Abstract
The aim of this work is a study of the encapsulation and intermolecular non-bonded interaction of an anticancer drug Talzenna into carbon nanotube [CNT(8,8-7)] and boron nitride nanotube [BNNT(8,8-7)]. The interaction effects of Talzenna with the CNT and BNNT on electronic and adsorption properties were theoretically investigated in the solvent phase at the M062X/6-311G* level of theory. With the encapsulation of Talzenna drug, the electronic properties of the CNT and BNNT nanotubes change significantly. The electronic spectra of the Talzenna drug, complexes CNT/Talzenna and BNNT/Talzenna in the solvent water were calculated by Time-Dependent Density Functional Theory (TD-DFT) for the study of adsorption effect. According to the natural bond orbital (NBO) results, all three molecules Talzenna, CNT and BNNT play as electrons donor and acceptors at the complexes CNT/Talzenna and BNNT/Talzenna. On the other hand, the charge transfer is occurred between the bonding, antibonding or nonbonding orbitals in molecules Talzenna, CNT and BNNT. Based on the results, CNT(8,8-7) and BNNT(8,8-7) can be used as a drug delivery system for the transportation of Talzenna as anticancer drug within the biological systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon nanotubes (CNTs) are drug delivery systems applicable in biology and medicine (Padma and Jain 2014; Bae et al. 2013). CNTs can deliver anticancer drugs into target cells through penetrating to cell membranes. After the interaction of drug with the surface of CNTs, they are delivered into the target cells (Kumar et al. 2017). CNTs are used to improve the pharmaceutical properties. The medicinal properties of drugs improve with interaction of drug on surface of CNTs and, therefore, the toxic effect of drugs decrease (Chakrabarti et al. 2015; Foldvari 2010). Therefore, the various researches have been carried out on the Boron nitride nanostructures. These materials are applicable in electronics devices, semiconductor with high thermal stability and nanowires. The energy gap of Boron nitride nanostructure is about 6 eV. The energy gap of Boron nitride nanotubes (BNNTs) is about 5.5 eV (Golberg et al. 2007). They have nontoxic property due to their high chemical and structural stability and high oxidation resistance, along with uniformity and stability in dispersion in solution (Yu et al. 2009).
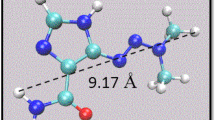
Talazoparib with trade name Talzenna (Fig. 1) has been developed by the United States Food and Drug Administration (FDA) for use in treatment advanced breast cancer (Exman et al. 2019). Talzenna acts as an inhibitor of poly-ADP ribose polymerase (PARP) which aids in single-strand DNA repair (Huang et al. 2015; Murai et al. 2012, 2014). Talzenna was confirmed in 2018 in the United States and 2019 in the Europe for advanced or metastatic breast cancer treatment (Exman et al. 2019; Domchek et al. 2019).
Performing Density functional theory (DFT) calculations, one may obtain useful information about the interaction between various nanotubes and drug molecules. The adsorption and interaction of anticancer drugs, such as cisplatin (Feazell et al. 2007), carboplatin (Dhar et al. 2008), paclitaxel (Liu et al. 2008), methotrexate (Pastorin et al. 2006) and doxorubicin (Ali-Boucetta et al. 2008) with BNNT, have already been studied. We have already studied the interaction between a new azomethine derivative and BN(6,6-7) nanotube by DFT calculations (Sheikhi et al. 2017). We discussed on the electronic properties, chemical shift tensors, electronic spectra natural charge of the azomethine and BN(6,6-7) nanotube in the complex BN(6,6-7)/azomethine. Recently, we have also investigated the adsorption and non-bonded interaction between various drugs, such as Syndros (Sheikhi et al. 2018a), Resveratrol (Sheikhi et al. 2018b), Alectinib (Sheikhi et al. 2019a), Rubraca (Sheikhi et al. 2019b), Curcumin (Shahab et al. 2019a) and Tyrphostin AG528 (Sheikhi et al. 2019c), over a variety of carbon nanotubes. In the current study, the encapsulation of Talzenna into the CNT(8,8-7) and BNNT(8,8-7) has been investigated by DFT method. We have studied changes in geometric parameters, thermodynamic parameters, frontier molecular orbitals (FMOs), quantum-chemical molecular descriptors, charge transfer analysis according to natural bond orbital (NBO) analysis and electronic structure and excited states.
