Abstract
Tramadol is a centrally acting analgesic drug, used in therapy as a racemic mixture of its trans enantiomers: (1R,2R) and (1S,2S). A simple capillary electrophoresis (CE) method was developed for the chiral separation of tramadol enantiomers using carboxymethyl-β-CD (CM- β-CD) as chiral selector. Influence of buffer pH and concentration, CD concentration, capillary temperature, applied voltage and injection parameters on the chiral separation was evaluated. Optimization of the analytical conditions was performed using a preliminary “one factor at time” approach followed by a faced-centred central composite design. The best results were obtained when using 25 mM sodium tetraborate buffer at pH 11.0, 5 mM CM-β-CD as chiral selector, + 17.5 kV voltage, 15 °C temperature, and 30 mbar/1 s hydrodynamic injection. The analytical performance of the method was evaluated in terms of precision, linearity, accuracy, detectability and robustness. The method was applied successfully for the determination of tramadol enantiomers from pharmaceutical formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tramadol (trans-(±)-2-(dimethylaminomethyl)-1-(m-methoxyphenyl) cyclohexanol) is an opioid analgesic drug, used for the treatment of moderate to severe pain. It has two distinct synergistic mechanisms of action, binding to the µ-opioid receptors, and inhibiting the reuptake of serotonin and norepinephrine (Scott and Perry 2000).
Statistically it is one of the most frequently used opioid drug worldwide, having one-tenth of the potency of morphine and being equally potent when compared with codeine; it has a relatively safe pharmacological profile and its risk of abuse is low (Miotto et al. 2017).
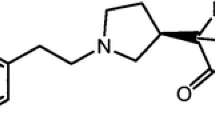
Tramadol presents two asymmetric carbon atoms in its structure, which leads to the existence of four stereoisomers: (1R,2R), (1S,2S), (1R,2S) and (1S,2R); however, it is used in therapy as a racemic mixture of (1R,2R) and (1S,2S) enantiomers. The chemical structure of tramadol enantiomers is presented in Fig. 1.
There are several differences in binding, activity, and metabolism associated with the two enantiomers; as it is acknowledged that the (+) (1R,2R) enantiomer has higher affinity for the µ-opioid receptors and preferentially inhibits serotonin uptake, the (−) (1S,2S) preferentially inhibits norepinephrine reuptake by stimulating α2-adrenergic receptors (Shipton 2000). The complementary and synergistic actions of the two enantiomers improve the analgesic efficacy and tolerability profile of the racemate (Grond and Sablotzki 2004).
Taking into consideration the aspects regarding the stereoselectivity of tramadol enantiomers presented above, it is clear that the development of new methods for its chiral analysis represents a necessity and a challenge. Capillary electrophoresis (CE) is an interesting option in the analysis of chiral pharmaceutical substances, with advantages related to the high efficiency of the separation, short migration times, rapid method development and especially low cost of operation due to the consumption of small quantities of analytes, reagents and chiral selectors (Chankvetadze 2018).
Furthermore, in CE usually a direct method of separation is used by directly adding the chiral selector in the background electrolyte (BGE). Many chiral selectors are currently available in CE; but among them cyclodextrins (CDs) are by far the most efficient and frequently used, due to their commercial availability, good stability over a wide pH range, relatively low price and UV transparency. Chiral recognition with CD involves the inclusion of the bulky hydrophobic part of the chiral analyte in its cavity and interactions of the hydroxyl groups or with other polar constituents on the upper rim such as hydrogen bonds and dipole–dipole interactions with the analyte (Rezanska et al. 2014).
The enantiomeric separation of tramadol and its major metabolites was achieved by capillary zone electrophoresis (CZE) in a phosphate buffer at pH 2.5 containing methyl-β-CD (M-β-CD), urea and hydroxypropylmethyl cellulose (Chan and Ho 1998). CZE was applied for the enantiomeric resolution of tramadol from pharmaceutical products using a polyacrylamide-coated capillary and carboxymethyl-β-CD (CM-β-CD) as chiral selector in a phosphate buffer at pH 2.5 (Rudaz et al. 1998). The previously mentioned method was also used for the chiral determination of tramadol and its main phase I metabolites in urine (Rudaz et al. 1999).
