Abstract
Pd-catalyzed coupling reactions like the Sonogashira coupling reaction are very useful tools for the formation of new carbon–carbon bonds under mild reaction conditions. Coupling reactions are also used for elaboration of organic compounds in drug and material discovery. Terminal alkynes have a very critical role in Sonogashira coupling reaction. Therefore, design and synthesis of new terminal alkynes are very important for the preparation of organic compounds. In the present study, a novel alkyne 3-ethynyl-2-(thiophen-2-yl)benzo[b]thiophene 13 was synthesized, and it was tested for Sonogashira coupling reaction with different iodoaryl compounds. It was investigated whether our terminal alkyne 13 having a special construction might be a useful precursor for the synthesis of potentially active organic molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heteroaromatic compounds have a very important role in discovery and development of new drug and material candidates due to their interesting properties (Brasholz et al. 2009; Katritzky et al. 1998; Kivrak and Larock 2010). They have been used as anti-parasitic (Coa et al. 2015), antibacterial (Pathak et al. 2012; Shakdofa et al. 2014), anti-cancer (Rahmouni et al. 2016), anti-fungal (Pathak et al. 2012), anti-inflammatory (Kazemizadeh et al. 2016), and antioxidant (Richardson et al. 2009) drugs. In addition, conjugated heterocyclic compounds have been used in solar cells, transistor, LEDs, biosensors, sensors, and electrochromic devices.
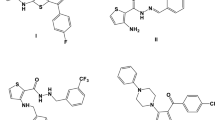
Thiophenes and benzothiophenes are well-known five-membered heterocyclic compounds present in organic chemistry. Their core is present in many natural and pharmacologically active compounds (Scheme 1). They have been used for the treatment of a variety of diseases. For example, Raloxifene 1 (Meixner et al. 2017; Weiser et al. 2017), Zileuton 2 (Sarret et al. 2017), and Sertaconazole 3 (Croxtall and Plosker 2009) are commercially available drugs. Raloxifene is used for the treatment of breast cancer. Moreover, Raloxifene has less side effects than Tamoxifen which has similar biological activities (Vogel et al. 2006). Moreover, the stability of thiophenes and benzothiophenes 4 plays an important role in increasing and preparing different types molecules for the material (Guo et al. 2018) and pharmaceutical areas. Recently, we also reported the novel biological activities of alkynyl-substituted benzothiophene derivatives (Algso et al. 2018). It was investigated whether benzothiophene derivatives have a high antibacterial activity against S. aureus ATCC 25923. Moreover, they may also be used for the treatment of fungal diseases.
Benzothiophenes are generally obtained from intramolecular cyclization and Claisen rearrangement reactions. For example, benzothiophenes were regioselectively synthesized via intramolecular cyclization reaction of o-alkinylthioanisoles (Sun et al. 2011). Recently, Mohanakrishnan et al. published a new method for the synthesis of benzothiophene and dibenzothiophene from thiophenes and 2,5-dimethoxy-THF using Lewis acid catalyst (Rafiq et al. 2014).
Organic reactions including cyclization reactions, condensation reactions, oxidation reaction, etc., have been used for the synthesis of organic molecules. Therefore, many scientists have been tried to find easy and effective methodologies for the formation of novel compounds. In the last decades, coupling reactions were mostly applied for the synthesis of organic molecules via a new carbon–carbon bond formation. One of the important coupling reactions is the palladium-catalyzed Sonogashira coupling reaction discovered in 1975. Sonogashira coupling reaction needs very easy reaction conditions such as, lower temperature, shorter reaction time and higher yields. Palladium-catalyzed Sonogashira coupling reaction needs a terminal alkyne and halo-substituted aromatics. Therefore, terminal alkynes are very important for the formation of a new C–C bond in designed molecules.
In the present study, a novel terminal alkyne-substituted benzothiophene was synthesized using electrophilic cyclization reactions and palladium-catalyzed coupling reaction. Then, our terminal alkyne was tested for Sonogashira coupling reaction using different halo-substituted aromatics, heteroaromatics and polyaromatics in the presence of Pd catalyst.
Experimental section
General information
All organic compounds were analyzed by 1H and 13C NMR. 1H and 13C NMR spectra which were recorded on an Agilent NMR (400 MHz) spectrometer. Chemical shifts are reported in parts per million (ppm) downfield from an internal TMS (trimethylsilane) reference. Coupling constants (J) are reported in hertz (Hz). In addition, spin multiplicities are presented by the following symbols: s (singlet), brs (broad singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). Flash chromatography was performed using thick-walled glass columns and ‘flash grade’ silica 60 (Merck 230–400 mesh). Commercially prepared 0.25 mm silica gel plates (Silica gel 60, F254) was used for thin layer chromatography (TLC) and visualization was affected by short wavelength UV lamp. Solvents, reagents, and chemicals used for reactions were purchased from commercial suppliers. All commercially available reagents were used directly without purification unless otherwise stated. The inert atmosphere was created by slight positive pressure (ca. 0.1 psi) of argon. All the glasswares were dried before using for reactions.