Computational methods and equipment
In this work, we have studied the encapsulation and non-bonded interaction between Talzenna drug with the CNT(8,8-7) and BNNT(8,8-7) by computational research. The quantum chemical calculations have been performed using the density functional theory (DFT) at the M062X method and 6-311G* basis set (Yoosefian and Jahani 2019; Rahmani et al. 2019) by the Gaussian 09 W program (Frisch et al. 2009) for the optimization of the molecule Talzenna drug, CNT(8,8-7), BNNT(8,8-7) and complexes CNT/Talzenna and BNT/Talzenna. The Polarized Continuum Model (PCM) (Frisch et al. 2009) was used for the calculations of solvent effect. The adsorption energies (Ead) (Sheikhi et al. 2018a) of Talzenna drug over CNT and BNNT are obtained by the following equations:
in which ECNT/Talzenna and EBNNT/Talzenna are the adsorption energies of the Talzenna on the carbon nanotube and BN nanotube, respectively. EBNNR and EAlNNR are energies of the BNNR(8,8-7) and AlNNR(8,8-7), respectively. Also, ETalzenna is the energy of Talzenna molecule.
Geometrical parameters, thermodynamic parameters, NBO analysis (Shahab et al. 2017a), density of states (DOS), and FMO analysis (Sheikhi et al. 2018c) were calculated using M062X/6-311G* level of theory. The molecular orbital (MO) calculations of the title compounds, such as EHOMO, ELUMO, energy gap between LUMO and HOMO (Eg = ELUMO – EHOMO), were computed. The optimized molecular structures, DOS results and the molecular orbitals’s graphs were visualized by and GaussView program (Shahab et al. 2017b). TD-DFT method (Frisch et al. 2000) was used for the calculation of electronic transitions of the Talzenna drug and the complexes CNT/Talzenna and BNNT/Talzenna. UV–Vis experimental absorption spectra of the complexes were measured by Cary 60 UV–Vis scanning spectrophotometer.
Results and discussion
Optimized structures
We calculated the optimized structures of the Talzenna drug, CNT(8,8-7), and BNNT(8,8-7) using DFT method in a solvent water. These structures are shown in Fig. 2.
In the first step, we have considered five states for encapsulation of the Talzenna into the CNT(8,8-7) and BNNT(8,8-7). The optimized states (S1, S2, S3, S4, S5) of the CNT(8,8-7) and BNNT(8,8-7) were calculated at the PM6 method (Fig. 3). The energies (E) for the investigated states S1, S2, S3, S4, and S5 are reported in Table 1. The calculated energies show that the most stable state of CNT(8,8-7) and BNNT(8,8-7) is state S5.
Consequently, we have considered the encapsulation of the Talzenna into the CNT(8,8-7) and BNNT(8,8-7) at the state S5 and have optimized using M062X/6-311G* level of theory in a solvent water. The optimized structures are shown in Figs. 4, 5.
Geometrical parameters play an important role to illustrate the adsorption of the molecules on nanotubes in drug delivery systems. The calculated selective bond lengths of the optimized structures of Talzenna, CNT(8,8-7), BNNT(8,8-7), complexes CNT(8,8-7)/Talzenna and BNNT(8,8-7)/Talzenna at the interacting locations are reported in Tables S1 and S2 (Atoms numbering is according to Figs. 4, 5). As shown in Tables S1 and S2, some geometrical parameters of Talzenna drug, carbon and BN nanotube are changed after the intermolecular non-bonded interaction drug with nanotubes and the formation of the complex, although these changes are not significant.
The thermochemical parameters, such as the sum of electronic and thermal energies (E + T), the sum of electronic and thermal enthalpies (E + H), the sum of electronic and thermal free energies (E + G), and entropy (S) of the Talzenna drug, CNT(8,8-7), BNNT(8,8-7), complexes CNT/Talzenna and BNNT/Talzenna, calculated using M062X/6-311G* level of theory. The results were reported in Table 2. With the encapsulation of the Talzenna drug in CNT(8,8-7) and BNNT(8,8-7), the Thermal, Gibbs and Enthalpy energies values decrease. The computed energy values show the reduced reactivity and increasing the stability of Talzenna drug in non-bonded interaction with CNT(8,8-7) and BNNT(8,8-7). The calculated energies for the complex BNNT/Talzenna are more negative rather than the complex CNT/Talzenna; also, the relative values of energies for BNNT/Talzenna are smaller than CNT/Talzenna; therefore, the complex BNNT/Talzenna has the most stability comparing with the complex CNT/Talzenna.