The enantioseparation of tramadol and five metabolites in serum samples by on-line capillary zone electrophoresis–electrospray ionization mass spectrometry (CZE–ESI–MS) was resolved using a negatively charged CD, sulfobutyl ether β-CD (SBE-β-CD) (Rudaz et al. 2000). The enantiodetermination of tramadol in human urine was resolved by CE with laser-induced native fluorescence detection (CE-LIF) using a dual CD system composed of CM-β-CD and M-β-CD (Soetebeer et al. 2001).
Tramadol enantioseparation was evaluated with highly sulphated cyclodextrins (HS-CDs); the best results were obtained when using HS-γ-CD in a phosphate buffer at pH 7.0 (Zandkarimi et al. 2005). Ultrashort partial filling technique was used to obtain infinite resolution of tramadol enantiomers and its metabolites with HS-γ-CD (Rudaz et al. 2004).
Tramadol was used as a model compound in the evaluation of synergistic effect of amino acids on the enantioresolution of basic analytes, in a BGE-containing CDs; the best results were obtained when using HP-α-CD, aspartic acid and a phosphate buffer at pH 3.0 (Chalavi et al. 2018). Tramadol was used as a model compound for the evaluation of sulfated maltodextrin as a chiral selector in CE (Tabani et al. 2015); maltodextrin was also used as a chiral selector for the stability evaluation of tramadol enantiomers in commercial tablets (Mohammadi et al. 2011) and for the enantioseparation of tramadol and methadone in tablets, urine and plasma samples (Naghdi and Fakhari 2018).
The aim of our study was the development of a new alternative CE chiral separation method for the enantioseparation of tramadol enantiomers using CD as chiral selectors and a rational approach for method optimization by applying an experimental design strategy to establish the optimal analytical conditions.
Experimental
Chemicals and reagents
(±)-(1S,2S, 1R,2R)-Tramadol hydrochloride racemic mixture and pure enantiomers (−)-(1S,2S)-tramadol and (+)-(1R,2R)-tramadol hydrochloride were purchased from Cayman Europe (Estonia). The racemic mixture was of pharmaceutical grade while the pure enantiomers were analytical standards.
Phosphoric acid 85% (Chimopar, Romania), disodium phosphate, monosodium phosphate, sodium tetraborate (Merck, Germany), methanol, and sodium hydroxide (Lach-Ner, Czech Republic) were used in the determinations. All reagents were of analytical grade. Deionised water was produced by a Milli-Q system (Millipore, USA) and was used throughout for the preparations of solutions.
The following CD were used as chiral selectors in the screening process: native neutral CDs (β-CD, γ-CD), derivatized neutral CDs (hydroxypropyl-β-CD—HP-β-CD, randomly methylated β-CD—RAMEB, heptakis(2,6-di-O-methyl)-β-CD—DIMEB, heptakis (2,3,6-tri-O-methyl)-β-CD—TRIMEB) and derivatized anionic CDs (carboxymethyl-β-CD—CM-β-CD, sulfobutyl ether-β-CD SBE-β-CD). All CDs were obtained from Cyclolab (Hungary) except for SBE-β-CD (Captisol®) which was kindly donated by Cydex (USA).
For determination from pharmaceutical preparations, Tramadol capsules containing 50 mg tramadol and tramadol modified release tablets containing 100 mg tramadol (Krka, Slovenia) were used.
Instrumentation
Separations were performed on an Agilent 1600 CE system (Agilent Technologies, Germany) equipped with DAD detector. Data acquisition was performed with Chemstation 7.01 software (Agilent Technologies, Germany). An uncoated bare fused-silica capillary of 30 cm length (22 cm effective length) × 50 µm (Agilent Technologies, Germany) was used during determinations. Buffer pH was determined using a Terminal 740 pH meter (Inolab, Germany).
In the process of method optimization, Design Expert 7.0 statistical software (Stat-Ease Inc., USA) was used for constructing the experimental design and data evaluation.