Synthesis of trimethyl((2-(methylthio)phenyl)ethynyl)silane (6)
To a stirred mixture of the 2-iodothioanisole 5 (2.5 mol, 620 mg), THF (8 mL), ethynyltrimethylsilane (3 mol, 303 mg), triethylamine (12.4 mol, 1.72 mL), PdCl2(PPh3)2 (0.06 mol, 42.1 mg) was added CuI (0.06 mol, 11.4 mg) under Argon atmosphere. The resulting mixture was stirred at room temperature for 12 h. Then, the mixture was quenched with water (30 mL), then extracted with DCM (3 × 30 mL). The organic phase was dried over anhydrous MgSO4 and filtered. The solvent was removed under vacuum, and the residue was purified by column chromatography over silica gel with hexane to afford trimethyl((2-(methylthio)phenyl)ethynyl)silane (6) as a light yellow oil in an 96% yield. 1H NMR (400 MHz, CDCl3) 7.43 (dd, J =7.7, 1.5 Hz; 1H), 7.27 (td, J =7.5, 1.5 Hz; 1H), 7.11 (d, J =7.4 Hz; 1H), 7.05 (td, J = 7.5, 1.2 Hz; 1H), 2.46 (s, 3H), 0.32 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 142.2, 132.7, 129.1, 124.1, 123.9, 121.1, 102.3, 101.4, 15.0, 0.13; IR (ATR) vmax (cm−1): 3060, 2981, 2921, 2891, 2155 (\({\text{C}} \equiv {\text{C}}\)), 1436, 1248 (Si–CH3), 838, 748, 685 (S–C). The spectral data were in agreement with those reported previously for this compound (Chen et al. 2014; Algso et al. 2018).
Synthesis of 1-ethynyl-2-(methylsulfanyl)benzene (7)
To a stirred mixture of 6 (500 mg, 2.27 mol), methanol (60 mL), and THF (20 mL) K2CO3 (939 mg, 6.81 mol) was added. The mixture was stirred at room temperature for 60 min. The reaction mixture extracted with EtOAc (3 × 15 mL). The organic extracts were dried over anhydrous MgSO4 and filtered. The solvents was removed under vacuum, and the residue was purified by column chromatography over silica gel with hexane to afforded 1-ethynyl-2-(methylsulfanyl)benzene (7) as yellow oil in an 91% yield: 1H NMR (400 MHz, CDCl3) δ 7.46 (dd, J =7.6, 1.5 Hz; 1H), 7.30 (td, J =8.2, 1.4 Hz; 1H), 7.15 (d, J =8.0 Hz; 1H), 7.08 (td, J = 8.5, 1.0 Hz; 1H), 3.5 (s, 1H, alkyne), 2.48 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 142.0, 133.1, 129.3, 124.2, 124.1, 120.1, 83.7, 81.0, 60.3; IR (ATR) vmax (cm−1): 3058, 2982, 3281 (\(\equiv\) C–H), 2920, 2102 (\({\text{C}} \equiv {\text{C}}\)), 1433, 748, 615 (S–C); HRMS calcd for C9H9S, 149.0425 [M+H]+ found 149.0248 [M+H]+. The spectral data were in agreement with those reported previously for this compound (Chen et al. 2014; Algso et al. 2018).
Synthesis of 2-((2-(methylthio) phenyl)ethynyl) thiophene 10
Method A: to a solution of 2-bromothiophene 9 (456.4 mg, 2.8 mol) in 1,4-dioxane (6 mL), CuI (32.4 mg, 0.17 mmol), PdCl2(PhCN)2 (65.2 mg, 0.17 mol) 1-ethynyl-2-(methylsulfanyl)benzene 7 (3.0 mol, 444 mg), diisopropylamine (1.38 g, 13.7 mol), and P(t-Bu)3 (52.9 mg, 0.33 mol) were successively added at room temperature under argon. The mixture was stirred at room temperature for 12 h, and then extracted with DCM (3 × 20 mL). The organic layer was dried over anhydrous MgSO4 and filtered. The solvent was removed under vacuum, and the residue was purified by column chromatography over silica gel with Hexane/EtOAc (10:1) to afford 2-((2-(methylthio) phenyl)ethynyl)thiophene (10) as orange oil in an 77% yield. 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J =7.7 Hz; 1H), 7.36–7.30 (m, 3H), 7.19 (d, J = 8,0 Hz; 1H), 7.13 (td, J = 8.0, 1.1 Hz; 1H), 7.04 (m, 1H), 2.53 (s, 3H).13C NMR (100 MHz, CDCl3) δ 141.8, 132.3, 132.2, 129.1, 127.8, 127.3, 124.4, 124.3, 123.2, 121.1, 90.7, 89.2, 15.3; IR (ATR) vmax (cm−1): 3086, 2912, 2981, 2200 (\({\text{C}} \equiv {\text{C}}\)), 1431, 753, 699 (S–C); HRMS calcd for C13H11S2, 231.0302 [M+H]+ found 231.0313 [M+H]+. The spectral data were in agreement with those reported previously for this compound (Lu and Wu 2007; Algso et al. 2018).