Electronic properties
The HOMO and LUMO orbitals (FMO) have a significant role in the chemical reactions and charge transfer phenomenon in molecules (Sheikhi et al. 2018a; Shahab et al. 2018). The HOMO and LUMO energies display the ability of orbital to donate and obtain an electron, respectively (Sheikhi et al. 2018b). The energy gap between HOMO and LUMO orbitals is a major factor in designation the electrical transport properties in molecules (Sheikhi et al. 2018c). We have investigated the non-bonded intermolecular interaction effects between Talzenna drug with CNT(8,8-7) and BNNT(8,8-7) on the electronic properties. The calculated results are reported in Table 3.
The adsorption energies (Ead) of the Talzenna drug over the CNT(8,8-7) and BNNT(8,8-7) are computed about − 40.78 eV and − 36.39 eV, respectively; therefore, the encapsulation of the Talzenna drug on the CNT(8,8-7) and BNNT(8,8,-7) is exothermic (Table 3). The adsorption energy of the Talzenna drug on the CNT(8,8-7) is greater than the adsorption energy of the Talzenna drug on the BNNT(8,8-7). Thus, it indicated a strong adsorption in complex CNT/Talzenna rather than BNNT/Talzenna complex.
After the encapsulation of Talzenna into CNT(8,8-7), the energies of HOMO and LUMO are decreased from − 5.81 and − 2.54 eV in carbon nanotube to − 5.82 and − 2.56 eV, respectively, in the complex CNT/Talzenna. The energy of HOMO is increased from − 8.11 eV in BNNT to − 7.65 eV in the complex BNNT/Talzenna, whereas the energy of LUMO is decreased from 0.65 eV to − 1.04 eV. The frontier orbitals graphs of the molecules Talzenna, CNT(8,8-7), BNNT(8,8-7), CNT/Talzenna and BNNT/Talzenna are presented in Fig. 6. Figure 6 shows that the electron density of HOMO and LUMO orbitals in the CNT(8,8-7) is mainly localized on the double bonds C=C of carbon nanotube. The HOMO orbital of the BNNT(8,8-7) mainly situated on nitrogen atoms, whereas the LUMO orbital localized on boron atoms. The electron density of HOMO and LUMO orbitals in the CNT(8,8-7)/Talzenna is mainly localized on the double bonds C=C of carbon nanotube, while the electron density of HOMO and LUMO orbitals in the BNNT(8,8-7)/Talzenna is mainly localized on the Talzenna drug.
The energy gaps values of the title molecules are reported in Table 3. The calculated DOS plots (Shahab et al. 2019b) in Fig. 7 also display the energy gaps of the investigated compounds. According to results of Table 3, the energy gaps are decreased from 3.27 and 7.46 eV in CNT and BNNT to 3.26 and 6.61 eV in the complexes CNT/Talzenna and BNNT/Talzenna, respectively. These results show increase in electrical conductivity of the complexes rather than the pure CNT and BNNT. Thus, we found that the CNT can be a better nanotube for encapsulation of the Talzenna drug due to the lower energy gap (3.27 eV) rather than BNNT (7.46 eV).
The quantum molecular descriptors for the investigated compounds including ionization potential (I), electron affinity (A), global hardness (η), electronegativity (χ), electronic chemical potential (µ), electrophilicity (ω) and chemical softness (S) are computed according to following equations, respectively (Shahab et al. 2019c):
in which are summarized in Table 3. The stability of the molecules is determined to hardness factor that shows chemical reactivity (Shahab et al. 2019d). According to Table 3, the values of global hardness (η) for CNT and BNNT are 1.63 eV and 3.73 eV. After capsulation of Talzenna drug into CNT and BNNT, the global hardness of in complex CNT/Talzenna has not changed (1.63 eV), whereas the global hardness of complex BNNT/Talzenna decreases to 3.30 eV. Also, the reactivity of CNT is the higher rather than BNNT due to the low η of CNT (1.63 eV) comparing with the η of BNNT (3.73 eV) that shows CNT is more reactive than BNNT. The electronic chemical potential (µ) index of the complexes CNT/Talzenna and BNNT/Talzenna also decreased rather than the pure CNT and BNNT. Therefore, the complexes have a high chemical activity, low chemical stability and, therefore, they are a soft system.