Electrophoretic conditions
The fused-silica capillary was conditioned by flushing for 30 min with 0.1 N sodium hydroxide, 15 min with deionized water and 15 min with the buffer solution. Before each run, the capillary was preconditioned 1 min with 0.1 N sodium hydroxide, 1 min with water and 2 min with the buffer solution.
Tramadol stock solutions having a concentration of 0.3 mg/mL were prepared in methanol and diluted with the same solvent to the desired concentrations. Samples and buffers were sonicated in an ultrasonic bath for 5 min prior to use. All the samples were kept in the refrigerator when not in use.
In the preliminary analysis, the following electrophoretic conditions were used: BGE concentration 25 mM, + 20 kV applied voltage, 25 °C system temperature, hydrodynamic injection 50 mbar for 1 s, UV detection at 210 and 270 nm. The calculated sample injection volume was 2.87 nL. The pH of the BGE was adjusted using a 0.1 N sodium hydroxide solution. To avoid problems related with high generated current, the developed current was kept below 150 µA.
Pharmaceutical sample preparation
Ten tablets/capsules from each sample were weighed on an analytical balance and grounded into a homogenous fine powder in a mortar. A quantity corresponding to the medium weight of the pharmaceutical formulation was quantitatively transferred into a volumetric flask, dissolved in methanol, sonicated in an ultrasonic bath for 5 min and filtered through a 0.45-µm cellulose membrane filter. The solutions were further diluted with methanol to the desired concentration before being injected in the CE system using the same electrophoretic conditions as in the case of standard tramadol solutions.
Results and discussion
Preliminary analysis
Tramadol is a basic analyte with a pKa of 9.41 consequently is positively charged at acidic and neutral pH values of the BGE because of the ionization of its side-chain amino group; however, due to the presence of a hydroxyl group, it can be charged also in a basic environment (Mohammadi et al. 2011). Consequently, tramadol can be detected over a large pH range between 2.0 and 11.0 in an achiral environment; however, in basic BGE it will migrate very close to the EOF.
Initial electrophoretic determinations were performed at four pH levels: 2.0, 5.0, 7.0 and 9.0. Both charged and uncharged CDs were selected to find the most appropriate chiral selector by adding 10 mM neutral CDs and 5 mM ionized CDs in the BGE. The best results using the initial electrophoretic conditions were obtained when using CM-β-CD. When using CM-β-CD, chiral recognition was observed at all pH levels, but the best results in terms of migration times and chiral resolution were achieved in a basic BGE.
CM-β-CD is an anionic derivatized CD, which is negatively charged at pH values above 5.0, as its carboxylic functions are deprotonated and consequently have its own electrophoretic mobility (Fang et al. 2017). When the pH of the BGE increased, inducing a charge on the chiral selector, the differences between the electrophoretic mobilities of tramadol enantiomers increased. Also, migration times were lower when using a basic environment.
The chiral interactions were evaluated taking into consideration the separation factor (α) calculated as the ratio of the migration times of the optical isomers and the chiral resolution (Rs) calculated using Rs = 2(t2 − t1)/(w1 + w2) equation, where the migration times (t1 and t2) and the peak-widths (w1 and w2) were marked for the slow and fast migrating enantiomers, respectively.
Method optimization
After a preliminary “one factor at time” (OFAT) approach a face-centred central composite design (FCCD) was used to establish the optimum electrophoretic conditions.
Initially, the influence of buffer concentration, buffer pH, CM-β-CD concentration, temperature, voltage and injection parameters on the enantioseparation was evaluated with the OFAT technique.
In an OFAT optimization, one electrophoretic parameter is varied while the others are maintained constant (Bezerra et al. 2008). This approach was used to clarify the influence of each parameter on the migration times and chiral resolution.
pH plays an important role in chiral separation by CE as the mobility of the analyte depends on it. Moreover, the magnitude of the EOF, the mobility of the charged chiral selector and the chiral recognition ability are also pH dependent. In our case by increasing buffer pH an increase of the chiral resolution was observed.
The increase of BGE concentration leads to a longer migration time due to the slower EOF and to a slight decrease of the chiral resolution. This may be attributed to the fact that an increase of BGE concentration will lead to an increased current generation as well as Joule heating.