Method B: to a mixture of 2-iodothiophene (707 mg, 3.37 mol), 1-ethynyl-2-(methylsulfanyl)benzene 7 (500 mg, 3.37), DMF (6 mL), PdCl2(PPh3)2 (118 mg, 0.17 mol), and triethylamine (1.9 mL, 13.7 mol) at 0 °C under argon gas CuI (32.1 mg, 0.17 mmol) was successively added. The mixture was stirred at room temperature for 4 h. The mixture was extracted with ether (3 × 20 mL). The organic layer was dried over anhydrous MgSO4 and filtered. The solvent was removed under vacuum, and the residue was purified by column chromatography over silica gel with Hexane/EtOAc (10:1) to afford compound 10 as orange oil in a 71% yield.
Synthesis of 3-iodo-2-(thiophen-2-yl)benzo[b]thiophene (11)
To a solution of 2-((2-(methylthio) phenyl)ethynyl) thiophene (10) (230 mg, 1 mol) in CH2Cl2 (10 mL) I2 (762 mg, 3 mol) was added at room temperature. After stirring for 30 min, the saturated aqueous solution of Na2S2O3 was added subsequently into the reaction mixture and extracted with EtOAc (3 × 20 mL). The combined organic phase was dried over anhydrous MgSO4 and filtered. The solvent was removed under vacuum, and the residue was purified by column chromatography over silica gel with hexane/EtOAc (19:1) to afford 3-iodo-2-(thiophen-2-yl)benzo[b]thiophene (11) as light yellow solid in a 99% yield. 7.82 (d, J = 8.1 Hz; 1H), 7.75 (d, J = 9.1 Hz; 1H), 7.62 (dd, J = 4.0, 1.1 Hz; 1H), 7.48-7.44 (m, 2H), 7.39 (td, J = 8.0, 1.2 Hz; 1H), 7.17 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 142.4, 138.1, 136.0, 135.9, 128.8, 127.5, 127.4, 126.4, 125.9, 125.8, 122.0, 79.5; IR (ATR) vmax (cm−1): 699, 815, 1419, 2912, 2981, 3086; HRMS calcd for C12H7S2, 341.9034 [M]+ found; 341.9059 [M]+ (Algso et al. 2018).
Synthesis of trimethyl((2-(thiophen-2-yl)benzo[b]thiophen-3-yl)ethynyl)silane 12
To a stirred mixture of the 3-iodo-2-(thiophen-2-yl)benzo[b]thiophene 11 (0.7 mol, 239.5 mg), dimethylformamide (DMF) (7.5 mL), ethynyltrimethylsilane (0.75 mol), triethylamine (3.0 mL), PdCl2(PPh3)2 (0.035 mol, 24.5 mg) under argon atmosphere CuI (0.035 mol, 6.6 mg) was added. The resulting mixture was stirred at room temperature for 12 h. Then, the reaction mixture was quenched with water (30 mL) and extracted with DCM (3 × 30 mL). The organic layer was dried over anhydrous MgSO4 and filtered. The solvent was removed under vacuum, and the residue was purified by column chromatography over silica gel with Hexane/EtOAc (19/1) to afford trimethyl((2-(thiophen-2-yl)benzo[b]thiophen-3-yl)ethynyl)silane 12 in a 89% yield as a red oil. 1H NMR (400 MHz, CDCl3) δ 7.90 (d, J = 10,0 Hz; 1H), 7.74 (d, J = 8.0 Hz; 1H), 7.68 (d, J = 4.0 Hz; 1H), 7.47-7.41 (m, 2H), 7.36 (td, J =8.0, 0.9 Hz; 1H), 7.14-7.11 (m, 1H), 0.40 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 141.1, 140.9, 136.8, 136.4, 127.4, 127.2, 127.0, 125.6, 125.3, 123.4, 122.0, 112.9, 104.1, 99.2, 0.13; IR (ATR) vmax (cm−1): 2958, 2148, 1438, 1247, 909, 839, 756, 696; HRMS calcd for C17H17S2Si, 313.0541 [M+H]+ found; 313.0537 [M+H]+(Algso et al. 2018).
