Abstract
A novel triphenylamine-base derivative L containing pyridine and terpyridine was designed and synthesized. Compound L exhibited distinct aggregation-induced emission (AIE) behavior in water–ethanol and also displayed a threefold increase in the intensity of luminescence at 608 nm. Furthermore, confocal microscopy imaging demonstrated that compound L displays low toxicity and brights red fluorescence in mitochondria in living HepG2 cells. Inherent from the mitochondrial-targeting ability of pyridine moiety and the AIE characteristic of triphenylamine group, compound L could be employed as a fluorescent probe in the near-infrared region for living cell imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluorescent organic materials have attracted increasing interest due to their potential applications in bioimaging and chemosensing (Zhang et al. 2017; Huang et al. 2016; Chang et al. 2016). However, a majority of fluorescence organic materials face the problem of aggregation-induced quenching (ACQ) because of strong π–π stacking interactions in the enlarged π-conjugated system (Thomas et al. 2007), resulting in limitation of their practical applications. Aggregation-induced emission (AIE), as a novel phenomenon, was first discovered by Tang’s group (Luo et al. 2001). The compound exhibits very weak fluorescence in solution but emits strong fluorescence in the aggregated state. Currently, AIE materials have gained widespread attentions for their potential application in various fields, for instance bioimaging, chemosensors and organic light-emitting diodes (Gui et al. 2017; Mei et al. 2015). However, the working mechanism of the AIE processes is very complicated, and the reason is still unclear. At present, some theories or hypothesises are proposed to explain the AIE phenomenon, e.g., intramolecular restrictions, J-type aggregates, restriction of intramolecular rotation, twisted intramolecular charge transfer (Li et al. 2017a ; Kwork et al. 2015; Ding et al. 2013). Therefore, a large number of compounds with AIE properties have been developed. To further enlarge the family of specific AIE-active compounds, it is necessary to carry out more extensive investigations in this field.
Herein, we developed a novel A–D–π–A type triphenylamine-based derivative (Fig. 1), which displays AIE behavior in water–ethanol system. First, triphenylamine moiety with a strong electron donor can help to delocalize the electron distribution and extend the conjugation system (Liu et al. 2014). Second, owing to the strong electron-withdrawing ability and large π-conjugation system, terpyridine ligand exhibits good optical properties (Wang et al. 2018a). Third, the pyridinium cation moiety with high membrane potential is able to target intracellular mitochondria (Tian et al. 2017). Compound L displays good AIE characteristic and emits red fluorescence. As expected, compound L is capable of targeting mitochondria.
Experimental section
Materials and measurements
All of the reagents were available commercially and used without further purification. 1H NMR and 13C NMR spectra were obtained on Bruker 400 Ultrashield spectrometer. Mass spectrum was measured on HRMS-LTQ Obritrap XL (ESI source). IR spectra (KBr pressed pellets) were obtained on Nicolet FT-IR-870SX spectrophotometer. UV–Vis absorption and one-photon fluorescence spectra were obtained on UV-1700 and Hitachi F-4600 fluorescence spectrophotometer, respectively.
Synthesis of L
M1 (Wang et al. 2016) (0.50 g, 1 mmol) were dissolved in ethanol (50 mL), M2 (Tian et al. 2017) (0.25 g, 1 mmol) and 3 drops piperidine were subsequently added. The reaction mixture was refluxed for 24 h. The solid was filtered off and washed with ethanol. The product was recrystallized from methanol to afford red solids. IR (cm−1) selected bands: 2918, 2135, 1796, 1646, 1585, 1510, 1468, 1439, 1418, 1389, 1323, 1292, 1175, 972, 875, 841, 793, 699, 660, 621, 557, 540. 1H NMR (d6-DMSO) δ: 8.77 (m, 4 H), 8.70 (m, 4 H), 8.16 (d, J = 6.9 Hz, 2 H), 8.05 (t, J = 7.8 Hz, 2 H), 7.97 (m, 3 H), 7.69 (d, J = 8.7 Hz, 2 H), 7.54 (m, 2 H), 7.44 (t, J = 7.9 Hz, 2 H), 7.36 (d, J = 16.2 Hz, 1 H), 7.23 (m, 5 H), 7.12 (d, J = 8.7 Hz, 2 H), 4.23 (s, 3 H). 13C NMR (d6-DMSO) δ: 156.1, 155.4, 149.7, 145.2, 140.7, 137.9, 130.4, 130.1, 128.6, 126.2, 125.4, 124.9, 124.8, 123.4, 122.7, 121.5, 121.3, 117.7, 56.4. MS: 594.26 ([M-PF6]+).
