.62 μg g−1 and 0.24—0.71 μg g−1, respectively. The concentration ranges of Hg were found as 1.63–2.3 μg g−1 and 1.03–2.31 μg g−1 for dry black and brown snuff, respectively. The extractable Hg in ASE of green and brown moist snuff samples is found in the range of 0.053–0.243 μg g−1 and 0.146–0.277 μg g−1, respectively. The resulted data indicated that extractable Hg in ASE was found to be 30 to 48% of total Hg contents in all snuff products (Table 5), whereas extracted Hg in ASE of dry black and brown snuff samples was found as 0.424 to 0.598 μg g−1 and 0.304–0.682 μg g−1 corresponding to 23 to 34% of total Hg contents (Table 5).
Risk assessment
Estimated daily intake (EDI) is based on the concentration of Hg in chewing/sniffing products and the ingested amount of the selected SLT products. To calculate the EDI, the following equation was used. The calculations are based on the standard assumption recommended by United States Environmental Protection Agency (USEPA). Hg content in the snuff samples is expressed in µg Kg−1; average body weight is expressed in Kg; and amount of snuff was taken as 0.01 kg of snuff/person/day.
The EDI values intended for brown moist, green moist, dry black and dry brown snuff (10 g day−1) were calculated as 0.042–0.118, 0.022–0.103, 0.532–0.740, 0.512–0.823 µg kg−1bw, respectively. The resulted EDI values correspond to 8–15%, 2–13%, 21.5–48% and 33–48% of the proximal tolerable daily intake (PTDI) of Hg for brown moist snuff, green moist snuff, dry black and dry brown snuff, respectively. All calculated EDI values for Hg were found to be lower than the PTDI of Hg recommended by (Joint and Ng 2010). Highest intake of Hg is coming from consumption of dry black snuff, whereas lowest comes from green moist snuff sample. Maximum weekly intake of total Hg for adult persons is recommended as 5.6 µg kg−1bw (1.6 µg kg−1bw of methylmercury and 4 µg kg−1bw of inorganic mercury) by Joint FAO/WHO expert committee for provisional tolerable weekly intake (Joint and Ng 2010). Average daily dose (ADD) of Hg depends on both the amount of consumption of SLT and the metal concentration in SLT. The ADD of Hg was calculated by means of its average concentration in SLT by its consumed weight via an individual (body weight 60 kg for an adult in Pakistan). The equation used for the determination of ADD of Hg in SLT for adults is given below:
where C signifies the concentration of Hg (mg g −1) in snuff products; exposure frequency (EF) and exposure duration (ED) signify exposure period of 365 days per year and 30 year for adults, respectively, for the present work. Ingestion rate (mg day−1) is represented by IngR; BW is adult’s body weight (60 kg); for non-carcinogenic risk, average time (AT) is calculated as ED*EF; however, conversion factor is signified as CF (10 −6 mg/kg). All these factors are available in the earlier intelligences of US EPA and in our formerly published work (Akhtar et al. 2016; Means 1989; Part 2011).
Non-carcinogenic risk associated with chronic exposure of Hg through SLT consumption for SLT consumers was evaluated based on the Hazard Quotient (HQ). HQ value < 1 signifies that population is improbable to have the risk. HQ was calculated by following equation (EPA 1992).
where ADD represents the average daily dose of Hg (mg kg−1 day−1) via snuff consumption and reference dose (RfD) for Hg is 0.0003 mg kg−1 day−1 which is recommended by the United States Environmental Protection Agency (EPA. 2013). The ADD values intended for brown moist, green moist, dry black and dry brown snuff samples based on consumption of 10 g day−1 are calculated as 0.07–0.123, 0.02–0.1, 0.17–0.39, 0.27–0.38 µg kg−1bw, respectively. FAO/WHO Joint Expert Committee on Food Additives (JECFA) and WHO, 2010, recommended Provisional Tolerable intake (PTI) of Hg, 5.6 µg Kg−1 body weight/week or 0.8 µg Kg−1 body weight/day (Joint 2003). The calculated ADD values for Hg were found to be lower than the tolerable daily intake level in brown and green moist snuff. However, consumption of higher weight and multiple time of snuff products might be created adverse impact on consumers. Highest intake of Hg is coming from consumption of dry black and dry brown snuff. The ADD values are shown in Table 6. The HQ values are found to be greater than 1 for dry black and dry brown snuff products, whereas for green and brown moist snuff HQ values were found to be < 1.0. It was indicated in the literature that the HQ values > 1 suggest adverse risk of human being (Akhtar et al. 2016; EPA 1992). Carcinogenic risk assessment in terms of cancer risk could not be performed due to unavailability of cancer slope factor for Hg.