Synthesis of 3-ethynyl-2-(thiophen-2-yl)benzo[b]thiophene (13)
To a solution of trimethyl((2-(thiophen-2-yl)benzo[b]thiophen-3-yl)ethynyl)silane (12) (56 mg, 0.17 mol) in methanol (15 mL) and THF (5 mL) K2CO3 (74.3 mg, 0.53 mol) was added at room temperature for 1 h. When the starting compound was gone, solvents were removed under reduced pressure and the residue was extracted with DCM. The solvent was removed under vacuum, and the residue was purified by column chromatography over silica gel with Hexane/EtOAc (19/1) to afford 3-ethynyl-2-(thiophen-2-yl)benzo[b]thiophene (13) as a red oil in a 83% yield. 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J =8.0 Hz; 1H), 7.77-7.70 (m, 2H), 7.48–7.34 (m, 3H), 7.16–7.10 (m, 1H), 3.70 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 141.4, 140.1, 136.9, 136.0, 127.7, 127.4, 127.1, 125.7, 125.4, 123.2, 122.1, 111.8, 85.8, 78.3; IR (ATR) vmax (cm−1): 3286, 3101, 2922, 2851, 2095, 1940, 1791, 1438, 1189, 752, 690, 647, 584; HRMS calcd for C14H9S2, 241.0146 [M+H]+ found; 241.0139 [M+H]+ (Algso et al. 2018).
General procedure of Sonogashira cross-coupling reaction between compound 13 and aryl iodide (14A–H)
To a stirred mixture of the terminal alkyne 13 (0.7 mol), dimethylformamide (DMF) (7.5 mL), aryl iodide compound (0.75 mol), triethylamine (3.0 mL), PdCl2(PPh3)2 (0.035 mol) under argon atmosphere CuI was added (0.035 mmol). The resulting mixture was stirred at room temperature for 10 h. Then, the reaction mixture was quenched with water (30 mL) and extracted with EtOAc (3 × 30 mL). The organic layer was dried over anhydrous MgSO4 and filtered. The solvent was removed under vacuum, and the residue was purified by column chromatography over silica gel with Hexane–EtOAc.
Compound 14A
The terminal alkyne (13) 170 mg (0.7 mol), dimethylformamide (DMF) (7.5 mL), iodobenzene, 142.8 mg (0.75 mol), triethylamine (3.0 mL), PdCl2(PPh3)2, 24.5 mg (0.035 mol), and CuI 7 mg (0.035 mmol) were employed to afford 68% yield as yellow-green solid of the indicated product. 1H NMR (400 MHz, CDCl3) δ 7.99 (d, J = 7.4 Hz; 1H), 7.77 (d, J = 8.0 Hz; 1H), 7.74–7.70 (m, 2H), 7.66 (d, J = 3.7 Hz; 1H), 7.49–7.37 (m, 6H), 7.13–7.15 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 140.8; 140.2, 137.0, 136.5, 131.8, 128.8, 128.7, 127.5, 127.2, 127.16, 125.7, 125.3, 123.5, 123.3, 122.2, 113.0, 97.9, 84.2; IR (ATR) vmax (cm−1): 3063, 2980, 2922, 2199, 1438, 1237, 746, 684; HRMS: calcd for C20H13S2, 317.0419 [M+H]+ found, 317.0457 [M+H]+.
Compound 14B
The terminal alkyne (13), 170 mg (0.7 mol), dimethylformamide (DMF) (7.5 mL), 4-iodotoluene, 152.6 mg (0.75 mol), triethylamine (3.0 mL), PdCl2(PPh3)2, 24.5 mg (0.035 mol), and CuI, 6.66 mg (0.035 mmol) were employed to afford 80% yield as yellow solid of the indicated product. 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 8.0 Hz; 1H), 7.76 (d, J = 7.9 Hz; 1H), 7.66 (d, J = 3.8 Hz; 1H), 7.60 (d, J = 8.0 Hz; 2H), 7.48–7.36 (m, 3H), 7.24 (d, J = 7.9 Hz; 2H), 7.15–7.12 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 140.8, 139.8, 139.0, 137.0, 136.6, 131.7, 129.5, 127.4, 127.04, 127.01, 125.6, 125.2, 123.3, 122.1, 120.4, 113.1, 98.2, 83.6, 21.8; IR (ATR) vmax (cm−1): 3069, 2981, 2918, 1437, 807, 752; HRMS: calcd for C21H15S2, 331.0615 [M+H]+ found 331.0699 [M+H]+.
Compound 14C
The terminal alkyne (13), 170 mg (0.7 mmol), dimethylformamide (DMF) (7.5 mL), 4-iodoaniline, 153 mg (0.75 mol), triethylamine (3.0 mL), PdCl2(PPh3)2, 24.5 mg (0.035 mmol), and CuI, 7 mg (0.035 mmol) were employed to afford 55% yield as yellow solid of the indicated product. 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 10.0 Hz; 1H), 7.76 (d, J = 9.6 Hz; 1H), 7.65 (dd, J =3.7 Hz, 1.12 Hz; 1H), 7.51 (d, J =8.7 Hz; 2H), 7.47–7.35 (m, 3H), 7.14–7.11 (m, 1H), 6.70 (d, J = 8.7 Hz; 2H), 3.9 (brs, 2H). 13C NMR (100 MHz, CDCl3) δ 147.2, 140.9, 138.8, 136.9, 136.8, 133.2, 127.4, 126.8 (including 2C), 125.6, 125.2, 123.4, 122.1, 115.1, 113.6, 112.8, 98.9, 82.2; IR (ATR) vmax (cm−1): 3648, 3378.68, 2956, 2922, 2194, 1603, 1614, 1288, 1174, 906, 825, 729, 696; HRMS:calcd for C20H14S2, 332.0568 [M+H]+ found 332.0559 [M+H]+.