Computational details
The ground state geometries of the molecules are optimized at the time-dependent density functional theory (TD-DFT)/CAM-B3LYP (Yannai et al. 2004) functional level without any symmetry restraint (Wang et al. 2012) using Gaussian 09 program (Frisch et al. 2009). The UV–Vis absorption properties are obtained based on the optimized structures with CAM-B3LYP functional utilizing Gaussian 09 package. The basis set of 6-31G* is chosen for all atoms. An analytical frequency confirms evidence that the calculated species represents a true minimum without imaginary frequencies on the respective potential energy surface. The lowest 25 spin-allowed singlet–singlet transitions, up to energy of about 5 eV, were taken into account in the calculation of the absorption spectra. The molecular orbitals were visualized using GaussView 5.0.9 (Dennington et al. 2009).
Results and discussion
Photophysical properties
The UV–Vis absorption and one-photon fluorescence spectra of L in different polar solvents were initially investigated. The corresponding photophysical data were summarized in Table 1. As depicted in Fig. 2, compound L exhibited two strong absorption bands at about 460 nm and 275 nm in different solvents, which can be assigned to the π–π* transition mixed with intramolecular charge transfer process (ICT) and πterpyridine–πterpyridine* transition, respectively. Weak solvatochromism was observed in the maximum absorption band, indicative of the little difference in dipoles between the ground and the excited states of the compound. Increasing the solvent polarity from benzene to DMF (Fig. 2 and Table 1), the fluorescence maxima of L shifted from 593 to 608 nm and the fluorescence was quenched at the same time, which might be assigned to ICT. Moreover, the Stokes’ shifts also exhibited an increasing tendency with the increase of the solvent polarity. To better understand the ICT process, we performed theoretical calculations with the density functional of CAM-B3lyp/6-31 g(d) (Fig. 3 and Table 2). From Fig. 3, the electron cloud of the highest occupied molecular orbital (HOMO) was dominated by the triphenylamine unit, –C=C– moiety and pyridine unit. Meanwhile, the lowest unoccupied molecular orbital (LUMO) was localized on the pyridine group due to the strong electron-withdrawing ability. Due to the HOMO → LUMO transition, the absorption band at 484 nm was assigned as the ICT transition mixed with π–π* transition, keeping consistent with the experimental ones.
Aggregation-induced emission (AIE) properties
Compound L could be dissolved in many organic solvents, such as THF, ethanol, acetonitrile and DMSO, but has poor solubility in water. To determine whether L is AIE active and considering the biological application, the UV–Vis absorption and fluorescence spectra of L in water–ethanol mixtures with different fraction of water (fw) were investigated. From Fig. 4, we can see that compound L displayed three absorption bands in dilute ethanol solution. As the water fraction increased to 90%, the band at about 460 nm gradually decreased, accompanying with slightly blue shift. Moreover, the absorption curves decayed further from zero in the long wavelength region with high water fraction, indicating the existence of aggregative species in these solutions.