The amount of dipole moment CNT(8,8-7) is increased after capsulation of Talzenna drug from 0.031 Debye to 5.633 Debye in complex CNT/Talzenna and the amount of dipole moment of BNNT(8,8-7) is increased after interaction with Talzenna from 0.005 Debye to 5.565 Debye in complex BNNT/Talzenna (see Table 3). The change of dipole moment after encapsulation of Talzenna drug into carbon and BN nanotubes displays a charge transfer between Talzenna drug and the title nanotubes.
NBO analysis
NBO analysis is an important method for studying intra- and inter-molecular bonding and interaction between bonds in molecular systems (Sheikhi et al. 2018d). The electron delocalization from donor orbitals (full NBOs) to acceptor orbitals (empty NBOs) describes a conjugative electron transfer process between them (Sheikhi et al. 2018d). For each donor orbital (i) and acceptor orbital (j), the stabilization energy E(2) associated with the delocalization i → j is calculated by the following equation (Weinhold and Landis 2001):
The stabilization energy (E(2)) describes the amount of the participation of electrons in the resonance between atoms of the molecular structure (Frisch et al. 2009). The bigger E(2), the more donation tendency from electron donor orbital to electron acceptor orbital (Sheikhi et al. 2018d). The NBO analysis for complex CNT/Talzenna and BNNT/Talzenna has been performed by M062X/6-311G* level of theory and the results are summarized in Tables S3 and S4 (Atoms numbering is according to Figs. 4, 5).
The σ → π*, π → π* and π* → π* transitions from Talzenna to CNT(8,8-7) occur in complex CNT/Talzenna. According to results of NBO analysis in Table S3, the σ → π* transitions from Talzenna to CNT(8,8-7) take place as σ(N2-H29) → π*(C128–C129) and σ(C7–H30) → π*(C88–C96) with stabilization energies (E(2)) about 0.11, 0.14 kcal/mol, respectively. The π → π* transitions are, such as π(N3–C4) → π*(C114–C122), π(C1–C5) → π*(C104–C105), π(C1–C5) → π*(C112–C120), π(C6–C7) → π*(C88–C96), π(C6–C7) → π*(C104–C105), π(C22–C23) → π*(C86–C87), interactions with resonance energies (E(2)) about 0.13, 0.12, 0.25, 0.15, 0.25, 0.13 kcal/mol, respectively. The important n → π* transition is observed for n1(N2) → π*(C112–C120) and n1(N2) → π*(C128–C129) interactions with stabilization energies (E(2)) about 0.44, 0.53 kcal/mol, respectively. According to results of Table S3, the π* → π* transitions have the highest resonance energies comparing with other transitions from Talzanna to CNT(8,8-7) including π*(N3–C4) → π*(C112–C120), π*(N3–C4) → π*(C121–C123), π*(C6–C7) → π*(C104–C105), π*(N16-C17) → π*(C125-C127), π*(N16-C17) → π*(C132-C133) with stabilization energies (E(2)) about 1.04, 1.35, 1.77, 1.82, 2.12 kcal/mol, respectively. The NBO analysis shows that π → σ* transitions from CNT(8,8-7) to Talzenna occur in complex CNT/Talzenna. The main π → σ* electron charge transfer is observed for π(C88–C96) → σ*(C7–H30), π(C94–C95) → σ*(C23–H40), π(C128–C129) → σ*(N2–H29) with high stabilization energies (E(2)) 0.91, 0.50 and 0.98 kcal/mol, respectively. Thus, Talzenna and CNT(8,8-7) act as both electron donor and electron acceptor and charge transfer takes place between Talzenna and CNT(8,8-7) in the complex CNT/Talzenna.
