Abstract
Facile preparation of one-dimensional spinel material is highly attractive for their widespread usage in energy storage devices. Herein, we report uniform one-dimensional CoMn2O4 nanorods were prepared by cetyl trimethyl ammonium bromide (CTAB) assisted hydrothermal technique followed by subsequent calcination process. The prepared materials were characterized to different types of physicochemical features to analyze the appropriateness of the material for the supercapacitor electrode application. The crystalline nature and bonding properties were examined by X-ray diffraction analysis and Fourier-transform infrared spectroscopic analysis. The structural and morphological features of prepared materials were analyzed using field emission scanning electron microcopy which reveals the edge curved one-dimensional nanorod structure. Furthermore, cyclic voltammetric, chronopotentiometric and electrochemical impedance spectroscopic analyses were employed to evaluate the electrochemical properties of the freshly prepared CoMn2O4 materials. The cyclicvoltammetric studies provide the specific capacitance of 895 Fg−1 at a scan rate of 5 mV s−1, whereas chronopotentiometric curves provide the highest specific capacitance of 802 Fg−1 at current density of 1 Ag−1. The cyclic stability analysis shows the excellent cyclic stability which retains 87% of initial capacitance after the continuous 5000 CV cycles at a scan rate of 100 mVs−1. The outstanding electrochemical features unquestionably make CoMn2O4 as a good candidate for supercapacitor devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid economic growth in recent decades has endorsed the alarming conflict between high requirement of fossil fuels and their restricted resources. To resolve this problem, researchers have been looking for efficient relevant technologies to fabricate renewable energy storage and conversion devices whereas conserving the surroundings(Zhu et al. 2015; Wang et al. 2016; Abbasi et al. 2020; Amiri et al. 2020; Moosavifard et al. 2020). Supercapacitors or electrochemical capacitors, as a new kind of energy storage device, have fascinated more consideration since their interesting characteristics such as high-power density than batteries and high energy density than conventional capacitors (Chen et al. 2014; Xu et al. 2014a, b). With a combination, consolidation of high power density and comparatively high energy density, supercapacitors have been generally utilized in various kinds of applications including memory backup systems, wireless networks, automotives, portable consumer electronics, motor starters and other self-powered micro/nano-electronics (Simon 2008; Zhu et al. 2008; Yesuraj et al. 2017). Supercapacitors can be categorized into two types such as electric double-layer capacitors (EDLC) and pseudocapacitors based on the energy storage mechanism (Zhang et al. 2017, 2019). In electric double-layer capacitors, the charge is stored by accumulation of charges at the electrode/electrolyte interface by achieving the formation of electric double layers (Makino et al. 2013; Wulan Septiani et al. 2020). Mostly, carbon-based materials (carbon nanotube, activated carbon, carbon aerogel and graphene oxide) exhibit the electric double layer energy storage mechanism. On the other hand, the pseudocapacitor materials such as metal oxides and conducting polymers can store charges through rapid and reversible surface redox reactions and it possesses much higher specific capacitance than electric double layer capacitor materials(Chen et al. 2013; Li et al. 2013; Wu et al. 2014). Therefore, developing of novel and proficient pseudocapacitor electrode materials with attractive electrochemical performances such as high electrical conductivity, electrochemical stability combined with novel structural features such as high surface area and porous structure is the hot research topic in recent years.
Transition metal oxides such as NiO (Yan et al. 2014), MnO2(Boisset et al. 2013), Co3O4(Xia et al. 2011), MoO3(Zhao et al. 2013), V2O5(Yang et al. 2013), SnO2(Geng et al. 2020) and ZnO (Naeem et al. 2020) materials have been examined intensively as the advanced electrode materials for supercapacitor device application since they exhibit multiple oxidation states for fast redox reactions, low-cost and eco-friendly nature. In contrast, these aforementioned materials often experience the poor cycle life, limited rate capability and poor mechanical stability when used as supercapacitor electrode materials (An et al. 2019; Li et al. 2019; Low et al. 2019). Also, they exhibit relatively low capacitance when compared with RuO2 (Hu et al. 2006) (identified as a potential supercapacitor electrode material) and these limitations have restricted their utilization in commercial supercapacitors. Consequently, it is a huge task to prepare electrode materials with superior electrochemical performances and fulfill the drawbacks of single transition metal oxide-based supercapacitor electrode materials.