Compound 14D
The terminal alkyne (13), 170 mg (0.7 mol), dimethylformamide (DMF) (7.5 mL), 2-iodothioanisole, 175 mg (0.75 mol), triethylamine (3.0 mL), PdCl2(PPh3)2, 24.5 mg (0.035 mol), and CuI, 7 mg (0.035 mmol) were employed to afford 93% yield as yellow solid of the indicated product. 1H NMR (400 MHz, CDCl3) δ 8.16 (d, J = 8.0 Hz; 1H), 7.75 (d, J = 7.96 Hz; 1H) 7.72 (dd, J = 3.7 Hz, 1.08 Hz; 1H), 7.66 (dd, J = 7.7 Hz, 1.32 Hz;1H), 7.49–7.33 (m, 4H) 7.27–7.24 (m, 1H), 7.22–7.11 (m, 2H),2.56 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 141.7, 141.0, 136.9, 136.5, 132.64, 130.0, 129.2, 127.6, 127.3, 127.2, 125.7, 125.5, 124.6, 124.5, 123.8, 122.1, 121.7, 113.0, 95.1, 90.5, 15.52; IR (ATR) vmax (cm−1): 3054, 2919, 2197, 1433, 1069, 745, 691; HRMS: calcd for C21H15S3, 363.0336 [M+H]+ found 363.0335 [M+H]+.
Compound 14E
The terminal alkyne (13), 170 mg (0.7 mol), dimethylformamide (DMF) (7.5 mL), 4-iodoanisole, 163 mg (0.75 mol), triethylamine (3.0 mL), PdCl2(PPh3)2 (24.5 mg, 0.035 mol), and CuI 7 mg (0.035 mmol) were employed to afford 80% yield as yellow-green solid of the indicated product. 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 10.2 Hz; 1H), 7.76 (d, J = 7.9 Hz, 1H), 7.66–7.63 (m, 3H), 7.45–7.35 (m, 3H), 7.14–7.11 (m, 1H), 6.96 (d, J = 14.4 Hz, 2H), 3.87 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 160.0, 140.8, 139.4, 136.9, 136.7, 133.3, 127.4, 127.0, 126.9, 125.6, 125.2, 123.4, 122.9, 115.6, 114.4, 113.3, 98.1, 82.9, 55.6; IR (ATR) vmax (cm−1): 3099, 3050, 2970, 2836, 2537, 2199, 1603, 1455, 1299, 1248, 1169, 1022, 834, 753, 689; HRMS: calcd for C21H14OS2, 346.0486 [M]+, found 346.0488 [M]+.
Compound 14F
The terminal alkyne (13), 168 mg (0.7 mol), dimethylformamide (DMF) (7.5 mL), 2-iodothiophene, 147 mg (0.75 mol), triethylamine (3.0 mL), PdCl2(PPh3)2, 24.5 mg (0.035 mol), and CuI 7 mg (0.035 mol) were employed to afford 74% yield as yellow-green solid of the indicated product. 1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 10.0 Hz, 1H), 7.7 (d, J = 10.0 Hz; 1H), 7.65–7.63 (d, J = 3.8, 1.2 Hz, 1H), 7.48–7.42 (m, 3H), 7.41–7.36 (m, 2H) 7.15–7.09 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 140.5, 140.4, 136.9, 136.4, 132.4, 128.1, 127.53, 127.50, 127.3, 127.2, 125.7, 125.4, 123.5, 123.3, 122.2, 112.6, 91.2, 88.0; IR (ATR) vmax (cm−1): 3112, 2980, 2809, 2199, 1670, 1508, 755, 700; HRMS: calcd for C18H10S3 [M]+, 321.9945; found 321.9948 [M]+.
Compound 14G
The terminal alkyne (13), 168.2 mg (0.7 mol), dimethylformamide (DMF) (7.5 mL), 5-iodofuran-2-carbaldehyde, 155 mg (0.75 mol), triethylamine (3.0 mL), PdCl2(PPh3)2, 24.5 mg (0.035 mol), and CuI, 7 mg (0.035 mmol) were employed to afford 63% yield as orange solid of the indicated product. 1H NMR (400 MHz, CDCl3) δ 9.67 (s, 1H), 7.90 (d, J = 6.08 Hz; 1H), 7.75(d, J = 0.76 Hz, 1H), 7.63 (d, J = 6.08 Hz; 1H), 7.45–7.42 (m, 2H), 7.40–7.35 (m, 1H), 7.30 (d, J = 3.8 Hz, 1H), 7.13–7.10 (m,1H), 6.89 (d, J = 3.7 Hz; 1H); 13C NMR (100 MH, CDCl3) δ 177.5, 152.8, 142.8, 142.1, 140.2, 136.8, 135.8, 127.83, 127.80, 127.78, 127.77, 126.0, 125.6, 123.1, 122.2, 117.7, 110.7, 91.5, 86.4; IR (ATR) vmax (cm−1): 359, 3113, 2981, 2808, 2199, 1670, 1506, 1386, 966, 761, 700; HRMS: calcd for C19H10O2S2 [M]+, 334.0122; found 334.0167 [M]+.