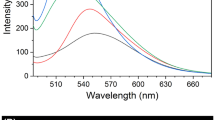
Figure 5 exhibited the changes of fluorescence intensity of L in aqueous mixtures. In dilute ethanol solution, compound L was nearly non-emissive. The fluorescence intensity of L obviously decreased with the gradual addition of water amounts into ethanol solution. This phenomenon could be reasonably explained by a twisted intramolecular charge transfer (TICT) mechanism. The fluorescence intensity was invigorated at fw = 80% and reached the maximum fluorescence intensity with λ = 618 nm at fw = 90%, which was a threefold enhancement to that of L in absolute ethanol solution, demonstrating the AIE characteristic of compound L. In this stage, compound L begins to aggregate because the solvating power of the aqueous mixture decrease, making the emission enhanced due to the restriction of intramolecular rotation effect. Meanwhile, the ICT effect is efficiently weakened. As depicted in Fig. 5b, when fw reached 95%, the fluorescence intensity of L in the water–ethanol mixture decreased. This phenomenon has often been detected in some dyes possessing AIE properties, but the reason is still unclear. Up to now, this phenomenon could be explained by two possible factors: (1) in the extensive aggregation state, the molecules on the nanoparticle surface contributes mainly to the emission, while the molecules in interior contributes faintly, resulting in a decrease of fluorescence intensity. (2) When adding water to the solution, the molecules could form amorphous particles and crystal particles. The former one could decrease the fluorescence intensity, while the latter will enhance the intensity of fluorescence (Zheng et al. 2013).
To further explore the enhanced emission of L in water–ethanol system, the aggregate process of L with different water fractions was investigated by scanning electron microscopy (SEM). As shown in Fig. 6a, a spherical particle of L formed immediately in the mixture of water–ethanol with fw of 40%. However, when fw = 90%, the AIE dots dispersed regularly and taken a block-like arrangement. These data demonstrated that the enhanced emission of L relies on the formation of nanoparticles. The comparative analysis of L with the previously reported AIE molecule was summarized in Table 3. Compared with the other reported AIE molecule, compound L exhibited long emission wavelength (> 600 nm), which encouraged us to explore its potential application in biological imaging.
Biological application
High cell viability is essential for a fluorescent probe in its biological application. To evaluate the cytotoxicity of L, HepG2 cells were treated with L, and its viability was subsequently determined by MTT assay. Figure 7 revealed that HepG2 cells incubated with 15 μM of L remained 90% viable after 24 h of feeding time, suggesting the low toxicity and good biocompatibility of L. To assess the cellular uptake properties, HepG2 cell was used as a model, whereby 10 μM of L was treated for 30 min followed by confocal microscopy imaging after washing with PBS. As shown in Fig. 8a, L could penetrate into the cell cytosol within the short incubation period and exhibited intensive red fluorescence. It is well-known that fluorophores possessing a pyridinium moiety can function as mitochondria probes. A co-staining experiment with Mitotracker deep red was performed to establish whether L could be internalized with membrane-rich mitochondria. As displayed in Fig. 8a, a clear mitochondria profile with strong green fluorescence was observed from the green channel, which was ascribed to Mitotracker deep red. A similar mitochondria profile with red fluorescence was obtained after treating with L. The signal of L overlaid very well with the fluorescence of Mitotracker deep red (Fig. 8c). Moreover, Pearson’s co-localization coefficient, which describes the correlation of the intensity distribution between these two channels, was calculated to be 0.92 (Fig. 8b), indicating compound L could selectively accumulate into the mitochondria. The above results revealed that compound L is a promising fluorescent probe for targeting mitochondria.
Conclusion
In summary, an A–D–π–A type red fluorescent compound was designed and synthesized. Compound L was almost non-emissive when dissolved in ethanol, but became strongly emissive when aggregated in water solution, demonstrating a typical AIE phenomenon. Confocal imaging experiment indicated that compound L was capable of targeting the mitochondrial in living HepG2 cells. Current results offer a platform for the development of new AIE materials with red emission for targeting mitochondria.