Recently, mixed metal oxides such as ZnCo2O4(Mary and Bose 2018), NiCo2O4(Pu et al. 2013), MnCo2O4(Che et al. 2016), CuCo2O4(Vijayakumar et al. 2017) and CoMn2O4 are demonstrated as efficient electrode materials for supercapacitors because of their mixed valence states that afford higher electrochemical activity and superior electronic conductivity than single metal oxides. Among them, Mn-based materials are of great interest because of their high electrical conductivity, low-cost and easily abundance and eco-friendly nature. Spinel structured CoMn2O4 exhibits high electrical conductivity and attractive electrochemical features when compared with corresponding single metal oxides such as Co3O4 and Mn3O4 (Yunyun et al. 2015). By altering the surface morphological features, the electrochemical properties of the CoMn2O4 materials could be considerably enhanced. Recently, Ji and Zhou et al. prepared cubic CoMn2O4 particles which directly grown on Ni foam through hydrothermal method. The prepared CoMn2O4 particles were used as binder-free electrode which provides a capacity of 140.6 mA h g−1 at 1 mA cm−2 in three electrode configuration and an asymmetric device exhibits a specific capacitance of 77.8 Fg−1 at 2 mA cm−2 (Chen et al. 2019a, b). Potheher et al. demonstrated CoMn2O4 material synthesized through hydrothermal technique. This material possessed the specific capacitance of 700 Fg−1 at a scan rate of 5 mV s−1(Vigneshwaran et al. 2016). Furthermore, Xuetang and Fan et al. established Spinel CoMn2O4 nanosheet arrays grown on Ni foam via hydrothermal route. The nanosheet arrays provide a specific capacitance of 1529 Fg−1 and good rate capability. The cyclic stability studies showed that the 100% stability after 3000 cycles (Yunyun et al. 2015). Wang et al. prepared both MnCo2O4 and CoMn2O4 nanowires using thermal decomposition of organometallic compounds. The MnCo2O4 and CoMn2O4 affords the specific capacitance of 2108 and 1342 Fg−1, respectively, at a current density of 1 Ag−1(Paquin et al. 2015). Consequently, it is important to take essential steps for enhancing the supercapacitive properties of the CoMn2O4 materials. The various synthetic routes are widely explored, including hydrothermal method, templates synthesis, co-precipitation method, thermal conversion, electrochemical deposition and many other methods. Among them, hydrothermal method has been recognized as a powerful method to provide size-controllable and well-ordered nanostructured morphology. In this endeavour, we followed the CTAB-assisted hydrothermal method to prepare CoMn2O4 materials (Dhand et al. 2015).
Herein, we report one-dimensional CoMn2O4 nanorods via CTAB-assisted hydrothermal technique followed by subsequent calcination process which also involved the variation of CTAB template concentration. Electrochemical measurements show that the prepared CoMn2O4 material had better supercapacitive features with a high specific capacitance of 895 Fg−1 at a scan rate of 5 mVs−1. In addition, cyclic stability studies shows that the prepared CoMn2O4 material retained 92% of specific capacitance after 2000 cycles. The outstanding electrochemical features made as the freshly prepared CoMn2O4 nanorod as a promising material for supercapacitor electrode applications.
Experimental section
Materials
All chemical reagents were analytical grade and utilized as received. Cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O), poly (vinylidene fluoride) (PVDF), carbon black and cetyltrimethylammonium bromide (CTAB) were procured from Sigma Aldrich. N-methyl- 2-pyrrolidone (NMP), potassium hydroxide (KOH), Manganese nitrate tetrahydrate (Mn(CH3COO)2.4H2O) and ethanol were purchased from SRL (India). In addition, acetone and nickel foil (0.025 mm thickness) were received from Alfa aesar.
Synthesis of CoMn2O4 materials
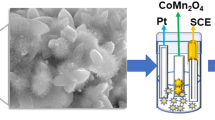
The CTAB-assisted hydrothermal synthetic method has been followed for the preparation of spinel CoMn2O4 materials. In a typical preparation of CoMn2O4 materials, 1 mM of cobalt nitrate hexahydrate and 2 mM of manganese nitrate tetrahydrate were dissolved in 70 ml of DI water at room temperature using magnetic stirrer with rotating speed of 450 rpm. Then, CTAB template was added to the above suspension and stirred for half an hour. After the complete dissolution of all precursors, the suspension was transferred to 100 ml of teflon lined stainless steel autoclave and kept in hot air oven. The hot air oven was heated to 180 °C for 24 h and then it is allowed to cool room temperature normally. The resultant precipitate was collected and washed with water and ethanol to remove impurities and unreacted species. Then, it is dried in a hot oven at 80 °C for 12 h and calcined in air at 300 °C for 3 h with ramping rate of 10 °C/min. In this endeavour, the CTAB concentration was varied to get the different type of nanostructure CoMn2O4 materials. The CTAB concentrations such as 0, 0.005, 0.01 and 0.02 M were used to prepare CoMn2O4-1, CoMn2O4-2, CoMn2O4-3 and CoMn2O4-4 samples, respectively. Figure 1 shows the schematic diagram of synthesis of CoMn2O4-4 material.