Compound 15
The terminal alkyne (13), 168.2 mg (0.7 mol), dimethylformamide (DMF) (7.5 mL), 3-iodo-2-(thiophen-2-yl)benzo[b]thiophene (11), 239.55 mg (0.75 mol), triethylamine (3.0 mL), PdCl2(PPh3)2, 24.5 mg (0.035 mol), and CuI, 6.66 mg (0.035 mmol) were employed to afford 34% yield as yellow-green solid of the indicated product 15. 1H NMR (400 MHz, CDCl3) δ 7.99 (d, J = 7.4 Hz, 2H), 7.78 (d, J = 8 Hz; 2H), 7.73 (dd, J = 4.0 Hz, 1.16 Hz; 2H), 7.50–7.45 (m, 4H), 7.40 (td, J = 8.04 Hz, 1.24 Hz; 2H), 7.15 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 143.9, 140.9, 137.3, 136.2, 127.8, 127.7, 127.5, 126.0, 125.7, 123.44, 123.4, 122.3, 82.6, 78.9; IR (ATR) vmax (cm−1): 3063, 2980, 2922, 2136, 1437, 1061, 748, 685; HRMS:calcd for C28H14S4, 477.9978; found 477.9971.
Results and discussion
First, 2-iodothioanisole 5 was allowed to react with trimethylsilylacetylene for the preparation of 2-ethynyltrimethylsilane 6 via palladium-catalyzed Sonogashira coupling reaction. The standard Sonogashira coupling condition was applied for the synthesis of 6 Pd(PPh3)2Cl2 which was used as a catalyst in the presence of CuI under basic (Et3N) reaction medium. After isolation of compound 6, 2-ethynylthioanisole 7 was obtained from the desilylation reaction of 2-ethynyltrimethylsilane 6 with potassium carbonate in methanol. When compound 7 was allowed to undergo coupling reaction with 2-bromothiophene in the presence of PdCl2(PhCN)2, CuI, (t-Bu)3P in Dioxane at room temperature for 12 h, desired compound 10 was isolated in 77% yield. Fascinatingly, 2-iodothiophene was used for the synthesis of 10 in the presence of Pd catalyst at room temperature, only the self-coupling reaction of terminal alkyne was formed. If the temperature was decreased to 0 °C, desired compound 10 was obtained in 72% yield (Scheme 2). In the last decades, electrophilic cyclization reactions have gained big importance for the synthesis of novel five- or six-membered heterocyclic compounds (Kumar et al. 2017; Yaragorla et al. 2017; Miao et al. 2017) such as indoles, benzothiophenes, and benzofurans. Recently, Zora et al. investigated a novel methodology for the synthesis of iodo-substituted pyrazoles via electrophilic cyclization under mild reaction conditions (Zora et al. 2011). For electrophilic cyclization reactions, moleculer iodine, monoiodochloride (ICl) and PhSeCl were used as electrophiles in a variety of organic solvents (Togo and Iida 2006; Yue and Larock 2004). Previous studies displayed that CH2Cl2 (DCM) and chloroform were among the most employed solvents for those kinds of cyclizations. Mild and nontoxic moleculer iodine was mostly used as electrophile which catalyzed various organic reactions with high efficiency and selectivity. In the light of the knowledge, when 10 was reacted with molecular iodine in DCM for 2 h at RT, a expected product 3-iodo-2-(thiophen-2-yl)benzo[b]thiophene (11) was obtained in good yield (99%) (Scheme 2).
For the synthesis of our designed terminal alkyne 13, intermediate 11 was allowed to react with ethynyltrimethylsilane for the formation of compound 12 which isolated in 89% yields via Sonogashira coupling reaction. Then, protecting TMS groups were removed using desilyation reaction with potassium carbonate in methanol to give the new benzothiophene-substituted terminal alkyne 13 (Scheme 3).
After isolation and characterization of our terminal alkyne precursor 13, the coupling properties were investigated using Sonogashira reaction in the presence of Pd catalyst (Scheme 4). As seen in Table 1, a variety of 3-alkynylic benzothiophene derivatives (14 A–H) were synthesized from coupling reaction. When 13 was allowed to react with iodobenzene in the presence of Pd(PPh2)2Cl2 and CuI in DMF/Et3N at RT, 14A was obtained in 68% yield (Table 1). If the same reaction condition was applied for the synthesis of 14B, the isolated yield was found as 79% yield. When the effect of heteroaromatic compounds were tested using 2-iodothiophene and 2-iodofuran, the 74% yield of 14F and 63% yield of 14G was found, respectively. Notably, 12C was obtained in moderate yield (55%). Interestingly, the reaction between 2-thioanisole and terminal alkyne 13 gave the highest yield as 93% yield. It was also tested for coupling reaction for poly-heteroaromatic compounds like compound 11, dibenzothiophene-substituted alkyne (Table 1, Entry 8) was not obtained. When our standard reaction condition was used for the formation of compound 14H, we obtained only self-coupling product 15 in 34% yield (Scheme 5). As a result, Sonogashira cross-coupling reaction was found to be general for a wide range of our terminal alkyne 13 and tolerated the presence of aromatic, poly-aromatic and heteroaromatic moieties with electron-withdrawing and electron-donating substituents (Table 1).