References
Chang ZF, Jing LM, Chen B, Zhang MS, Cai XL, Liu JJ, Ye YC, Lou XD, Zhao ZJ, Liu B, Wang JL, Tang BZ (2016) Rational design of asymmetric red fluorescent probes for live cell imaging with high AIE effects and large two-photon absorption cross sections using tunable terminal groups. Chem Sci 7:4527–4536. https://doi.org/10.1039/C5SC04920B
Dennington R, Keith T, Millam J (2009) GaussView, version 5. Semichem Inc, Shawnee Mission
Ding D, Li K, Liu B, Tang BZ (2013) Bioprobes based on AIE fluorogens. Acc Chem Res 46:2441–2453. https://doi.org/10.1021/ar3003464
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman Scalmani, Barone Mennucci B, Petersson G (2009) Gaussian 09, revision A. Gaussian Inc, Wallingford
Gui C, Zhao EG, Kwok RTK, Leung ACS, Lam JWY, Jiang MJ, Deng HQ, Cai YJ, Zhang WJ, Su HF, Tang BZ (2017) AIE-active theranostic system: selective staining and killing of cancer cells. Chem Sci 8:1822–1830. https://doi.org/10.1039/C6SC04947H
Hu QL, Gao M, Feng GX, Liu B (2014) Mitochondria-targeted cancer therapy using a light-up probe with aggregation-induced-emission characteristics. Angew Chem In Ed 53:14225–14229. https://doi.org/10.1002/anie.201408897
Huang YY, Zhang GX, Hu F, Jin YL, Zhao R, Zhang DQ (2016) Emissive nanoparticles from pyridinium-substituted tertraphenylethylene salts: imaging and selective cytotoxicity towards cancer cells in vitro and in vivo by varying counter anions. Chem Sci 7:7013–7019. https://doi.org/10.1039/C6SC02395A
Kwork RT, Leung CWT, Lam JWY, Tang BZ (2015) Biosensing by luminogens with aggregation-induced emission characteristics. Chem Soc Rev 44:4228–4238. https://doi.org/10.1039/C4CS00325J
Li LQ, Wang LY, Tang H, Cao DR (2017a) A facile synthesis of novel near-infrared pyrrolopyrrole aza-BODIPY luminogens with aggregation-enhanced emission characteristics. Chem Commun 53:8352–8355. https://doi.org/10.1039/C7CC04568A
Li XS, Jiang MJ, Lam JWY, Tang BZ, Qu JNY (2017b) Mitochondrial imaging with combined fluorescence and stimulated raman scattering microscopy using a probe of the aggregation-induced emission characteristic. J Am Chem Soc 139:17022–17030
Liu G, Yang MD, Wang LK, Zheng J, Zhou HP, Wu JY, Tian YP (2014) Schiff base derivatives containing heterocycles with aggregation-induced emission and recognition ability. J Mater Chem C 2:2684–2691. https://doi.org/10.1039/C3TC32591A
Lu HG, Zheng YD, Zhao XW, Wang LJ, Ma SQ, Han XQ, Xu B, Tian WJ, Gao H (2016) Highly efficient far red/near-infrared solid fluorophores: aggregation-induced emission, intramolecular charge transfer, twisted molecular conformation, and bioimaging applications. Angew Chem 128:163–167. https://doi.org/10.1002/anie.201507031
Luo JD, Xie ZL, Lam JWY, Cheng L, Chen HY, Qiu CF, Kwok HS, Zhan XW, Liu YQ, Zhu DB, Tang BZ (2001) Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaohenylsilole. Chem Commun. https://doi.org/10.1039/B105159H
Mei J, Leung NLC, Kwok RTK, Lam JWY, Tang BZ (2015) Aggregation-induced emission: together we shine, united we soar. Chem Rev 115:11718–11940. https://doi.org/10.1021/acs.chemrev.5b00263
Thomas SW III, Joly GD, Swager TM (2007) Chemical sensors based on amplifying fluorescent conjugated polymers. Chem Rev 107:1339–1386. https://doi.org/10.1021/cr0501339