Material characterization
The X-ray diffraction (XRD) patterns were acquired on a PANalytical X-pert PRO diffractometer instrument with Ni filtered Cu-Kα radiation (λ = 0.154060 nm) at a voltage of 40 kV and a current of 40 mA. Fourier transform infrared spectroscopy (FTIR) was recorded using Perkin-Elmer RX1′ spectrophotometer instrument with 4 cm−1 resolution for 20 scans and field-emission scanning electron microscope (FE-SEM, Hitachi SU6600) operated with an accelerating voltage of 15 kV for obtained morphological features.
Electrochemical measurements
For electrochemical measurement, the working electrode comprising of 70 wt% of active material, 10 wt% of carbon black and 10 wt% of poly (vinylidene fluoride) (PVDF). For electrochemical analysis, the above-mentioned materials made slurry with N-methyl-2- pyrrolidone (NMP) solvent. The resultant slurry was coated uniformly on the surface (two side) of nickel foil and dried in hot air oven at 60 °C for 12 h. The active material’s weight of the electrode is approximately 3 mg. The supercapacitor analysis was done on a biologic electrochemical workstation (Model VSP-150) in three electrode set up using 2 M KOH as an electrolyte. Standard calomel electrode and platinum foil were used as counter and reference electrodes, respectively. The supercapacitive properties of the CoMn2O4 material were examined by cyclic voltammetric (CV), chronopotentiometric (CP) or galvanostatic charge/discharge (GCD) and electrochemical impedance studies (EIS). Additionally, cyclic stability test was also carried out to demonstrate cycle life of the CoMn2O4 electrodes.
Results and discussion
X-ray diffraction analysis
The phase purity and crystallographic nature of the freshly prepared CoMn2O4 materials were examined by X-ray diffraction studies as shown in Fig. 2. The intensities and positions of the peaks are more consistent with the standard diffraction pattern of the tetragonal, spinel CoMn2O4 phase with JCPDS card no.- 01-077-0471. The distinctive peaks located at 29.3, 30.8, 33.3, 36, 36.8, 44.2, 52.7, 56.5, 58.3, 65.6, and 71.2° are indexed to the (112), (200), (103), (211), (202), (220), (105), (303), (321), (116) and (305) planes, respectively. All diffraction peaks are appeared as same, however, intensities only vary.
FTIR spectroscopic analysis
The internal structure and bonding properties of the synthesized materials such as CoMn2O4-1, CoMn2O4-2, CoMn2O4-3 and CoMn2O4-4 were evaluated using FTIR spectroscopic analysis as shown in Fig. 3. It is worth to note that the impurity peaks do not visible in FTIR spectrum confirms the formation of pure CoMn2O4 materials. The FTIR spectrum provides two high-intensity peaks which are the characteristic peak of the spinel CoMn2O4 materials. The peaks appeared at 652 and 540 cm−1 are due to bending vibration of cobalt oxide at tetrahedral sites and manganese oxide at octahedral sites, respectively (Hosseini et al. 2011; Vigneshwaran et al. 2016). The basic characterization results such as XRD and FTIR have established the formation, crystalline phase and internal structure of the CoMn2O4 in the present CTAB assisted hydrothermals synthetic method.
Morphological analysis
The morphological features of CoMn2O4 were evaluated using FE-SEM analysis as shown in Figs. 4, 5. Figure 4 a, b shows the lower and higher magnification FESEM images of CoMn2O4-1 materials which shows irregular-shaped nanoparticles, which was synthesized without CTAB template. The definite-shaped nanoparticles with the size of 90 ± 5 nm are formed when introducing of 0.005 M of CTAB template (CoMn2O4-2) as shown in Fig. 4 c and d. When the CTAB concentration increased to 0.01 M (CoMn2O4-3), the same morphology was obtained and the shape of the nanoparticles are getting elongated which shows in Fig. 5a, b. Figure 5c, d shows the lower and higher magnification images of CoMn2O4-4 material which shows the edge-curved nanorod morphology.
The size and length of the nanorods are 30 ± 5 nm and 150 ± 5 nm, respectively. These results signify that the CTAB template has more consequences on the morphology of the CoMn2O4 materials. The nanorods morphology is more favored for electrochemical process since it provides high surface area for electrochemical redox reactions.
Electrochemical analysis
The supercapacitive features of the freshly prepared CoMn2O4 electrodes such as CoMn2O4-1, CoMn2O4-2, CoMn2O4-3 and CoMn2O4-4 were examined in a three electrode configuration using 2 M KOH electrolyte. Usually, the electrochemical properties can be demonstrated using different techniques including cyclic voltammetry (CV), chronopotentiometry (CP) and electrochemical impedance spectroscopy (EIS).
A cyclic voltammetric study is utilized to evaluate the redox behavior, capacitance and reversibility nature. Figure 6a–d shows the cyclic voltammetric curves of CoMn2O4-1, CoMn2O4-2, CoMn2O4-3 and CoMn2O4-4, respectively, which were demonstrated at the scan rates from 5 to 100 mV s−1 within a potential window from 0 to 0.5 V. All the CV curves exhibit the redox peaks such as the anodic peak located at approximately 0.31 V. whereas the cathodic peak positioned at approximately 0.22 V which is due to the redox process and signifying the pseudocapacitor behavior of the CoMn2O4 materials (Cai et al. 2014; Senthilkumar and Kalai Selvan 2014).
