Abstract
A rapid and economical method using modified QuEChERS sample pretreatment coupled with high-sensitivity gas chromatography/triple quadrupole mass spectrometry was established to simultaneously determine ten pyrethroid pesticides in fruits and vegetables. All pesticides were detected within 20 min of one injection. Concurrent backflushing provided column protection, greatly facilitating instrument maintenance. For quantitation, matrix-matched calibration was used to compensate for signal-enhancement effects and to ensure the precision of the method. The limit of detection (LOD) was in the range of 0.3–4.9 μg/kg. The recovery rate was from 78.8 to 118.6%, with relative standard deviation (RSD) below 14.8%. The developed method is suitable for rapid and sensitive multi-residue analysis of pyrethroid pesticides in fruits and vegetables. It is good for users in professional institutions that implement safety controls for testing hundreds of agricultural product samples everyday.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyrethroid pesticides are synthetic derivatives of naturally occurring pyrethrums that can be extracted from the flowers of Chrysanthemum cinerariaefolium (Galera et al. 1996; Schettgen et al. 2002). For more than 30 years, pyrethroids have been widely used in agriculture to protect fruit and vegetable crops, in forestry and in household applications. Pyrethroids have broad spectrum of insecticidal activity, are relatively non-toxic to mammals and birds, break down quickly, and are non-persistent in the environment. However, excessive use or incorrect application, premature harvesting, and failure to allow adequate time for pesticide degradation before marketing may allow residues to exceed safe limits and pose a threat to consumer safety (Sánchez et al. 2010; Peng et al. 2012). Because of this risk to public health, many methods have been developed to analyze pyrethroid pesticides in different fruits and vegetables (McMahon and Hardin 1994; Bennett et al. 1997; Paoli et al. 1997; Andersson and Palsheden 1988).
Because of their weak polarity, moderate boiling point and easy gasification, pyrethroid pesticides have typically been detected using gas chromatography (GC). This method is, however, unsuitable for reliable qualitative and quantitative determination of pyrethroids in complex matrices, and samples must be purified before analysis to prevent interference by the matrix. GC coupled to mass spectrometry (GC/MS), in full scan or selected ion monitoring (SIM) mode, has also been widely used to measure pesticide residues. This method, however, has low sensitivity in full scan mode and may provide poor spectral information in SIM mode because of matrix interference by components in vegetables (Mahugija et al. 2017). Gas chromatography–tandem mass spectrometry (GC–MS/MS) coupled with multiple reaction monitoring (MRM) is able to provide simultaneous quantitative and qualitative analysis of trace levels of analytes in complex matrices (Plomley et al. 1994; Hayward et al. 1999). Many studies have shown that GC–MS/MS can be used routinely for trace level analysis, with high-sensitivity and good reliability (Jiang et al. 2014; Mo et al. 2014).
A potential problem when using GC is that less-volatile matrix components could block the system and result in gradual reduction of analyte signals, shifts in retention times, and broadening of target peaks (Mastovská et al. 2004). This problem can be minimized by column backflushing, where the column flow is reversed using a pressure-controlled tee (PCT) installed at the column outlet, before the MS port (Klee 2009). The combined use of GC–MS/MS and backflushing to reduce accumulation of high-boiling impurities in the chromatography column has been shown to work well, especially with dirty samples.
Several methods of sample preparation have been developed for the analysis of pyrethroid pesticides in fruits and vegetables. These include solid phase extraction (SPE), liquid–liquid extraction (LLE) (Barrionuevo and Lanças 2002; Farajzadeh and Khoshmaram 2014), gel permeation chromatography (GPC) (Wang et al. 2014), solid phase micro-extraction (SPME) (Tsoutsi et al. 2006), liquid–solid extraction (LSE) and matrix solid-phase dispersion (MSPD) (Sobhanzadeh et al. 2012; Li et al. 2013). Since most of these techniques are complicated, laborious, time-consuming, and require large amounts of solvents, a simpler and faster analytical procedure is much needed. The QuEChERS (quick, easy, cheap, effective, rugged, and safe) method for determination of pesticide residues is a widely used sample preparation method (Wu 2017). Because of its flexibility, QuEChERS provides a useful working template that can be modified to suit the properties of diverse analytes and matrices as well as different equipment and analytical techniques available in individual laboratories (Marchis et al. 2012). QuEChERS has been successfully used for the extraction of a broad range of analytes from many different matrices (Marchis et al. 2012; Rashid et al. 2010).