Conclusions
Pd-catalyzed coupling reactions have been used for the synthesis of important organic molecules via a new C–C bond formation. Sonogashira cross-coupling reaction also plays a critical role in the synthesis of alkynyl-substituted organic molecules. Terminal alkynes have a very critical role in Sonogashira coupling reaction, so the design of new terminal alkyne could be very important for Sonogashira coupling reaction. In this work, we have investigated a user-friendly methodology for the synthesis of novel alkynyl-substituted benzothiophene derivatives using Pd-catalyzed Sonogashira coupling reaction under mild reaction conditions. In the first part of study, a novel alkyne 13 was synthesized and characterized. Then, it was tested for coupling reactions with a variety of aryl iodide derivatives including aromatics, heteroaromatics and polyaromatics. As a result, it was found that our terminal alkyne 13 might be a useful precursor for the synthesis of potentially active novel organic molecules via coupling reactions in future studies.
References
Algso MAS, Kivrak A, Konus M, Yilmaz C, Kurt-Kizildogan A (2018) Synthesis and biological evaluation of novel benzothiophene derivatives. J Chem Sci. https://doi.org/10.1007/s12039-018-1523-3
Brasholz M, Reissig HU, Zimmer R (2009) Sugars, alkaloids, and heteroaromatics: exploring heterocyclic chemistry with alkoxyallenes. Acc Chem Res 42(1):45–56. https://doi.org/10.1021/ar800011h
Chen CC, Chen CM, Wu MJ (2014) Transition metal-catalyzed cascade cyclization of aryldiynes to halogenated benzo b naphtho 2,1-d thiophene derivatives. J Org Chem 79(10):4704–4711. https://doi.org/10.1021/jo500377v
Coa JC, Castrillon W, Cardona W, Carda M, Ospina V, Munoz JA, Velez ID, Robledo SM (2015) Synthesis, leishmanicidal, trypanocidal and cytotoxic activity of quinoline-hydrazone hybrids. Eur J Med Chem 101:746–753. https://doi.org/10.1016/j.ejmech.2015.07.018
Croxtall JD, Plosker GL (2009) Sertaconazole A review of ıts use in the management of superficial mycoses in dermatology and gynaecology. Drugs 69(3):339–359
Guo SH, He YW, Murtaza I, Tan JH, Pan JY, Guo YT, Zhu YN, He Y, Meng H (2018) Alkoxy substituted 1 benzothieno 3,2-b 1 benzothiophene derivative with improved performance in organic thin film transistors. Org Electron 56:68–75. https://doi.org/10.1016/j.orgel.2018.02.003
Katritzky AR, Karelson M, Sild S, Krygowski TM, Jug K (1998) Aromaticity as a quantitative concept. 7. Aromaticity reaffirmed as a multidimensional characteristic. J Org Chem 63(15):5228–5231. https://doi.org/10.1021/jo970939b
Kazemizadeh AR, Shajari N, Shapouri R, Adibpour N, Teimuri-Mofrad R (2016) Synthesis and anti-brucella activity of some new 1,3,4-oxadiazole derivatives containing a ferrocene unit. J Iran Chem Soc 13(7):1349–1355. https://doi.org/10.1007/s13738-016-0849-3
Kivrak A, Larock RC (2010) Synthesis of dihydrobenzisoxazoles by the 3 + 2 cycloaddition of arynes and oxaziridines. J Org Chem 75(21):7381–7387. https://doi.org/10.1021/jo101656c
Kumar S, Mujahid M, Verma AK (2017) Regioselective 6-endo-dig iodocyclization: an accessible approach for iodo-benzo a phenazines. Org Biomol Chem 15(21):4686–4696. https://doi.org/10.1039/c7ob00671c
Lu WD, Wu MJ (2007) Halocyclization of 2-alkynylthioanisoles by cupric halides: synthesis of 2-substituted 3-halobenzo b thiophenes. Tetrahedron 63(2):356–362. https://doi.org/10.1016/j.tet.2006.10.068
Meixner CN, Aref MW, Gupta A, McNerny EMB, Brown D, Wallace JM, Allen MR (2017) Raloxifene improves bone mechanical properties in mice previously treated with zoledronate. Calcif Tissue Int 101(1):75–81. https://doi.org/10.1007/s00223-017-0257-4
Miao MZ, Xu HP, Luo Y, Jin MC, Chen ZK, Xu JF, Ren HJ (2017) A modular approach to highly functionalized 3-sulfonylfurans via conjugate addition of 3-cyclopropylideneprop-2-en-1-ones with sodium sulfinates and sequential 5-endo-trig iodocyclization. Org Chem Front 4(9):1824–1828. https://doi.org/10.1039/c7qo00362e