The redox reaction may be described in the following Eqs. 1, 2 and 3. (Yunyun et al. 2015)
The broadness of CV curves is increasing from CoMn2O4-1 to CoMn2O4-4 confirming the superior pseudocapacitance behavior of the CoMn2O4-4 material. The diffusive and capacitive contributions in CV analysis (for CoMn2O4-4 material) were calculated by using the Eq. (4).
where I is the fitting current, \(v\) is the scan rate. The a and b are variables. The value of b is determined by plotting the log (i) versus log (\(v\)) curves as shown in Fig. 6e. The b value of 0.5 demonstrates diffusion controlled progression, whereas 1.0 indicates the surface capacitive controlled process. In the present investigation, the b values are 0.64 and 0.72 for anodic and cathodic current, respectively. This indicates that the present energy storage process is mixed behavior of diffusive and capacitive controlled process. (Chen et al. 2019a, b; Zhang et al. 2020).
The quasi symmetric feature of the redox peaks and cycles attributes the outstanding reversibility of the CoMn2O4 electrodes. when the scan rate is raised from 5 to 100 mV s−1, the current response enhance at the same time as the shape profile of the CV curves do not altered which suggesting enhanced mass transportation and rapid conduction of electrons in the electrode material during the electrochemical process(Jadhav et al. 2016). Interestingly, the peak appeared at the anodic current was shifted towards the positive region, whereas cathodic peak drift towards the more negative region which is due to ohmic resistance and electrical polarization behavior of CoMn2O4 electrodes(Cao et al. 2014). In addition, the area under the cyclic voltammetric curves is directly proportional to the specific capacitance of the corresponding electrodes. The CoMn2O4-4 provides higher area under the CV curves than other electrodes such as CoMn2O4-1, CoMn2O4-2 and CoMn2O4-3 confirming its superior pseudocapacitive behavior. The specific capacitance of the CoMn2O4 electrodes were calculated from the CV curves utilizing Eq. 5.
where ∫idV denotes the integral area of CV curve, ∆V potential limit (V), m mass of the active material (mg), and S the sweep rate (mV s−1) from 5 to 100 mV s−1. The obtained specific capacitance values are 232, 511, 670 and 895 Fg−1 for CoMn2O4-1, CoMn2O4-2, CoMn2O4-3 and CoMn2O4-4 electrodes, respectively, at a scan rate of 5 mV s−1. The specific capacitance of CoMn2O4-4 electrode (895 Fg−1) is approximately four-fold increases from the specific capacitance of the CoMn2O4-1electrode (232 Fg−1). Additionally, the specific capacitance of the.
CoMn2O4-4 electrode is higher than previously published literatures based on Co3O4, (Co3O4 nanoparticles (519 Fg−1)(Vijayakumar et al. 2013), hollow Co3O4 nanowire (599 Fg−1) (Xia et al. 2011) and Co3O4 nanowire (754 Fg−1) (Tu et al. 2012) MnO2 (MnO2 nanostructure (311 Fg−1) (Zhang et al. 2013), MnO2 nanorods (649 Fg−1) (Kumar et al. 2016) and α- MnO2 nanorod (166.2 Fg−1) (Li et al. 2011) and CoMn2O4 electrode materials (CoMn2O4 nanofibers (121 Fg−1) (Alkhalaf et al. 2017), cubic CoMn2O4 particles (77.8 Fg−1) (Chen et al. 2019a, b) and CoMn2O4 (700 Fg−1) (Vigneshwaran et al. 2016). The superior specific capacitance behavior of the CoMn2O4-4 electrode is explained as follows: (i) The one-dimensional nanorods exhibit high surface area which provides the more active sites for electrochemical reaction. (ii) The small dimensions of the nanorods not only enhance the transportation of electrolyte ions and also increase the absorption of ions during electrochemical analysis. (ii) It gives the short diffusion path for electron and ion transportations and remained their original structural natures.
The rate capabilities of all electrode materials were characterized through scan rate vs specific capacitance graph as shown in Fig. 6f. The specific capacitances decrease with the increasing of scan rate and this trend is explained as follows: at low scan rate, the larger time is allowed to perform electrochemical reaction actively in both inner and outer region of electrode materials and hence the specific capacitance increases. On the other hand, the outer region of electrode only participate in the electrochemical process during higher scan rate which is due to the shorter time and thus decrease the specific capacitance(Kim et al. 2016). The remaining characterizations such as electrochemical impedance spectroscopy, chronopotentiometry and cyclic stability studies were carried out only for CoMn2O4-4 electrode due to its better electrochemical properties.