The NBO analysis shows that σ → π*, π → π*, n → π* transitions from Talzenna to BNNT(8,8-7) occur in complex BNNT/Talzenna (Table S4). As shown in Table S4, the important σ → π* transitions from Talzenna to BNNR(8,8-7) take place as σ(C20–H37) → π*(B73–N74), σ(C20–H38) → π*(N75–B83), σ(C22-H39) → π*(B132–N133), σ(C25-H41) → π*(B101-N102), with resonance energies (E(2)) about 1.16, 0.98, 1.38, 1.64 kcal/mol, respectively. The π → π* transitions from Talzenna to BNNT(8,8-7) are, such as π(N2–H29) → π*(B56–N57), π(N3–C4) → π*(B49–N51), π(N16–C17) → π*(B60–N67), π(N16–C17) → π*(N62–B69), π(N16–C17) → π*(N68–B76), interactions with stabilization energies (E(2)) about 0.87, 0.66, 0.37, 0.73 and 0.64 kcal/mol, respectively. The important n → π* charge transfers from Talzenna to BNNT(8,8-7) are observed for n1(N2) → π*(B56–N57), n1(N3) → π*(N58–B65), n1(N16) → π*(N68–B76), n1(N16) → π*(B77–N78), n1(N18) → π*(B53–N55) interactions with stabilization energies (E(2)) about 2.75, 2.24, 4.22, 1.01, 1.12 kcal/mol, respectively. Also, the π → σ*, π → π* and π* → π* transitions from BNNT(8,8-7) to Talzenna occur in complex BNNT/Talzenna. According to the results of NBO analysis, the important interactions from BNNT(8,8-7) to Talzenna are, such as π(N64-B72) → σ*(N2-H29), π(B121-N129) → σ*(C7-H30), π*(B121-N129) → σ*(N13-H34), π*(B132-N133) → σ*(C22-H39), transitions with stabilization energies values (E(2)) of 2.81, 0.85, 0.61, 0.88 kcal/mol, respectively. Thus, Talzenna and BNNR(8,8-7) act as both electron donor and electron acceptor in the complex BNNT/Talzenna and, therefore, charge transfer takes place between Talzenna and BNNT(8,8-7).
Electronic Structure and Excited States
To investigate encapsulation effect of Talzenna into CNT(8,8-7) and BNNT(8,8-7) on the λmax, we have obtained the theoretical UV/Vis spectra of Talzenna and the complexes CNT/Talzenna and BNNT/Talzenna using TD-DFT calculations at M062X/6-311G* method. We investigated 20 excited states and reported the important transitions in Tables 4, 5, 6. Tables 4, 5 and 6 show the λmax, oscillator strength (f), and excitation energies (E). In the calculated UV spectrum of Talzenna drug, λmax appears at 159.55 nm (f = 0.94) (Table 4). The charge transfer at λmax = 159.55 nm is related to the excited state S0 → S12 with eight electron configurations, such as H-5 → L + 1 (35%), H-3 → L + 2 (15%), H-4 → L + 1 (6%), H-2 → L + 1 (5%), H-2 → L + 5 (5%), H-1 → L + 3 (9%), H → L (4%), H → L + 5 (7%), in which the main transition is the transition from HOMO-5 to LUMO + 1 [H-5 → L + 1 (35%)]. The excited state of S0 → S14 at 154.41 nm (f = 0.61) is also the other important excited state in the UV spectrum of Talzenna. The other excited states of Talzenna drug have a small intensity. The calculated UV spectrum of Talzenna drug in the solvent water is displayed on Fig. 8.
With the encapsulation of Talzenna into the CNT(8,8-7), λmax is appeared at 365.71 nm (f = 1.39) in electronic absorption spectrum of complex CNT(8,8-7) that is due to charge transfer of one electron into the excited state S0 → S3 and it describes by three configurations for one-electron excitation H-2 → L (38%), H → L + 2 (37%), H → L + 1 (3%) (Table 5). The main transition is including the transition from HOMO-2 to LUMO [H-2 → L (38%)]. The excited state of S0 → S2 at 371.57 nm (f = 1.27) is also the other important excited state in the theoretical UV/Vis spectrum of the complex CNT/Talzenna. The other excited states of CNT/Talzenna have very small intensity and do not play any role in the formation of electron spectrum of the title complex (Table 5). The theoretical UV spectrum of CNT/Talzenna in the solvent water displayed on in Fig. 8.
After the encapsulation of Talzenna into the BNNT(8,8-7), λmax appears at 162.44 nm (f = 0.47) in the calculated UV spectrum of BNNT/Talzenna. The charge transfer at λmax = 162.44 nm is related to the excited state S0 → S11 and is defined by ten configurations including H-22 → L + 1 (13%), H-7 → L + 2 (14%), H-24 → L + 1 (8%), H-20 → L + 1 (4%), H-13 → L + 1 (2%), H-3 → L + 1 (5%), H-2 → L + 3 (3%), H-1 → L + 3 (7%), H → L (3%), H → L + 15 (3%) (Table 6). Excitation of an electron from H-7 → L + 2 (14%) gives the main contribution to the formation of the absorption band at 162.44 nm. The other important excited state is excitation of one electron at 191.66 nm (f = 0.44) that belonged to the transition into the excited state S0 → S7. The other excited states of the title complex have small intensity. The calculated UV spectrum of the complex BNNT/Talzenna displayed on in Fig. 8.