CD concentration is considered one of the most important parameters in chiral separations. Since it has an optimum value, the difference in the apparent mobility between the two enantiomers will reach a maximum at a certain CD concentration which depends on the affinity of the enantiomers towards the CD and will decrease at higher concentrations.
It was noticed that as the temperature increased, both analysis time and chiral resolution decreased, due to the lower viscosity of the BGE.
An increase of the applied voltage leads to shorter migration times, but on the other hand generation of Joule heat may affect negatively chiral resolution.
By increasing injection times, the sensitivity increases, but the chiral resolution decreases due to peak broadening.
In recent years, the experimental design has been widely used to understand the effects of and interactions of input factors on the output responses of analytical methods (Fukuda et al. 2018). In this study, we applied a FCCD, used in response surface technology for building quadratic models for the response variables (Candioti et al. 2014).
After preliminary determinations, three separation parameters, system temperature (A), buffer pH (B) and CD concentration (C), were chosen as significant to observe the effect of separation responses. The responses were the chiral resolution between peaks and the migration time of the second migrating enantiomer.
A total of 15 experiments were carried out, each factor had two levels (coded as − 1 and 1), and five additional injections at the center point of the experimental plan. The levels of the selected parameters were chosen based on the previous screening analysis: temperature (15, 17.5, 20 °C), buffer pH (9.0, 10.0, 11.0), CD concentration (3, 5, 7 mM). The rest of the parameters, considered to be less important based on OFAT results, were held constant in the optimization process: BGE concentration 25 mM, applied voltage 20 kV, injection pressure/time 50 mbar/1 s. The experimental design and the response factor are presented in Table 1.
Mathematical models were obtained through statistical analysis, we evaluated to include or to exclude the coefficients of linear terms (A, B, C), interaction terms (AB, AC, BC), and quadratic terms (A2, B2, C2) using a backward elimination strategy based on analysis of variance (ANOVA). Based on ANOVA, the following regression models were obtained:
Multiple regression model adjustment was assessed based on determination coefficients, R2 and R2-adj. The following values of the determination coefficients were obtained: for resolution R2 = 0.9466 and R2-adj = 0.9253, while for analysis time R2 = 0.9819 and R2-adj = 0.9683. The values obtained suggest that our models are suitable for navigation in the design space.
3-D response surface plots for both responses are presented in Fig. 2.
The error provided by regression model lack-of-fit is significantly lower than random pure error, indicating that the regression models are fitted.
Derringer’s desirability functions were used for maximization and target optimization of the output responses. The numerical optimization feature of the statistical software was used to set goals for each response to generate optimal conditions.
An optimum solution was generated for buffer pH, CD concentration, and system temperature; and further fine-tuning for buffer concentration, applied voltage and injection parameters was made based on previous observations in the OFAT approach preliminary optimization.
Finally, the following parameters were selected as optimum for the separation: 25 mM sodium tetraborate BGE, pH 11.0, 5 mM CM-β-CD chiral selector, system temperature 15 °C, applied voltage + 17.5 kV, injection pressure/time 30 mbar/1 s. Applying the optimized conditions, we succeeded in the chiral separation of tramadol enantiomers in approximately 7 min, with a Rs of 2.80 and a α 1.08.
The migration order was established based on the differential migration time of the two enantiomers in a chiral system and by spiking the racemate with a solution of a pure enantiomer. The order of migration was (−)-(1S,2S)-tramadol followed by (+)-(1R,2R)-tramadol. The migration order depends on the affinity of the enantiomers towards the chiral selector, the enantiomer which is complexed more strongly by the CD migrating slower.
A typical electropherogram for tramadol standard solution is presented in Fig. 3.
Analytical performance
The analytical performances of the method were verified in terms of precision, linearity, accuracy, detectability and robustness according to ICH guidelines (Parr and Schmidt 2018).
To verify intra- and inter-day precision, a sample of 0.3 mg/mL standard tramadol solution was analyzed. The intra-day variation was determined by analyzing six replicates on the same day while inter-day variation was conducted over three consecutive days. Intra- and inter-day precision were expressed as RSD (%) for migration time, peak area and peak height. The results are presented in Table 2.