The electrochemical impedance spectroscopic analyses were carried out to analyze intrinsic electrochemical properties of the CoMn2O4-4 electrode as shown in Fig. 7. Two main distinctive properties observed in the high- and low-frequency regions are ascribed to different resistance and capacitance phenomena during different interfacial processes in Faradaic reactions. The semicircle at high-frequency region is the characteristic of resistance at the solid oxide or electrode materials/liquid electrolyte interface owing to discontinuity in the charge transfer process. The straight line obvious at low-frequency region attributes the confirmation of diffusive charge transfer mechanism. The intercept of the real axis is due to internal resistances which are originated from the resistance produced from all the active electrode materials, the resistance of the electrolyte and the resistance created between material/current collector interfaces (contact resistance) (Bengoechea et al. 2002; Lu et al. 2016).
The more vertical line of the curve attributes the more facile electrolyte diffusion to the surface due to the small nanoparticle size and large surface area of the compared to the CoMn2O4 -4 sample(Xu et al. 2014a, b). Figure 8a shows the chronopotentiometric curves of the CoMn2O4-4 electrode at a current densities of 1, 2, 3, 4, 5, 10 and 20 Ag−1. The potential limits from 0 to 0.45 V was chosen for chronopotentiometric analysis and it shown in Fig. 7. The non-linear behavior of the charge discharge curves confirms the pseudocapacitive nature which is more consistent with the CV analysis. It is interesting to note that there are two sections of CP curves, including the straight line and curved line which signifies the characteristic faradaic nature(Zhao et al. 2016). The specific capacitances were calculated by CP curves, using the Eq. 6.
where I denotes discharge current density (A), ∆t is the discharge time (s), ∆V the potential window (V) and m is the mass of the active material (mg). The CoMn2O4-4 electrode exhibit a specific capacitance of 802, 649, 580, 524, 466, 222 and 177Fg −1 at a current densities of 1, 2, 3, 4, 5, 10 and 20 Ag−1, respectively. The specific capacitance vs. current density graph is shown in Fig. 8b. The specific capacitance values decrease with increasing of current density which is due to the time limitation process and it is more reliable with the CV curves (Yesuraj et al. 2019).
Long-time cyclic stability behavior of the materials is also great importance for high performance supercapacitor application. Cyclic stability of CoMn2O4-4 electrode is further evaluated by using continuous 5000 CV cycles at high scan rate of 100 mVs−1 as shown in Fig. 9. The inset of Fig. 8 shows the 1st and 5000th cycles of cyclic stability studies. Initially, the amount of specific capacitance increases at 900th of cycles which is attributed to the rising of efficient interfacial area between the electrodes and electrolyte and along with the slow activation process of the CoMn2O4-4 electrode(Hou et al. 2016). After that, it gradually decreases and retained 87% of initial capacitance after 5000 cycles. The superior cyclic stability property is due to the nanorods structural features of CoMn2O4-4 electrode material. The superior specific capacitance and cyclic stability features implies probable application of CoMn2O4-4 nanorod in electrode material for supercapacitors.
Conclusion
The work established a simple CTAB assisted hydrothermal synthetic technique for the preparation of edge curved one-dimensional CoMn2O4 nanorods for pseudocapacitor electrode application followed by annealing process. In this process, the CTAB template plays a key role to control the morphological features of CoMn2O4 materials. The synthesized materials were characterized by XRD, FTIR and FESEM techniques to evaluate their formation, crystalline phase, bonding properties and structural features. The high concentration of CTAB provides edge curved one-dimensional nanorods structure. The electrochemical properties of the CoMn2O4 materials were evaluated using cyclic voltammetric, chronopotentiometric and electrochemical impedance spectroscopic analysis. The CV curves provide the specific capacitance of 895 Fg−1 at a scan rate of 5 mV s−1, whereas CP curves provide the specific capacitance of 802 Fg−1 at a current density of 1 Ag−1. Furthermore, CoMn2O4 electrode retained 87% of initial capacitance after 5000 CV cycles at a scan rate of 100 mV s−1.The outstanding electrochemical features and superior cyclic stability facilitate the obtained CoMn2O4 material to be an important material for supercapacitor device applications.