The matrix in agricultural products is typically complex and samples may still contain impurities even after pretreatment. These impurities can compete with pesticide analytes in key sites such as the injection port and column head during analysis, thus enhancing the effects of the matrix. Matrix-enhanced effects lead to a high and incorrect recovery rate (usually > 100%). Many methods have been described for reducing matrix effects. These include multiple purification methods, specialized injection techniques, correction factor calibration, and the addition of analyte protectants (Huang et al. 2006; Poole 2007; Wang et al. 2012). Matrix matching of standards is recognized as one of the most effective ways to solve the problem of matrix interference. A blank substrate solution can easily be prepared in the laboratory to maintain the same level of compensation in the response of pesticide in the standard solution and sample solution.
In the present study, a simple, rapid and interference-free method for the determination and confirmation of trace and larger amounts of pyrethroid pesticides in different fruits and vegetables has been developed and validated. Our new method is based on a modified QuEChERS approach and was tested using GC–MS/MS equipped with a backflushing column. The method combines the key benefits of the QuEChERS approach and GC–MS/MS to achieve the best results. Performance was evaluated in terms of linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision, and matrix effects. The new method was then tested by using it for the analysis of pyrethroid pesticides in real samples.
Experimental
Materials
Standards
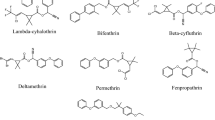
Ten pyrethroid pesticide standards (purity ≥ 95%) were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). The structures of these standards are shown in Fig. 1. Standard stock solutions (1 g/L) in acetone/toluene solution (3:7, v/v) were prepared and stored at − 80 °C. A series of mixed working solutions were prepared by stepwise dilution of the stock solutions with acetonitrile and stored at − 20 °C.
Reagents
Pesticide-analysis-grade acetonitrile (purity ≥ 99.9; Tedia Company, Inc., Fairfield, OH, USA) was used as the extractant. Analytical-grade sodium chloride (NaCl, purity ≥ 99.99%; CNW Technologies GmbH, Düsseldorf, Germany) and anhydrous magnesium sulfate (MgSO4, 20–50 mesh, 97% purity; Acros Organics, Shanghai, China) were used for salting out. Anhydrous MgSO4 (100 mesh, 97% purity; Sigma-Aldrich Co., Ltd., Steinheim, Germany) was used for dehydration. Primary secondary amine (PSA) and C18 sorbents (40 μm particle size) were purchased from ANPEL Scientific Instrument Co., Ltd. (Shanghai, China).
Sample preparation
Fresh fruits (pear and waxberry) and vegetables (tomato, cucumber, and cowpea) were purchased from local supermarkets. Pyrethroid-free samples were used as the blank matrix to prepare matrix-matched standards for calibration. Samples were prepared by quartering and homogenizing the samples in a food processor (Model HR-7633; Philips, Zhuhai, China).
Samples were extracted following the typical QuEChERS procedure, with some modifications. In brief, homogenized samples (25 ± 0.01 g) were weighed into 250 mL polypropylene bottles. Control samples were spiked at two concentrations (0.05 and 0.1 mg/L). Acetonitrile (25 mL) was then added, and the mixture was homogenized at room temperature for 2 min using an IKA T25 homogenizer (IKA, Guangzhou, China). Anhydrous MgSO4 (20–50 mesh, 10 g) and NaCl (1 g) were added, and the mixture was homogenized for 1 min and then centrifuged at 2,500×g for 3 min.
A sample of the supernatant (4.0 mL) was removed and added to a 15 mL centrifuge tube containing PSA (100 mg), C18 (100 mg), and anhydrous MgSO4 (100 mesh, 1200 mg). The mixture was shaken at low speed for 1 min and then centrifuged at 3,000×g for 5 min. A sample of the supernatant (0.8 mL) was added into a sample bottle, and subsequently 1 µL injected into the GC–MS/MS equipment for analysis.