In the UV spectrum of isolated Talzenna drug, λmax appeared at 159.55 nm, while after encapsulation of Talzenna into CNT(8,8-7) and BNNT(8,8-7) is changed to 365.71 nm and 162.44 nm, respectively. According to these results, we found that encapsulation of Talzenna drug into CNT and BNNT enhances the value of λmax and it can be recognized as a bathochromic shift.
Conclusion
In this work, we have studied the adsorption energies and electronic properties of capsulation of Talzenna drug into CNT(8,8-7) and BNNT(8,8-7) using DFT calculations and UV/Vis spectroscopy investigations. The results show that Talzenna drug has the higher adsorption energy (-40.78 eV) with CNT rather than BNNT (36.39 eV). The CNT(8,8-7) with the lower energy gap (3.27 eV) rather than the BNNT (7.46 eV) can be a better choice for encapsulation of the Talzenna drug. Also, the values of η parameter of CNT (1.63 eV) and BNNT (3.73 eV) specified that the reactivity of CNT is higher rather than BNNT that shows the Talzenna increasing the reactivity of CNT comparing with BNNR. NBO analysis predicted a charge transfer from the Talzenna drug to CNT and BNNT and from CNT and BNNT to Talzenna. The encapsulation of Talzenna drug into the CNT and BNNT increases the value of λmax (from 159.55 nm to 365.71 nm in CNT/Talzenna and from 159.55 nm to 162.44 nm in BNNT/Talzenna) and it can be considered as a bathochromic shift. We hope that results of our research can be used in drugs delivery to cancer cells and to support decreased drug interaction with healthy tissue.
References
Ali-Boucetta H, Al-Jamal KT, McCarthy D, Prato M, Bianco A, Kostarelos K (2008) Multiwalled carbon nanotube-doxorubicin supramolecular complexes for cancer therapeutics. Chem Comm 4:459–461. https://doi.org/10.1039/B712350G
Bae YH, Mrsny RJ, Park K (eds) (2013) Cancer targeted drug delivery: an elusive dream. Springer Science & Business Media.
Chakrabarti M, Kiseleva R, Vertegel A, Ray SK (2015) Carbon nanomaterials for drug delivery and cancer therapy. J Nanosci Nanotechnol 15:5501–5511. https://doi.org/10.1166/jnn.2015.10614
Dhar S, Liu Z, Thomale J, Dai H, Lippard SJ (2008) Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J Am Chem Soc 130:11467–21176. https://doi.org/10.1021/ja803036e
Domchek S, Postel-Vinay S, Im SA, Park YH, Delord JP, Italiano A, Alexandre J, You B, Bastian S, Krebs MG, Waqar S, Lanasa M, Angell HK, Tang M, Gresty C, Opincar L, Herbolsheimer P, Kaufman B (2019) Abstract PD5–04: an open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): updated results in patients with germline BRCA-mutated (gBRCAm) metastatic breast cancer (MBC). Cancer Res 79:PD5–04. https://doi.org/10.1158/1538-7445.SABCS18-PD5-04
Exman P, Barroso-Sousa R, Tolaney SM (2019) Evidence to date: talazoparib in the treatment of breast cancer. Onco Targets Ther 12:5177–5187. https://doi.org/10.2147/OTT.S184971
Feazell RP, Nakayama-Ratchford N, Dai H, Lippard SJ (2007) Soluble single-walled carbon nanotubes as longboat delivery systems for platinum (iv) anticancer drug design. J Am Chem Soc 129:8438–8439. https://doi.org/10.1021/ja073231f
Foldvari M (2010) Formulating nanomedicines: focus on carbon nanotubes as novel nanoexcipients. Key engineering materials. Trans Tech Publ 441:53–74. https://doi.org/10.4028/www.scientific.net/KEM.441.53