Calibration curve was constructed using nine different concentrations over a concentration range of 0.06–0.6 mg/mL and was performed in triplicates. Correlation coefficients of over 0.99 suggest a good linearity of the method (Table 3).
Limits of detection (LOD) and quantification (LOQ) were calculated as the standard deviation of regression equation/slope of the regression equation multiplied by 3.3 and 10, respectively (Table 3).
The robustness of the method was studied using a Plackett–Burmann experimental plan. Plackett–Burmann designs are two-level fractional factorial designs, which allow to study up to N-1 input factors with N experiments (Bezerra et al. 2008); in the current experiment, we studied five input factors with 12 experiments. The five variables were the following: BGE concentration (22 mM, 28 mM), BGE pH (10.8, 11.2), CD concentration (4.5 mM, 5.5 mM), capillary temperature (14 °C, 16 °C), applied voltage (16 kV, 19 kV); each value investigated at a lower and an upper level with regard to the optimized values. As response factors chiral resolution and migration times of both enantiomers were recorded. According to the statistical evaluation of the data using ANOVA, none of the five studied variables influenced significantly the three experimental responses; consequently, the developed method can be considered robust.
The accuracy of the method was determined by performing recovery tests. An appropriate amount of tramadol capsule powder was weighed and spiked with known amounts of racemic tramadol (0.075, 0.15, 0.30 mg/mL); samples were analyzed in triplicates. The accuracy values ranged from 98.12 to 99.25% for (S,S)-tramadol and 98.01 to 98.77 for (+)-(R,R)-tramadol respectively. The good recovery values are an indication of good accuracy of the method.
The developed method was applied for the determination of tramadol in pharmaceutical products. Good agreement between the obtained and the manufacturer’s claimed values were found for all samples (Table 4). Figure 4 shows typical electropherograms of pharmaceutical formulation samples.
Conclusions
We have developed a simple and cost-effective CZE analytical procedure for stereoselective analysis of tramadol in pharmaceutical formulations. A complex screening of several CDs at four different pH levels was made to establish the optimum chiral selector. The best results were obtained when using an anionic CD, CM-β-CD in a basic BGE.
Under basic conditions, tramadol is uncharged and transported towards cathode while the anionic selector migrating towards anode will decelerate the more strongly complexed enantiomer.
After a preliminary univariate optimization, we optimized the analytical method using a FCCD. In comparison with the classical OFAT approach the use of experimental design allows to evaluate the existence of interaction between the factors which may lead to a more adequate method development and optimization.
The analytical performance of the optimized method was verified according to ICH guideline and can be considered suitable for tramadol preliminary chiral analysis. Based on this assumption, the newly developed technique can be used to determine the enantiomeric ratio of tramadol in formulations as well as to quantify the tramadol enantiomer content.
References
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Candioti LV, De Zan MM, Camara MS, Goichoechea HC (2014) Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 124:123–138. https://doi.org/10.1016/j.talanta.2014.01.034
Chalavi S, Fakhari AR, Nojavan S, Mirzaei P (2018) Evaluation of the synergistic effect with amino acids for enantioseparation of basic drugs using capillary electrophoresis. Electrophoresis 39:2022–2029. https://doi.org/10.1002/elps.201800128
Chan ECY, Ho PC (1998) Enantiomeric separation of tramadol hydrochloride and its metabolites by cyclodextrin-mediated capillary zone electrophoresis. J Chromatogr B 707:287–294. https://doi.org/10.1016/S0378-4347(97)00605-1
Chankvetadze B (2018) Contemporary theory of enantioseparations in capillary electrophoresis. J Chromatogr A 1567:2–25. https://doi.org/10.1016/j.chroma.2018.07.041