Instrument conditions
GC–MS/MS analysis was carried out using a model 7890A gas chromatography and a model 7683B auto injector coupled with a 7000A triple quadrupole (QQQ) mass spectrometer (Agilent Technologies, USA). An HP-5 ms Ultra Inert capillary column (325 °C, 30 mm × 250 μm × 0.25 μm; J&W Scientific, USA) was used for GC separations. It was preceded by a deactivated guard column (2 m × 250 μm × 0 μm; J&W Scientific) and followed by a deactivated-current-limiting column (retention gap, 450 °C, 0.65 m × 150 μm × 0 μm; J&W Scientific).
Helium (purity ≥ 99.999%) was used as the carrier gas, and the column head pressure was held at 0.20 MPa. The program was as follows: 90 °C held for 0 min, ramped to 280 °C at 15 °C/min, and maintained for 10 min, followed by backflushing at 300 °C and 0.41 MPa for 5 min. The GC injection port temperature was 250 °C, and the transfer-line temperature was 280 °C. The injection volume was 1 μL in splitless mode.
The mass spectrometer was used in MRM mode. The ionization voltage was 70 eV in electron ionization (EI) mode. The ion source and MS1 and MS2 quadruple temperatures were 230, 150, and 150 °C, respectively. Helium (purity ≥ 99.999%) and nitrogen (purity ≥ 99.999%) were used as the quench gas (2.25 mL/min flow rate) and the collision gas (1.5 mL/min flow rate), respectively.
Method validation
Analytical figures of merit were evaluated in accordance with the regulations of the National Standards of the People’s Republic of China and the European Commission. The parameters evaluated were linearity, LOD, LOQ, accuracy, precision, and matrix effects (Chen et al. 2013).
Linearity was evaluated by analyzing standard solutions of mixed pesticides at six different concentrations. LODs and LOQs were calculated using the method recommended by the American Chemical Society (ACS) (1980). LODs were calculated as thrice the signal-to-noise (S/N) ratio obtained by analyzing non-spiked tomato samples, and LOQs were calculated as tenfold of the S/N ratio. Accuracy was assessed by measuring analytes in fruit and vegetable samples spiked at three different concentrations. Six replicates were performed for each experiment. Precision was measured in terms of intraday and interday variability. Intraday precision was determined on 1 day by analyzing pesticides added at the intermediate spiking level in seven replicate tomato and pear samples; interday precision was measured by carrying out the analyses once a day for 2 weeks. To evaluate the matrix effect, matrixes of blank fruit and vegetable (pesticide-free samples) were spiked with ten pyrethroid pesticides at low level (~ LOQ), intermediate level (~ 10 × LOQ), and high level (~ 100 × LOQ). The matrix effect was calculated by comparing the signal peak area of pesticides in the sample with that of the pesticides in the standard solution (n = 5).
Results and discussion
A modified QuEChERS method coupled with GC–MS/MS, which requires low volumes of organic solvents, was established and used to determine ten pyrethroid pesticides in fruits and vegetables. All of the tested pesticides could be extracted and determined in ~ 2 h, and the method was evaluated scientifically and was found to perform well with tests on the practical samples.
Optimization of GC–MS/MS conditions using MRM and backflushing
Different mass spectrometer settings and chromatographic conditions were evaluated to determine optimal conditions for rapid and sensitive determination. For each pesticide, ions were collected in single reaction monitoring mode (SRM) to meet the requirements of four identification points for positive samples, as laid out in the Official Journal of the European Union (2002).
Overlapping sample peaks obtained by GC that cannot be separated effectively inevitably complicate the confirmation of positive samples. However, this problem can be solved by mass spectroscopy. In the present study, optimized MRM transitions were chosen to improve the selectivity and sensitivity of the GC–MS/MS method (Table 1). Ten components were conclusively identified, although the peaks for the isomers of fenvalerate and tau-fluvalinate overlapped in the total ion chromatogram (TIC) mode (Fig. 2). Using the extracted ion mode (m/z 167 > 125 and 167 > 89 for fenvalerate, m/z 250 > 55 and 250 > 200 for tau-fluvalinate), the problem of overlapping peaks was completely resolved. All of the pesticide standards were detected efficiently in 20 min (Fig. 2); the entire analysis including the extraction procedure could be completed in ~ 2 h. A firm foundation was thus established for the rapid identification and quantification of pesticides in practical samples.