Frisch A, Nielson AB, Holder AJ (2000) GAUSSVIEW User Manual. Gaussian Inc., Pittsburgh
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 revision A02. Gaussian Inc, Wallingford CT
Golberg D, Bando Y, Tang CC, Zhi CY (2007) Boron nitride nanotubes. Adv Mater 19:2413–2432. https://doi.org/10.1002/adma.200700179
Huang J, Wang L, Cong Z, Amoozgar Z, Kiner E, Xing D, Orsulic S, Matulonis U, Goldberg MS (2015) The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1−/− murine model of ovarian cancer. Biochem Biophys Res Commun 463:5551–556. https://doi.org/10.1016/j.bbrc.2015.05.083
Kumar B, Jalodia K, Kumar P, Gautam HK (2017) Recent advances in nanoparticle-mediated drug delivery. J Drug Deliv Sci Technol 41:260–268. https://doi.org/10.1016/j.jddst.2017.07.019
Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, Dai H (2008) Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 68:6652–6660. https://doi.org/10.1158/0008-5472.CAN-08-1468
Murai J, Huang SYN, Das BB, Renaud A, Zhang Y, Doroshow JH, Ji J, Takeda S, Pommier Y (2012) Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 72:5588–5599. https://doi.org/10.1158/0008-5472.CAN-12-2753
Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S, Morris J, Teicher B, Doroshow JH, Pommier Y (2014) Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther 13:433–443. https://doi.org/10.1158/1535-7163.MCT-13-0803
Padma VD, Jain S (eds) (2014) Targeted drug delivery: concepts and design advances in delivery science and technology. Springer.
Pastorin G, Wu W, Wieckowski S, Briand JP, Kostarelos K, Prato M, Bianco A (2006) Double functionalisation of carbon nanotubes for multimodal drug delivery. Chem Comm 11:1182–1184. https://doi.org/10.1039/B516309A
Rahmani Z, Edjlali L, Vessally E, Hosseinian A, Delir Kheirollahi Nezhad P (2019) A density functional theory outlook on the possible sensing ability of boron nitride nanotubes and their Al- and Si-doped derivatives for sulfonamide drugs. J Sulfur Chem 41:82–95. https://doi.org/10.1080/17415993.2019.1687702
Shahab S, Filippovich L, Sheikhi M, Kumar R, Dikusar E, Yahyaei H, Muravsky A (2017) Polarization, excited states, trans-cis properties and anisotropy of thermal and electrical conductivity of the 4-(phenyldiazenyl)aniline in PVA matrix. J Mol Struct 1141:703–709. https://doi.org/10.1016/j.molstruc.2017.04.014
Shahab S, Sheikhi M, Filippovich L, Dikusar Anatol’evich E, Yahyaei H (2017) Quantum chemical modeling of new derivatives of (E, E)-azomethines: synthesis, spectroscopic (FT-IR, UV/Vis, polarization) and thermophysical investigations. J Mol Struct 1137:335–348. https://doi.org/10.1016/j.molstruc.2017.02.056
Shahab S, Sheikhi M, Filippovich L, Khaleghian M, Dikusar E, Yahyaei H, Yousefzadeh Borzehandani M (2018) Spectroscopic studies (geometry optimization, E→ZIsomerization, UV/Vis, excited states, FT-IR, HOMO-LUMO, FMO, MEP, NBO, Polarization) and anisotropy of thermal and electrical conductivityof new azomethine dyes in stretched polymer matrix. Silicon 10:2361–2385. https://doi.org/10.1007/s12633-018-9773-8
Shahab S, Sheikhi M, Khaleghian M, Balakhanava I, Azarakhshi F (2019) DFT Study of Physisorption Effect of the Curcumin on CNT(8,0-6) Nanotube for Biological Applications. Chin J Struct Chem 38:37–52. https://doi.org/10.14102/j.cnki.0254-5861.2011-2052.