Exposure of Hg in population consumed snuff products
The Hg levels in hair samples of adult male subjects consuming different types of snuff were higher at 95% confidence interval (CI: 4.64–5.52 μg g−1) than referents male subjects not use any cigarette or/smokeless products have (CI: 1.68–2.09 μg g−1). The Hg levels in blood samples of adult male subjects consuming different snuff products were higher at 95% confidence interval (CI: 7.70–9.82 μg g−1), than referents male subjects not use any cigarette or/smokeless products have (CI: 4.23–6.17 μg g−1). The contents of Hg in hair samples of the population consuming different types of snuff have significantly higher levels than those who were not using any snuff product (p = 0.03–0.04). The resulted data indicate a correlation of sniffing habit with Hg content in biological samples of the adult population. The average values of Hg content in hair and blood samples of adult male subjects with standard deviation are given in Table 7.
Conclusion
An innovative ultrasonically modified cloud point extraction method which eliminates the surfactant’s effects using acidic solutions and long ultrasonic-assisted shaking/heating on the extraction efficiency was developed for the preconcentration of Hg, whereas the acidic ASE of each snuff products was directly subjected for complexation with DDTP. In addition to this, two dispersion modes, i.e., ultrasound energy and vortex shaking, were compared. Dispersion of the micelle by the ultrasound energy for the proposed method improves the extraction efficiency immensely due to increased interaction of metal chelate with micelles formed after cloud point. Ultrasound energy improves the extraction efficiency by increasing the contact area between both phases (aqueous and surfactant rich phase) which ease the analytes mass transfer into the micelles enriched phase from the aqueous phase. This preconcentration method with higher extraction efficiency and valuable parameters facilitates the tough task of Hg determination in the ASE of smokeless tobacco products. Average Hg concentration in each SLT product indicates that the source of Hg may be related with preparation of SLT products or mixing unidentified constituents. Daily intake of Hg was greater than the tolerable daily intakes, which shows the significant risk related to the consumption of these products. The findings of the current study would be advantageous for health specialists and individuals consume SLT frequently. Moreover, extraction rate of the Hg from the snuff samples by the saliva is different for each type (dry and moist). It was observed that dry snuff, especially black, contained higher levels of Hg than moist green and brown snuff.
References
Akhtar A, Afridi HI, Kazi TG et al (2017) Chromium Exposure in the Adult Population, Consuming Different Types of Smokeless Tobacco Products in Pakistan. Biol Trace Elem Res 175:312–321
Akhtar A, Kazi TG, Afridi HI et al (2016) Vortex-assisted ionic liquid-based dispersive liquid–liquid microextraction for assessment of chromium species in artificial saliva extract of different chewing tobacco products. Environ Sci Pollut Res 23:25288–25298
Ali J, Tuzen M, Kazi TG (2017) Evaluation of Mercury in Environmental Samples by a Supramolecular Solvent-Based Dispersive Liquid-Liquid Microextraction Method Before Analysis by a Cold Vapor Generation Technique. J AOAC Int 100:782–788
Arain SS, Kazi TG, Arain JB, Afridi HI, Brahman KD (2014) Preconcentration of toxic elements in artificial saliva extract of different smokeless tobacco products by dual-cloud point extraction. Microchem J 112:42–49
Bloomfield MM, Stephens LJ (1996) Chemistry and the living organism. J. Wiley, New York
Boffetta P, Hecht S, Gray N, Gupta P, Straif K (2008) Smokeless tobacco and cancer. Lancet Oncol 9:667–675
Bolewska J, Holmstrup P, Moller-Madsen B, Kenrad B, Danscher G (1990) Amalgam associated mercury accumulations in normal oral mucosa, oral mucosal lesions of lichen planus and contact lesions associated with amalgam. J Oral Pathol Med 19:39–42
Borges DLG, da Veiga MAMS, Frescura VLA, Welz B, Curtius AJ (2003) Cloud-point extraction for the determination of Cd, Pb and Pd in blood by electrothermal atomic absorption spectrometry, using Ir or Ru as permanent modifiers. J Anal At Spectrom 18:501–507
Bull S (2011) HPA compendium of chemical hazards inorganic mercury/elemental mercury. Health Protection Agency CRCE HQ, HPA p 33
Carabias-Martınez R, Rodrıguez-Gonzalo E, Moreno-Cordero B, Pérez-Pavón J, Garcıa-Pinto C, Laespada EF (2000) Surfactant cloud point extraction and preconcentration of organic compounds prior to chromatography and capillary electrophoresis. J Chromatogr A A 902:251–265
Chiha M, Hamdaoui O, Ahmedchekkat F, Pétrier C (2010) Study on ultrasonically assisted emulsification and recovery of copper (II) from wastewater using an emulsion liquid membrane process. Ultrason Sonochem 17:318–325
Coelho LM, Arruda MAZ (2005) Preconcentration procedure using cloud point extraction in the presence of electrolyte for cadmium determination by flame atomic absorption spectrometry. Spectrochim Acta B At Spectrosc 60:743–748
Dhaware D, Deshpande A, Khandekar R, Chowgule R (2009) Determination of toxic metals in Indian smokeless tobacco products. Sci World J 9:1140–1147
Dressler VL, Flores ÉM, Pozebon D, Kaercher LE (2002) On-line pre-concentration of Hg in blood and urine and determination by CVAAS. J Anal At Spectrom 17:790–793
Emerging SCo, Risks NIH (2008) Health effects of smokeless tobacco products. Health & Consumer Protection DG, European Commission Brussels
EPA E (1992) risk assessment forum: Guidelines for exposure assessment [FRL-4129–5] Washington, DC.