For dirty samples, multiple injections lead to gradual accumulation of residual impurities on the column, which eventually causes serious contamination of the chromatographic system. One method of solving this problem is increasing the temperature of the columns; but this method increases run times, reduces efficiency, and decreases the lifespan of the column. Column backflushing has been shown to significantly reduce long-term problems associated with the injection of dirty samples, both in pesticide residue analysis and in other applications (Chen et al. 2009; Mezcua et al. 2009). In the present study, a time-efficient backflushing approach was used to alleviate problems associated with the injection of dirty samples. Upon elution of the final analyte of interest from the HP-5 ms Ultra Inert capillary column, a PCT installed between the two columns allowed a rapid backflush, which took only a few minutes to complete, and removed less-volatile matrix components (Mastovskaa and Wylie 2012). A time-saving and cost-effective method was used for reducing contamination of both the chromatographic system and detector by high-boiling residues. The service life of the chromatographic system have also been extends. The benefits of backflushing on long-term system performance were evaluated by repeated injections of various food samples. Backflushing clearly removed high-boiling matrix components and prevented contamination of the GC system and the MS sources (Fig. 3).
Extraction and purification of samples
A modified QuEChERS method was used to purify fruit and vegetable samples. Preliminary experiments for optimizing the key parameters were carried out using anhydrous MgSO4 and the sorbents PSA and C18. Moisture in the substrate can be removed by anhydrous MgSO4. Anhydrous MgSO4 with 100 mesh size is dust-free and is best for salting out, whereas anhydrous MgSO4 with 20–50 mesh size is better for dehydration. PSA is used mainly to remove sugars and fatty acids present in the matrix. As a departure from other QuEChERS methods, C18 sorbent was used to remove most of the nonpolar fats, as well as impurities present in the samples, such as fat-soluble pigments and sterols.
The following conditions were compared to optimize extraction efficiency: (1) 5, 10, 15, and 20 g of anhydrous MgSO4 (20–50 mesh); and (2) 0.25, 0.5, 1, and 2 g of NaCl. Parameters used to optimize purification efficiency were (1) 50, 100, 150, and 200 mg of PSA; and (2) 50, 100, 150, and 200 mg of C18. The amount of adsorbent was found to affect purification efficiency; insufficient adsorbent led to incomplete removal of impurities, whereas excess adsorbent resulted in lower recovery of the pesticides. The best recovery rates (> 95%) for most pesticides were obtained with 10 g of anhydrous MgSO4 (20–50 mesh) and 1 g of NaCl; however, the recovery rate was not significantly improved by increasing the amounts of anhydrous MgSO4 and NaCl. The capacity of PSA to adsorb impurities was improved with increasing amounts of PSA from 50 to 200 mg, although some of the pesticides were absorbed when the amount of PSA reached 150 mg. A similar result was also observed with C18. The optimal parameters for the extraction process were 10 g of anhydrous MgSO4 (20–50 mesh) and 1 g of NaCl, and the optimal parameters for the purification process were 1200 mg of anhydrous MgSO4 (100 mesh), 100 mg of PSA, and 100 mg of C18.
In the present study, 25 mL of acetonitrile, a good solvent for all of the target pesticides, was used for the extraction. The pretreatment process, injection, and GC–MS/MS detection can all be completed within 2 h. The method can meet the requirements of real-time analysis of pesticide residues since it provides a low-cost, simple, rapid, and efficient analytical method, with little pollution. The extracted solution was injected directly into the GC–MS/MS system without the need for rotary evaporation and redissolution. As compared with the literature (Jardim et al. 2014), the purification steps was reduced, pretreatment time was saved, and satisfactory results was still provided (Table 2).
Evaluation of the new method
Linearity, LOD, and LOQ
Quantitative characteristics of the new method were obtained under optimized conditions. Since a matrix-matched standard would be used for the analysis of practical samples, the suitability of the new method in a typical matrix (tomato) was also demonstrated. Quantitative parameters such as linearity, R values, LODs, and LOQs are shown in Table 3. Good linearity with high correlation coefficients (R2 ≥ 0.990) was observed for the different compounds. Overall, the linearity was considered fit for purpose, given the range of pesticide concentrations in practical fruit and vegetable samples. The estimated LODs based on an S/N ratio of 3 were in the range of 0.3–4.9 μg/kg. LOQs based on an S/N ratio of 10 were ~ 10 μg/kg, except for three pyrethroids (bifenthrin, 1 μg/kg; cyfluthrin, 13 μg/kg; and cypermethrin, 15 μg/kg). Extraction ion chromatograms near the LOQs for the ten pyrethroid pesticides in the cucumber extract are shown in Fig. 4. The determination of the target analytes and the qualitative or quantitative analysis of pesticides in practical actual samples did not show any interference from the background of the blank sample.