References
Abbasi L, Arvand M, Moosavifard SE (2020) Facile template-free synthesis of 3D hierarchical ravine-like interconnected MnCo2S4 nanosheet arrays for hybrid energy storage device. Carbon N Y 161:299–308. https://doi.org/10.1016/j.carbon.2020.01.094
Alkhalaf S, Ranaweera CK, Kahol PK et al (2017) Electrochemical energy storage performance of electrospun CoMn2O4nanofibers. J Alloys Compd 692:59–66. https://doi.org/10.1016/j.jallcom.2016.09.005
Amiri M, Davarani SSH, Kaverlavani SK et al (2020) Construction of hierarchical nanoporous CuCo2V2O8 hollow spheres as a novel electrode material for high-performance asymmetric supercapacitors. Appl Surf Sci 527:146855. https://doi.org/10.1016/j.apsusc.2020.146855
An C, Zhang Y, Guo H, Wang Y (2019) Metal oxide-based supercapacitors: progress and prospectives. Nanoscale Adv 1:4644–4658. https://doi.org/10.1039/c9na00543a
Bengoechea MR, Aliev FM, Pinto NJ (2002) Effects of confinement on the phase separation in emeraldine base polyaniline cast from 1-methyl-2-pyrrolidinone studied via dielectric spectroscopy. J Phys Condens Matter 14:11769–11778. https://doi.org/10.1088/0953-8984/14/45/318
Boisset A, Athouël L, Jacquemin J et al (2013) Comparative performances of birnessite and cryptomelane MnO2 as electrode material in neutral aqueous lithium salt for supercapacitor application. J Phys Chem C 117:7408–7422. https://doi.org/10.1021/jp3118488
Cai D, Liu B, Wang D et al (2014) Facile hydrothermal synthesis of hierarchical ultrathin mesoporous NiMoO4 nanosheets for high performance supercapacitors. Electrochim Acta 115:358–363. https://doi.org/10.1016/j.electacta.2013.10.154
Cao Y, Li W, Xu K et al (2014) MnMoO4·4H2O nanoplates grown on a Ni foam substrate for excellent electrochemical properties. J Mater Chem A 2:20723–20728. https://doi.org/10.1039/c4ta04019h
Che H, Liu A, Mu J et al (2016) Template-free synthesis of novel flower-like MnCo2O4 hollow microspheres for application in supercapacitors. Ceram Int 42:2416–2424. https://doi.org/10.1016/j.ceramint.2015.10.041
Chen LY, Kang JL, Hou Y et al (2013) High-energy-density nonaqueous MnO2@nanoporous gold based supercapacitors. J Mater Chem A 1:9202–9207. https://doi.org/10.1039/c3ta11480e
Chen W, Rakhi RB, Hedhili MN, Alshareef HN (2014) Shape-controlled porous nanocarbons for high performance supercapacitors. J Mater Chem A 2:5236–5243. https://doi.org/10.1039/c3ta15245f
Chen F, Wang Z, Huo S et al (2019a) Cubic CoMn2O4 particles directly grown on Ni foam as binder-free electrode for asymmetric supercapacitors. Mater Lett 237:209–212. https://doi.org/10.1016/j.matlet.2018.11.100
Chen J, Yang B, Hou H et al (2019b) Disordered, large interlayer spacing, and oxygen-rich carbon nanosheets for potassium ion hybrid capacitor. Adv Energy Mater 9:1–9. https://doi.org/10.1002/aenm.201803894
Dhand C, Dwivedi N, Loh XJ et al (2015) Methods and strategies for the synthesis of diverse nanoparticles and their applications: a comprehensive overview. RSC Adv 5:105003–105037. https://doi.org/10.1039/c5ra19388e
Geng J, Ma C, Zhang D, Ning X (2020) Facile and fast synthesis of SnO2 quantum dots for high performance solid-state asymmetric supercapacitor. J Alloys Compd 825:153850. https://doi.org/10.1016/j.jallcom.2020.153850
Hosseini SA, Salari D, Niaei A et al (2011) Chemical-physical properties of spinel CoMn2O4 nano-powders and catalytic activity in the 2-propanol and toluene combustion: effect of the preparation method. J Environ Sci Heal Part A Toxic/Hazardous Subst Environ Eng 46:291–297. https://doi.org/10.1080/10934529.2011.539093
Hou L, Bao R, Chen Z et al (2016) Comparative investigation of hollow mesoporous NiCo2S4 ellipsoids with enhanced pseudo-capacitances towards high-performance asymmetric supercapacitors. Electrochim Acta 214:76–84. https://doi.org/10.1016/j.electacta.2016.08.038
Hu CC, Chang KH, Lin MC, Wu YT (2006) Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors. Nano Lett 6:2690–2695. https://doi.org/10.1021/nl061576a
Jadhav HS, Pawar SM, Jadhav AH et al (2016) Hierarchical mesoporous 3D Flower-like CuCo2O4/NF for high-performance electrochemical energy storage. Sci Rep 6:2–13. https://doi.org/10.1038/srep31120