EPA. U (2013) Regional screening level (RSL) summary table. United States Environmental Protection Agency Washington (DC).
Farrand P, Rowe R, Johnston A, Murdoch H (2001) Community dentistry: prevalence, age of onset and demographic relationships of different areca nut habits amongst children in Tower Hamlets London. Br Dent J 190:150
Filik H, Şener İ, Cekic SD, Kiliç E, Apak R (2006) Spectrophotometric determination of paracetamol in urine with tetrahydroxycalix [4] arene as a coupling reagent and preconcentration with Triton X-114 using cloud point extraction. Chem Pharm Bull 54:891–896
García-Rico L, Leyva-Perez J, Jara-Marini ME (2007) Content and daily intake of copper, zinc, lead, cadmium, and mercury from dietary supplements in Mexico. Food Chem Toxicol 45:1599–1605
Gnamuš A, Byrne AR, Horvat M (2000) Mercury in the soil-plant-deer-predator food chain of a temperate forest in Slovenia. Environ Sci Technol 34:3337–3345
Guiñez M, Bazan C, Martinez LD, Cerutti S (2018) Determination of nitrated and oxygenated polycyclic aromatic hydrocarbons in water samples by a liquid–liquid phase microextraction procedure based on the solidification of a floating organic drop followed by solvent assisted back-extraction and liquid chromatography–tandem mass spectrometry. Microchem J 139:164–173
Gupta PC, Ray CS (2003) Smokeless tobacco and health in India and South Asia. Respirology 8:419–431
Hussein HS, Ruiz ON, Terry N, Daniell H (2007) Phytoremediation of mercury and organomercurials in chloroplast transgenic plants: enhanced root uptake, translocation to shoots, and volatilization. Environ Sci Technol 41:8439–8446
Joint F (2003) Summary and conclusions of the 61st meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). FAO/WHO, Rome.
Joint F, Ng J, Additives WECoF (2010) Joint FAO/WHO Expert Committee on Food Additives. Seventy-second meeting: Summary and conclusions
Ju T, Jiang J, Meng Y, Yan F, Xu Y, Gao Y, Aihemaiti A (2020) An investigation of the effect of ultrasonic waves on the efficiency of silicon extraction from coal fly ash. Ultrason Sonochem 60:104765
Kazi TG, Arain SS, Afridi HI, Brahman KD, Kolachi NF, Mughal MA (2013) Analysis of cadmium, nickel, and lead in commercial moist and dry snuff used in Pakistan. Environ Monit Assess 185:5199–5208
Khan M et al (2017) Application of ultrasonically modified cloud point extraction method for simultaneous enrichment of cadmium and lead in sera of different types of gallstone patients. Ultrason Sonochem 39:313–320
Khawaja MI, Shafiq M, Nusrat R, Khawaja MR (2005) Preventing the oral cavity cancer epidemic. Asian Pac J Cancer Prev 6:420
Manzoori JL, Bavili-Tabrizi A (2002a) The application of cloud point preconcentration for the determination of Cu in real samples by flame atomic absorption spectrometry. Microchem J 72:1–7
Manzoori JL, Bavili-Tabrizi A (2002b) Cloud point preconcentration and flame atomic absorption spectrometric determination of Cd and Pb in human hair. Anal Chim Acta 470:215–221
Mazahir S et al (2006) Socio-demographic correlates of betel, areca and smokeless tobacco use as a high risk behavior for head and neck cancers in a squatter settlement of Karachi Pakistan. Subst Abuse Treat Prev Policy 1:10
Means B (1989) Risk-assessment guidance for superfund. Volume 1. Human health evaluation manual. Part A. Interim report (Final). Environmental Protection Agency, Washington, DC (USA). Office of Solid Waste.