Shahab S, Sheikhi M, Khaleghian M, Kaviani S (2019) Theoretical study of interaction between apalutamide anticancer drug and thymine by DFT method. Chin J Struct Chem 3s8:1645–1663. https://doi.org/10.14102/j.cnki.0254-5861.2011-2282
Shahab S, Sheikhi M, Filippovich L, Dikusar E, Rouhani M, Pazniak A, Kumar R (2019) Molecular investigations of the new synthesized azomethines as antioxidants: theoretical and experimental studies. Current Mol Med 19:419–433. https://doi.org/10.2174/1566524019666190509102620
Shahab S, Sheikhi M, Filippovich L, Ignatovich Z, Muravsky A, Alnajjar R, Kaviani S, Strogova A (2019) Optimization, Spectroscopic (FT-IR, Excited States, UV/Vis) Studies, FMO, ELF, LOL, QTAIM and NBO analyses and electronic properties of two new pyrimidine derivatives. Chin J Struct Chem 38:1615–1639. https://doi.org/10.14102/j.cnki.0254-5861.2011-2251
Sheikhi M, Shahab S, Filippovich L, Khaleghian M, Dikusar E, Mashayekhi M (2017) Interaction between new synthesized derivative of (E, E)-azomethines and BN(6,6–7) nanotube for medical applications: geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, excited state), FMO, MEP and HOMO-LUMO investigations. J Mol Struct 1146:881–888. https://doi.org/10.1016/j.molstruc.2017.06.017
Sheikhi M, Shahab S, Khaleghian M, Kumar R (2018) Interaction between new anti-cancer drug syndros and CNT (6, 6–6) nanotube for medical applications: geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, Excited state), FMO, MEP and HOMO-LUMO investigation. Appl Surf Sci 434:504–513. https://doi.org/10.1016/j.apsusc.2017.10.154
Sheikhi M, Shahab S, Khaleghian M, Hajikolaee FH, Balakhanava I, Alnajjar R (2018) Adsorption properties of the molecule resveratrol on CNT (8, 0–10) nanotube: geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, Excited State), FMO, MEP and HOMO-LUMO investigations. J Mol Struct 1160:479–478. https://doi.org/10.1016/j.molstruc.2018.01.005
Sheikhi M, Shahab S, Filippovich L, Yahyaei H, Dikusar E, Khaleghian M (2018) New derivatives of (E, E)-azomethines: Design, quantum chemical modeling, spectroscopic (FT-IR, UV/Vis, polarization) studies, synthesis and their applications: experimental and theoretical investigations. J Mol Struct 1152:368–385. https://doi.org/10.1016/j.molstruc.2017.09.108
Sheikhi M, Shahab S, Filippovich L, Dikusar E, Khaleghian M (2018) DFT investigations (geometry optimization, UV/Vis, FT-IR, NMR, HOMO-LUMO, FMO, MEP, NBO, Excited States) and synthesis of new pyrimidine dyes. Chin J Struc Chem 37:1201–1222. https://doi.org/10.14102/j.cnki.0254-5861.2011-1887
Sheikhi M, Shahab S, Alnajjar R, Ahmadianarog M (2019) Adsorption properties of the new anti-cancer drug alectinib on CNT(6,6–6) nanotube: geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, Excited State), FMO, MEP and HOMO-LUMO investigations. J clust sci 30:83–96. https://doi.org/10.1007/s10876-018-1460-9
Sheikhi M, Shahab S, Khaleghian M, Ahmadianarog M, Kumar R (2019) Investigation of the adsorption rubraca anticancer drug on the CNT(4,4–8) nanotube as a factor of drug delivery: a theoretical study based on DFT method. Curr Mol Med 19:473–486. https://doi.org/10.2174/1566524019666190506143152
Sheikhi M, Shahab S, Alnajjar R, Ahmadianarog M, Kaviani S (2019) Investigation of adsorption tyrphostin AG528 anticancer drug upon the CNT(6,6–6) nanotube: a DFT study. Curr Mol Med 19:91–104. https://doi.org/10.2174/1566524019666190226111823
Weinhold F, Landis CR (2001) Natural Bond Orbitals and Extensions of Localized. Chem Educ Res Pract 2:91–104. https://doi.org/10.1039/B1RP90011K
Yoosefian M, Jahani M (2019) A molecular study on drug delivery system based on carbon nanotube for the novel norepinephrine prodrug, Droxidopa. J Mol Liq 284:258–264. https://doi.org/10.1016/j.molliq.2019.04.016
Yu J, Chen Y, Cheng BM (2009) Dispersion of boron nitride nanotubes in aqueous solution with the help of ionic surfactants. Solid State Commun 149:763–766. https://doi.org/10.1016/j.ssc.2009.03.001
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Azarakhshi, F., Sheikhi, M., Shahab, S. et al. Investigation of encapsulation of Talzenna drug into carbon and boron-nitride nanotubes [CNT(8,8-7) and BNNT(8,8-7)]: a DFT study. Chem. Pap. 75, 1521–1533 (2021). https://doi.org/10.1007/s11696-020-01407-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01407-8