Fang L, Du Y, Hu X, Luo L, Guo X, Yu J (2017) Carboxymethyl β-cyclodextrin as chiral selector in capillary electrophoresis: enantioseparation of 16 basic chiral drugs and its chiral recognition mechanism associated with drugs' structural features. Biomed Chromatogr 31:e3991. https://doi.org/10.1002/bmc.3991
Fukuda IM, Pinto CFF, Moreira CS, Saviano AM, Lourenco FR (2018) Design of experiments (DoE) applied to pharmaceutical and analytical quality by design (QbD). Braz J Pharm Sci 54:e01006. https://doi.org/10.1590/s2175-97902018000001006
Grond S, Sablotzki A (2004) Clinical pharmacology of tramadol. Clin Pharmacokinet 43:879–923. https://doi.org/10.2165/00003088-200443130-00004
Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R (2017) Trends in tramadol: pharmacology, metabolism and misuse. Anesth Analg 124:44–51. https://doi.org/10.1213/ANE.0000000000001683
Mohammadi A, Nojavan S, Rouini M, Fakhari AR (2011) Stability evaluation of tramadol enantiomers using a chiral stability-indicating capillary electrophoresis method and its application to pharmaceutical analysis. J Sep Sci 34:1613–1620. https://doi.org/10.1002/jssc.201100021
Naghdi E, Fakhari AR (2018) Simultaneous chiral separation of tramadol and methadone in tablets, human urine, and plasma by capillary electrophoresis using maltodextrin as chiral selector. Chirality 30:1161–1168. https://doi.org/10.1002/chir.23008
Parr MK, Schmidt AH (2018) Life cycle management of analytical methods. J Pharm Biomed Anal 147:506–517. https://doi.org/10.1016/j.jpba.2017.06.020
Rezanska P, Navratilova K, Rezanska M, Kral V, Sykora D (2014) Application of cyclodextrins in chiral capillary electrophoresis. Electrophoresis 35:2701–2721. https://doi.org/10.1002/elps.201400145
Rudaz S, Veuthey JL, Desiderio C, Fanali S (1998) Use of cyclodextrins: resolution of tramadol enantiomers. Electrophoresis 19:2883–2889. https://doi.org/10.1002/elps.1150191614
Rudaz S, Veuthey JL, Desiderio C, Fanali S (1999) Simultaneous stereoselective analysis by capillary electrophoresis of tramadol enantiomers and their main phase I metabolites in urine. J Chromatogr A 846:227–237. https://doi.org/10.1016/S0021-9673(99)00028-X
Rudaz S, Cherkaoui S, Dayer P, Fanali S, Veuthey JL (2000) Simultaneous stereoselective analysis of tramadol and its main phase I metabolites by on-line capillary zone electrophoresis–electrospray ionization mass spectrometry. J Chromatogr A 868:295–303. https://doi.org/10.1016/S0021-9673(99)01257-1
Rudaz S, Le Saux T, Prat J, Gareil P, Veuthey JL (2004) Ultrashort partial-filling technique in capillary electrophoresis for infinite resolution of tramadol enantiomers and its metabolites with highly sulfated cyclodextrins. Electrophoresis 25:2761–2771. https://doi.org/10.1002/elps.200406052
Scott LJ, Perry CM (2000) Tramadol: a review of its use in perioperative pain. Drugs 60:139–176. https://doi.org/10.2165/00003495-200060010-00008
Shipton EA (2000) Tramadol-present and future. Anaesth Intensive Care 28:363–374
Soetebeer UB, Schierenberg MO, Schulz H, Andresen P, Blaschke G (2001) Direct chiral assay of tramadol and detection of the phase II metabolite O-demethyl tramadol glucuronide in human urine using capillary electrophoresis with laser-induced native fluorescence detection. J Chromatogr B 765:3–13. https://doi.org/10.1016/S0378-4347(01)00366-8
Tabani H, Mahyari M, Sahragard A, Fakhari AR, Shaabani A (2015) Evaluation of sulfated maltodextrin as a novel anionic chiral selector for the enantioseparation of basic chiral drugs by capillary electrophoresis. Electrophoresis 36:305–311. https://doi.org/10.1002/elps.201400370
Zandkarimi M, Shafaati A, Foroutan SM, Oliaee NH, Eslamparast A (2005) Separation of tramadol enantiomers by capillary electrophoresis using highly sulfated cyclodextrins. DARU 13:143–147
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarkany, A., Hancu, G., Cârje, A. et al. Chiral separation of tramadol enantiomers by capillary electrophoresis using cyclodextrins as chiral selectors and experimental design method optimization. Chem. Pap. 73, 2363–2370 (2019). https://doi.org/10.1007/s11696-019-00789-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00789-8