Tian XH, Hussain S, Wang H, Zhang Q, Zhao M, Chen JY, Zhang H, Zhou HP, Chen Y, Tian YP (2017) A series of water-soluble pyridinium derivatives with two-photon absorption in the near infrared region for mitochondria targeting under stimulated emission depletion (STED) nanoscopy. Dyes Pigm 147:90–98. https://doi.org/10.1016/j.dyepig.2017.07.025
Viglianti L, Leung NLC, Xie N, Gu XG, Sung HYH, Miao Q, Williams ID, Licandro E, Tang BZ (2017) Aggregation-induced emission mechanistic study of the clusteroluminescence of tetrathienyethene. Chem Sci 8:2629–2639. https://doi.org/10.1039/c6sc05192h
Wang XC, Tian XH, Zhang Q, Sun PP, Wu JY, Zhou HP, Jin BK, Yang JX, Zhang SY, Wang CK, Tao XT, Jiang MH, Tian YP (2012) Assembly, two-photon absorption, and bioimaging of living cells of a cuprous cluster. Chem Mater 24:954–961. https://doi.org/10.1021/cm2029855
Wang H, Tian XH, Du W, Zhang Q, Guan LJ, Wang AD, Zhang YJ, Wang CK, Zhou HP, Wu JY, Tian YP (2016) A two-photon fluorescent RNA probe screened from a series of oxime-functionalized 2,2′:6′,2′′-terpyridine ZnX2 (X = Cl, Br, I) complexes. J Mater Chem B 4:4818–4825. https://doi.org/10.1039/C6TB01202G
Wang H, Hu L, Du W, Tian XH, Hu ZJ, Zhang Q, Zhou HP, Wu JY, Uvdal K, Tian YP (2018a) Mitochondria-targeted iridium (III) complexes as two-photon fluorogenic probes of cysteine/homocysteine. Sens Actuators B 255:408–415. https://doi.org/10.1016/j.snb.2017.08.074
Wang Y, Mao PD, Wu WN, Mao XJ, Fan YC, Zhao XL, Xu ZQ, Xu ZH (2018b) New pyrrole-based single-molecule multianalyte sensor for Cu2+, Zn2+, and Hg2+ and its AIE activity. Sens Actuators B 255:3085–3092. https://doi.org/10.1016/j.snb.2017.09.133
Xie HT, Wu YL, Zeng F, Chen JJ, Wu SZ (2017) An AIE-based fluorescent test strip for the portable detection of gaseous phosgene. Chem Commun 53:9813–9816. https://doi.org/10.1039/c7cc05313d
Yannai T, Tew DP, Handy NC (2004) A new hybrid exchange-correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57. https://doi.org/10.1016/j.cplett.2004.06.011
Zhang JN, Kang H, Li N, Zhou SM, Sun HM, Yin SW, Zhao N, Tang BZ (2017) Organic solid fluorophores regulated by subtle structure modification: color-tunable and aggregation-induced emission. Chem Sci 8:577–582. https://doi.org/10.1039/C6SC02875F
Zheng Z, Yu ZP, Yang MD, Jin F, Zhang Q, Zhou HP, Wu JY, Tian YP (2013) Substituent group variations directing the molecular packing, electronic structure, and aggregation-induced emission property of isophorone derivatives. J Org Chem 78:3222–3234. https://doi.org/10.1021/jo400116j
Acknowledgements
This study was supported by the Science Foundation of Department Education of Anhui Province (KJ2017ZD49), and the Talent Foundation of the Education Department of Anhui (gxbjZD2016111), Doctoral Startup Foundation of Wannan Medical College (wYRCQD201715).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, L., Sun, H., Zhou, L. et al. Design and synthesis of an AIE-active fluorogen with red emission and its biological application. Chem. Pap. 72, 2813–2819 (2018). https://doi.org/10.1007/s11696-018-0529-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0529-0