Kim T, Ramadoss A, Saravanakumar B et al (2016) Applied surface science synthesis and characterization of NiCo2O4 nanoplates as efficient electrode materials for electrochemical supercapacitors. Appl Surf Sci 370:452–458. https://doi.org/10.1016/j.apsusc.2016.02.147
Kumar A, Sanger A, Kumar A et al (2016) Sputtered synthesis of MnO2nanorods as binder free electrode for high performance symmetric supercapacitors. Electrochim Acta 222:1761–1769. https://doi.org/10.1016/j.electacta.2016.10.161
Li Y, Xie H, Wang J, Chen L (2011) Preparation and electrochemical performances of α-MnO2 nanorod for supercapacitor. Mater Lett 65:403–405. https://doi.org/10.1016/j.matlet.2010.10.048
Li X, Li X, Wang G et al (2013) Flexible supercapacitor based on MnO2 nanoparticles via electrospinning. J Mater Chem A 1:10103–10106. https://doi.org/10.1039/c3ta11727h
Li Y, Han X, Yi T et al (2019) Review and prospect of NiCo2O4 -based composite materials for supercapacitor electrodes. J Energy Chem. https://doi.org/10.1016/j.jechem.2018.05.010
Low WH, Khiew PS, Lim SS et al (2019) Recent development of mixed transition metal oxide and graphene/mixed transition metal oxide based hybrid nanostructures for advanced supercapacitors. J Alloys Compd 775:1324–1356. https://doi.org/10.1016/j.jallcom.2018.10.102
Lu Y, Zhang Z, Liu X et al (2016) NiCo2S4/carbon nanotube nanocomposites with a chain-like architecture for enhanced supercapacitor performance. CrystEngComm 18:7696–7706. https://doi.org/10.1039/c6ce01556e
Makino S, Yamauchi Y, Sugimoto W (2013) Synthesis of electro-deposited ordered mesoporous RuOx using lyotropic liquid crystal and application toward micro-supercapacitors. J Power Sources 227:153–160. https://doi.org/10.1016/j.jpowsour.2012.11.032
Mary AJC, Bose AC (2018) Surfactant assisted ZnCo2O4 nanomaterial for supercapacitor application. Appl Surf Sci 449:105–112. https://doi.org/10.1016/j.apsusc.2018.01.117
Moosavifard SE, Saleki F, Mohammadi A et al (2020) Construction of hierarchical nanoporous bimetallic copper-cobalt selenide hollow spheres for hybrid supercapacitor. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2020.114295
Naeem F, Naeem S, Zhao Z et al (2020) Atomic layer deposition synthesized ZnO nanomembranes: A facile route towards stable supercapacitor electrode for high capacitance. J Power Sources 451:227740. https://doi.org/10.1016/j.jpowsour.2020.227740
Paquin F, Rivnay J, Salleo A et al (2015) Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J Mater Chem C 3:10715–10722. https://doi.org/10.1039/b000000x
Pu J, Wang J, Jin X et al (2013) Porous hexagonal NiCo2O4 nanoplates as electrode materials for supercapacitors. Electrochim Acta 106:226–234. https://doi.org/10.1016/j.electacta.2013.05.092
Senthilkumar B, KalaiSelvan R (2014) Hydrothermal synthesis and electrochemical performances of 1.7V NiMoO4. xH2O||FeMoO4 aqueous hybrid supercapacitor. J Colloid Interface Sci 426:280–286. https://doi.org/10.1016/j.jcis.2014.04.010
Simon P (2008) Simon 2009. Nat Mater 7:845–854
Tu J, Gu C, Zhao X et al (2012) Freestanding Co3O4 nanowire array for high performance supercapacitors. RSC Adv 2:1835. https://doi.org/10.1039/c1ra00771h
Vigneshwaran P, Kandiban M, Senthil Kumar N et al (2016) A study on the synthesis and characterization of CoMn2O4 electrode material for supercapacitor applications. J Mater Sci Mater Electron 27:4653–4658. https://doi.org/10.1007/s10854-016-4343-6
Vijayakumar S, Kiruthika Ponnalagi A, Nagamuthu S, Muralidharan G (2013) Microwave assisted synthesis of Co3O4 nanoparticles for high-performance supercapacitors. Electrochim Acta 106:500–505. https://doi.org/10.1016/j.electacta.2013.05.121
Vijayakumar S, Nagamuthu S, Ryu KS (2017) CuCo2O4 flowers/Ni-foam architecture as a battery type positive electrode for high performance hybrid supercapacitor applications. Electrochim Acta 238:99–106. https://doi.org/10.1016/j.electacta.2017.03.178
Wang Y, Shen C, Niu L et al (2016) Hydrothermal synthesis of CuCo2O4/CuO nanowire arrays and RGO/Fe2O3 composites for high-performance aqueous asymmetric supercapacitors. J Mater Chem A 4:9977–9985. https://doi.org/10.1039/c6ta02950g
Wu Z, Zhu Y, Ji X (2014) NiCo2O4 -based materials for electrochemical supercapacitors. J Mater Chem A 2:14759–14772. https://doi.org/10.1039/c4ta02390k