Extracted-ion chromatograms (EIC) acquired in MRM mode by GC–MS/MS of 10 pyrethroid pesticides: spiking level was at 1 μg/L for bifenthrin, 20 μg/L for cyfluthrin and cypermethrin, and 10 μg/L for the other seven pyrethroid pesticides found in cucumber extract. Peak identification: 1 = bifenthrin; 2 = fenpropathrin; 3 = cyhalothrin; 4 = permethrin*; 5 = flucythrinate*; 6 = fenvalerate*; 7 = tau-fluvalinate*; 8 = deltamethrin*; 9 = cyfluthrin*; 10 = cypermethrin*. (* indicates multiple isomers)
Recoveries and precision of the method
The accuracy in terms of mean percentage recovery from the three vegetables and two fruits at three spike concentrations was determined. The samples were allowed to stand after addition of the ten pesticides to ensure complete absorption. Subsequent extraction, cleanup, and detection were carried out using our newly established method. Good recoveries were achieved for all analytes (Table 2). Average recoveries were 78.8–118.6%, and RSDs were < 20% (0.9–14.8%) at quality control levels. The results imply that the method can satisfy the requirements for pesticide residue analysis of fruit and vegetable samples in China (GB2763-2016), as well as meet the requirements stipulated in the European Union Guidance Document (SANCO 2003). The intraday precision for the ten pesticides in tomato and pear spiked at the intermediate level was 4.6–9.8%, and the interday precision over 2 weeks was 7.6–15.2%. These values are within the acceptable range and demonstrate the high precision of the method.
Matrix effects
To examine the matrix effect for the five samples (vegetables and fruits) and the blank solvent, the responses of matrix-matched standards with that of the blank solvent was compared. The matrix effects in the vegetables and fruits were 0.91–3.97 and 1.05–2.41, respectively, with RSDs of 0.8–18.2 and 2.7–14.3 (n = 5). There was a significant ion enhancement effect in all tested matrixes, and a higher substrate effect was often apparent at low pesticide concentrations (usually near the LOQs). On the basis of these results, matrix-matched calibration was used to compensate for signal-enhancement effects and to ensure precision of the analysis. For the analysis, using a high-sensitivity instrument whenever possible is important. To obtain reliable test results, the samples should be diluted sufficiently to greatly reduce the matrix effects of the extraction solvent.
Application to practical samples
The validated analytical method was used for the analysis of pyrethroid pesticides in 20 fruits and 20 vegetables samples obtained from a local supermarket. External calibration was used for quantification with matrix-matched standards (0.1 mg/L). Samples (tomato and pear) spiked with pyrethroid pesticides (0.05 mg/L of each compound) were used for quality control in each batch of samples. Relative recoveries obtained for samples at the spiking level were satisfactory, ranging from 93.6 to 113.8%, and in good agreement with acceptable RSDs (< 10%). The results indicate that practical sample matrixes have little effect on the new method, which is thus suitable for the analysis of trace levels of pyrethroid pesticides in typical samples.
From forty practical samples, pesticides were detected only in four samples; seven of the ten pesticides under study were not detected in any of the samples tested. The four positive samples and one of the negative samples (blank sample) are shown in Fig. 5. Bifenthrin (8 μg/L) and cyhalothrin (54 μg/L) were detected in one tomato sample (Fig. 5a); cypermethrin was detected in one kidney bean sample (30 μg/L) and one grape sample (298 μg/L) (Fig. 5b, c); and cyhalothrin (22 μg/L) was detected in one grape sample (Fig. 5d). Impurity peaks that have only one of the selected ions or an incorrect ion ratio are also shown in Fig. 4a–e. All parameters meet the requirements of the China National Food Safety Standard-Maximum Residue Limits for Pesticides in Food (GB2763-2016).