Mehrabi F, Dil EA (2017) Investigate the ultrasound energy assisted adsorption mechanism of nickel (II) ions onto modified magnetic cobalt ferrite nanoparticles: Multivariate optimization. Ultrason Sonochem 37:37–46
Miano AC, Ibarz A, Augusto PED (2016) Mechanisms for improving mass transfer in food with ultrasound technology: Describing the phenomena in two model cases. Ultrason Sonochem 29:413–419
Middleton J (2016) Worldwide news and comment. Tob Control 25:5
Mirbod SM, Ahing SI (2000) Tobacco-associated lesions of the oral cavity: Part I Nonmalignant lesions. J Can Dent Assoc 66:252–256
Pappas R, Stanfill S, Watson C, Ashley D (2008) Analysis of toxic metals in commercial moist snuff and Alaskan iqmik. J Anal Toxicol 32:281–291
Part F (2011) Supplemental guidance for inhalation Risk Assessment, vol. I EPA/540/1e89/002
Patra M, Sharma A (2000) Mercury toxicity in plants. Bot Rev 66:379–422
Patrick L (2002) Mercury toxicity and antioxidants: part I: role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. (Mercury Toxicity). Altern Med Rev 7:456–472
Popova L, Ling PM (2013) Alternative tobacco product use and smoking cessation: a national study. Am. J Public Health 103: 923–930
Rodu B, Cole P, Fisher MA, Taylor GW, Tilashalski KR (2005) Smokeless tobacco and periodontal Disease/The authors reply. J Dent Res 84:1086
Rodu B, Jansson C (2004) Smokeless tobacco and oral cancer: a review of the risks and determinants. Crit Rev Oral Biol Med 15:252–263
Schat H, Llugany M, Bernhard R (2000) Metal-specific pattern of tolerance, uptake, and transport of heavy metals in hyperaccumulating and non-hyperaccumulating metallophytes. In: Phytoremediation of contaminated soils and water. CRC Press LLC, pp 171–188.
Shah AQ, Kazi TG, Arain MB, Jamali MK, Afridi HI, Jalbani N, Baig JA (2009) Comparison of electrothermal and hydride generation atomic absorption spectrometry for the determination of total arsenic in broiler chicken. Food Chem 113:1351–1355
Shemirani F, Abkenar SD, Jamali MR (2005) Determination of cadmium (II), copper (II) and zinc (II) in water samples by flame atomic absorption spectrometry after cloud point extraction. Indian J Chem Sect A 44:1211–1214
Shirkhanloo H, Khaligh A, Mousavi HZ, Eskandari MM, Miran-Beigi AA (2015) Ultra-trace arsenic and mercury speciation and determination in blood samples by ionic liquid-based dispersive liquid-liquid microextraction combined with flow injection-hydride generation/cold vapor atomic absorption spectroscopy. Chem Pap 69:779–790
Stanisz E, Werner J, Matusiewicz H (2013) Mercury species determination by task specific ionic liquid-based ultrasound-assisted dispersive liquid–liquid microextraction combined with cold vapour generation atomic absorption spectrometry. Microchem J 110:28–35
Subramanian S, Nandy S, Kelly M, Gordon D, Smith GD (2004) Patterns and distribution of tobacco consumption in India: cross sectional multilevel evidence from the 1998–9 national family health survey. BMJ 328:801–806
Wrangsjo K, Alderling M, Lindahl G, Meding B (2015) Hand Eczema and Use of Snus (Moist Snuff)–a Population-based Study. Acta Derm Vener 95:3
Wu G-H, Cao S-S (2010) Mercury and cadmium contamination of irrigation water, sediment, soil and shallow groundwater in a wastewater-irrigated field in Tianjin, China. Bull Environ Contam Toxicol 84:336–341
Yu L-P (2005) Cloud point extraction preconcentration prior to high-performance liquid chromatography coupled with cold vapor generation atomic fluorescence spectrometry for speciation analysis of mercury in fish samples. J Agric Food Chem 53:9656–9662
Yuan C-G, Wang J, Jin Y (2012) Ultrasensitive determination of mercury in human saliva by atomic fluorescence spectrometry based on solidified floating organic drop microextraction. Microchim Acta 177:153–158
Acknowledgment
The authors would like to thank National Centre of Excellence in Analytical Chemistry (NCEAC) university of Sindh Jamshoro, for providing excellent research lab facilities to carryout research work.
Funding
This study was not funded by any funding agency.
Author information
Authors and Affiliations
Contributions
Dr Tasneem G Kazi made project and took part in designing the study, interpretation and check the manuscript. Asma Akhtar sampling the smokless tobacco products, preparation and perform experimental work. Dr Hassan Imran Afridi gathering and generating the data as well as proof reading of text.
Dr Syed Syed Ghulam Musharraf and Muhammad Balal Arain, took part in experiment and analysis of the mercury in smokless tobacco Products and biological samples.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akhtar, A., Kazi, T.G., Afridi, H.I. et al. Efficiency of different green shaking extraction methods for the preconcentration of trace quantity of mercury in artificial saliva extract of snuff products: impact on adult consumers. Chem. Pap. 75, 3005–3015 (2021). https://doi.org/10.1007/s11696-021-01545-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01545-7