Pathak RB, Chovatia PT, Parekh HH (2012) Synthesis, antitubercular and antimicrobial evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Bioorg Med Chem Lett 22(15):5129–5133. https://doi.org/10.1016/j.bmcl.2012.05.063
Rafiq SM, Sivasakthikumaran R, Mohanakrishnan AK (2014) Lewis acid/Bronsted acid mediated benz-annulation of thiophenes and electron-rich arenes. Org Lett 16(10):2720–2723. https://doi.org/10.1021/ol501006t
Rahmouni A, Souiei S, Belkacem MA, Romdhane A, Bouajila J, Ben Jannet H (2016) Synthesis and biological evaluation of novel pyrazolopyrimidines derivatives as anticancer and anti-5-lipoxygenase agents. Bioorg Chem 66:160–168. https://doi.org/10.1016/j.bioorg.2016.05.001
Richardson DR, Kalinowski DS, Lau S, Jansson PJ, Lovejoy DB (2009) Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochim Biophys Acta Gen Subj 1790(7):702–717. https://doi.org/10.1016/j.bbagen.2008.04.003
Sarret C, Pichard S, Afenjar A, Boespflug-Tanguy O (2017) Lack of long-term neurologic efficacy of zileuton in Sjogren–Larsson’s syndrome. Neuropediatrics 48(3):205–206. https://doi.org/10.1055/s-0037-1601856
Shakdofa MME, Shtaiwi MH, Morsy N, Abdel-rassel TMA (2014) Metal complexes of hydrazones and their biological, analytical and catalytic applications: a review. Main Group Chem 13(3):187–218. https://doi.org/10.3233/mgc-140133
Sun LL, Deng CL, Tang RY, Zhang XG (2011) CuI/TMEDA-catalyzed annulation of 2-bromo alkylbenzenes with Na2S: synthesis of benzo b thiophenes. J Org Chem 76(18):7546–7550. https://doi.org/10.1021/jo201081v
Togo H, Iida S (2006) Synthetic use of molecular iodine for organic synthesis. Synlett 14:2159–2175. https://doi.org/10.1055/s-2006-950405
Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Wade JL, Robidoux A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N, Nsabp (2006) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes—the NSABP study of tamoxifen and raloxifene (STAR) P-2 trial. JAMA J Am Med Assoc 295(23):2727–2741. https://doi.org/10.1001/jama.295.23.joc60074
Weiser M, Levi L, Burshtein S, Hagin M, Matei VP, Podea D, Miclutia I, Tiugan A, Pacala B, Grecu IG, Noy A, Zamora D, Davis JM (2017) Raloxifene plus antipsychotics versus placebo plus antipsychotics in severely Ill decompensated postmenopausal women with schizophrenia or schizoaffective disorder: a randomized controlled trial. J Clin Psychiatry 78(7):E758. https://doi.org/10.4088/jcp.15m10498
Yaragorla S, Pareek A, Dada R, Saini PL (2017) Single-step synthesis of 3-iodoquinolines from 2-aminophenyl ketones through a regioselective (6-endo-dig) electrophilic cyclization. Eur J Org Chem 31:4600–4608. https://doi.org/10.1002/ejoc.201700668
Yue DW, Larock RC (2004) Synthesis of 3-iodoindoles by electrophilic cyclization of N, N-dialkyl-2-(1-alkynyl)anilines. Org Lett 6(6):1037–1040. https://doi.org/10.1021/ol0498996
Zora M, Kivrak A, Yazici C (2011) Synthesis of pyrazoles via electrophilic cyclization. J Org Chem 76(16):6726–6742. https://doi.org/10.1021/jo201119e
Acknowledgements
The authors thank The Scientific and Technological Research Council of Turkey (Project no: 115Z020) for providing financial support for obtaining reactant and reagents, and Van Yüzüncü Yil University (Project no: FBA-2017-6007) for providing financial support for obtaining solvents, glasswares and salts. We thank University of Duhok for the scholarship given to Muheb A.S. Algso. The authors would also like to acknowledge networking contribution by the COST Action CM1407.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11696_2018_640_MOESM1_ESM.doc
Characterization of the new benzo[b]thiophene products including 1H NMR, 13C NMR, and FTIR spectra, as well as mass characterization data. (DOC 42003 kb)
Rights and permissions
About this article
Cite this article
Algso, M.A.S., Kivrak, A. New strategy for the synthesis of 3-ethynyl-2-(thiophen-2-yl)benzo[b]thiophene derivatives. Chem. Pap. 73, 977–985 (2019). https://doi.org/10.1007/s11696-018-0640-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0640-2