GC–MS/MS MRM chromatograms of (a) tomato, (b) kidney bean, (c) grape a, (d) grape b, (e) blank sample, and (f) standard solution (0.1 mg/L solution of each pesticide in acetonitrile). Peak identification: 1 = bifenthrin; 2 = fenpropathrin; 3 = cyhalothrin; 4 = permethrin*; 5 = cyfluthrin*; 6 = cypermethrin*; 7 = flucythrinate*; 8 = fenvalerate*; 9 = tau-fluvalinate*; 10 = deltamethrin*; 11 = impurity peak. (Asterisk indicates multiple isomers)
Conclusions
A rapid analytical method based on a modified QuEChERS method coupled with GC–MS/MS was developed for the analysis of ten pyrethroid pesticides in fruits and vegetables. The method showed satisfactory performance in terms of the efficiency and precision of rapidly quantifying pyrethroid pesticides in fruits and vegetables. Serious contamination of the GC system by the components of the complex sample matrix have been prevented by column backflushing, as well as reduced maintenance and cost. Compared with extraction and detection methods in the literature, developed method is more economical because of the low levels of required organic solvent. It showed other advantages, such as short extraction times, simplicity of operation, high-sensitivity, good recovery and low environmental impact. The newly developed method can potentially contribute to food safety control as a universal technique for detecting pesticide residues in various fruits and vegetables.
References
American Chemical Society (ACS) Subcommittee on Environmental Analytical Chemistry (1980) Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal Chem 52:2242–2249
Andersson A, Palsheden H (1988) Pesticide Analytical Methods in Sweden, Uppsala, Sweden. National Food Administration, Rapport 17/98, Part 1, pp 9-41
Barrionuevo WR, Lanças FM (2002) Comparison of liquid–liquid extraction (LLE), solid-phase extraction (SPE), and solid-phase microextraction (SPME) for pyrethroid pesticides analysis from enriched river water. Environ Contam Toxicol 69:123–128. https://doi.org/10.1007/s00128-002-0018-5
Bennett DA, Chung AC, Lee SM (1997) Multiresidue method for analysis of pesticides in liquid whole milk. J AOAC Int 80:1065–1077
Chen J, Meng CK, Naravan SB, Luan W, Bennett MJ (2009) The use of Deconvolution Reporting Software and backflush improves the speed and accuracy of data processing for urinary organic acid analysis. Clin Chim Acta 405:53–59. https://doi.org/10.1016/j.cca.2009.04.005
Chen MX, Cao ZY, Jiang Y, Zhu ZW (2013) Direct determination of glyphosate and its major metabolite, aminomethylphosphonic acid, in fruits and vegetables by mixed-mode hydrophilic interaction/weak anion-exchange liquid chromatography coupled with electrospray tandem mass spectrometry. J Chromatogr A 1272:90–99. https://doi.org/10.1016/j.chroma.2012.11.069
Commission decision of 12 August implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (2002/657/EC). Official Journal of the European Union, L 221, pp 16
Farajzadeh MA, Khoshmaram L (2014) A rapid and sensitive method for the analysis of pyrethroid pesticides using the combination of liquid–liquid extraction and dispersive liquid-liquid microextraction. CLEAN–soil air. Water 42:1–8. https://doi.org/10.1002/clen.201300663
Galera MM, Vidal JLM, Frenich AG, García MDG (1996) Determination of cypermethrin, fenvalerate and cis- and trans-permethrin in soil and groundwater by high-performance liquid chromatography using partial least-squares regression. J Chromatogr A 727:39–46. https://doi.org/10.1016/0021-9673(95)01151-X
Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed [Internet] Brussels (Belgium): SANCO (2003). [cited 2015 Mar 13]. Available from: http://ec.europa.eu/food/plant/pesticides/guidance_documents/docs/qualcontrol_en.pdf
Hayward DG, Hooper K, Andrzejewski D (1999) Tandem-in-time mass spectrometry method for the sub-parts-per-trillion determination of 2, 3, 7, 8-chlorine-substituted dibenzo-p-dioxins and-furans in high-fat foods. Anal Chem 71:212–220. https://doi.org/10.1021/ac980282+