Wulan Septiani NL, Kaneti YV, Fathoni KB et al (2020) Self-assembly of nickel phosphate-based nanotubes into two-dimensional crumpled sheet-like architectures for high-performance asymmetric supercapacitors. Nano Energy 67:104270. https://doi.org/10.1016/j.nanoen.2019.104270
Xia XH, Tu JP, Mai YJ et al (2011) Self-supported hydrothermal synthesized hollow Co3O4 nanowire arrays with high supercapacitor capacitance. J Mater Chem 21:9319–9325. https://doi.org/10.1039/c1jm10946d
Xu K, Li W, Liu Q et al (2014a) Hierarchical mesoporous NiCo2O4@MnO2 core-shell nanowire arrays on nickel foam for aqueous asymmetric supercapacitors. J Mater Chem A 2:4795–4802. https://doi.org/10.1039/c3ta14647b
Xu Y, Wang X, An C, Wang Y, Jiaoa L, Yuana H (2014b) Facile synthesis route of porous MnCo2O4 and CoMn2O4 nanowires and their excellent electrochemical properties in supercapacitor. J Mater Chem A 20:16480–16488. https://doi.org/10.1039/C4TA03123G
Yan X, Tong X, Wang J et al (2014) Synthesis of mesoporous NiO nanoflake array and its enhanced electrochemical performance for supercapacitor application. J Alloys Compd 593:184–189. https://doi.org/10.1016/j.jallcom.2014.01.036
Yang J, Lan T, Liu J et al (2013) Supercapacitor electrode of hollow spherical V2O5 with a high pseudocapacitance in aqueous solution. Electrochim Acta 105:489–495. https://doi.org/10.1016/j.electacta.2013.05.023
Yesuraj J, Samuel AS, Elaiyappillai E et al (2017) A facile sonochemical assisted synthesis of α-MnMoO4/PANI nanocomposite electrode for supercapacitor applications. J Electroanal Chem 797:78–88. https://doi.org/10.1016/j.jelechem.2017.05.019
Yesuraj J, Suthanthiraraj SA, Padmaraj O (2019) Materials science in semiconductor processing synthesis, characterization and electrochemical performance of DNA- templated Bi2MoO6 nanoplates for supercapacitor applications. Mater Sci Semicond Process 90:225–235. https://doi.org/10.1016/j.mssp.2018.10.030
Yunyun F, Xu L, Wankun Z et al (2015) Spinel CoMn2O4 nanosheet arrays grown on nickel foam for high-performance supercapacitor electrode. Appl Surf Sci 357:2013–2021. https://doi.org/10.1016/j.apsusc.2015.09.176
Zhang X, Sun X, Zhang H et al (2013) Microwave-assisted reflux rapid synthesis of MnO2 nanostructures and their application in supercapacitors. Electrochim Acta 87:637–644. https://doi.org/10.1016/j.electacta.2012.10.022
Zhang C, Huang Y, Tang S et al (2017) High-energy all-solid-state symmetric supercapacitor based on Ni3S2 mesoporous nanosheet-decorated three-dimensional reduced graphene oxide. ACS Energy Lett 2:759–768. https://doi.org/10.1021/acsenergylett.7b00078
Zhang C, Zheng B, Huang C et al (2019) Heterostructural three-dimensional reduced graphene oxide/CoMn2O4 nanosheets toward a wide-potential window for high-performance supercapacitors. ACS Appl Energy Mater 2:5219–5230. https://doi.org/10.1021/acsaem.9b00904
Zhang C, Peng Z, Chen Y et al (2020) Efficient coupling of semiconductors into metallic MnO2@CoMn2O4 heterostructured electrode with boosted charge transfer for high-performance supercapacitors. Electrochim Acta 347:136246. https://doi.org/10.1016/j.electacta.2020.136246
Zhao G, Zhang N, Sun K (2013) Porous MoO3 films with ultra-short relaxation time used for supercapacitors. Mater Res Bull 48:1328–1332. https://doi.org/10.1016/j.materresbull.2012.11.080
Zhao C, Ju P, Wang S et al (2016) One-step hydrothermal preparation of TiO2/RGO/Ni(OH)2/NF electrode with high performance for supercapacitors. Electrochim Acta 218:216–227. https://doi.org/10.1016/j.electacta.2016.09.122
Zhu M, Weber CJ, Yang Y et al (2008) Chemical and electrochemical ageing of carbon materials used in supercapacitor electrodes. Carbon N Y 46:1829–1840. https://doi.org/10.1016/j.carbon.2008.07.025
Zhu L, Chang Z, Wang Y et al (2015) Core-shell MnO2@Fe2O3 nanospindles as a positive electrode for aqueous supercapacitors. J Mater Chem A 3:22066–22072. https://doi.org/10.1039/c5ta05556c
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sandosh, T.A., Simi, A. Morphology controlled synthesis of one-dimensional CoMn2O4 nanorods for high-performance supercapacitor electrode application . Chem. Pap. 75, 2295–2304 (2021). https://doi.org/10.1007/s11696-020-01448-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01448-z