Huang BY, Pan CP, Zhang W, Wang YR, Cao J, Jiang SR (2006) Rapid determination of 45 pesticide residues in fruits and vegetables by dispersive PSA cleanup and gas chromatography-mass spectrometry with correction of matrix effects. J Instrum Anal 25(3):11–16
Jardim ANO, Mello DC, Goes FCS, Junior EFF, Caldas ED (2014) Pesticide residues in cashew apple, guava, kaki and peach: GC–μECD, GC–FPD and LC–MS/MS multiresidue method validation, analysis and cumulative acute risk assessment. Food Chem 164:195–204. https://doi.org/10.1016/j.foodchem.2014.05.030
Jiang Y, Zhong M, Ma Y (2014) The rapid selecting of precursor ions and product ions of thirty-four kinds of pesticide for content determination by GC-EI/MS/MS. Food Control 43:110–114. https://doi.org/10.1016/j.foodcont.2014.03.004
Klee MS (2009) Optimizing capillary column backflush to improve cycle time and reduce column contamination. J Sep Sci 32:88–98. https://doi.org/10.1002/jssc.200800434
Li J, Liu D, Wu T, Zhao W, Zhou Z, Wang P (2013) A simplified procedure for the determination of organochlorine pesticides and polychlorobiphenyls in edible vegetable oils. Food Chem 151:47–52. https://doi.org/10.1016/j.foodchem.2013.11.047
Mahugija JAM, Khamis FA, Lugwisha EHJ (2017) Determination of levels of organochlorine, organophosphorus, and pyrethroid pesticide residues in vegetables from markets in Dar es Salaam by GC-MS. Int J Anal Chem. https://doi.org/10.1155/2017/4676724 (In Press)
Marchis D, Ferro G, Brizio P, Squadrone S, Abete M (2012) Detection of pesticides in crops: A modified QuEChERS approach. Food Control 25:270–273. https://doi.org/10.1016/j.foodcont.2011.10.055
Mastovská K, Hajslová J, Lehotay SJ (2004) Ruggedness and other performance characteristics of low-pressure gas chromatography-mass spectrometry for the fast analysis of multiple pesticide residues in food crops. J Chromatogr A 1054:335–349. https://doi.org/10.1016/j.chroma.2004.08.061
Mastovskaa K, Wylie PL (2012) Evaluation of a new column backflushing set-up in the gas chromatographic–tandem mass spectrometric analysis of pesticide residues in dietary supplements. J Chromatogr A 1265:155–164. https://doi.org/10.1016/j.chroma.2012.09.094
McMahon B, Hardin N (1994) Pesticide analytical manual, vol 1, 3rd edn. US Food and Drug Administration, Washington
Mezcua M, Martinez-Uroz MA, Wylie PL, Fernandez-Alba AR (2009) Simultaneous screening and target analytical approach by gas chromatography-quadrupole-mass spectrometry for pesticide residues in fruits and vegetables. J AOAC Int 92:1790–1806
Mo W, He H, Xu X, Huang B, Ren Y (2014) Simultaneous determination of ethyl carbamate, chloropropanols and acrylamide in fermented products, flavoring and related foods by gas chromatography–triple quadrupole mass spectrometry. Food Control 43:251–257. https://doi.org/10.1016/j.foodcont.2014.03.033
Paoli MD, Barbina MT, Damiano V, Fabbro D, Bruno R (1997) Simplified determination of combined residues of prochloraz and its metabolites in vegetable, fruit and wheat samples by gas chromatography. J Chromatogr A 765:127–131. https://doi.org/10.1016/S0021-9673(96)00995-8
Peng Y, Li Y, Chen J (2012) Optical technologies for determination of pesticide residue. In: Theophile T (ed) Infrared spectroscopy-materials science, engineering and technology. InTech, Rijeka, pp 453–466
Plomley JB, Koester CJ, March RE (1994) Determination of N-Nitrosodimethylamine in complex environmental matrices by quadrupole ion storage tandem mass spectrometry enhanced by unidirectional ion ejection. Anal Chem 66:4437–4443
Poole CF (2007) Matrix-induced response enhancement in pesticide residue analysis by gas chromatography. J Chromatogr A 1158(1–2):241–250. https://doi.org/10.1016/j.chroma.2007.01.018
Rashid A, Nawaz S, Barker H, Ahmad I, Ashraf M (2010) Development of a simple extraction and clean-up procedure for determination of organochlorine pesticides in soil using gas chromatography tandem mass spectrometry. J Chromatogr A 1217:2933–2939. https://doi.org/10.1016/j.chroma.2010.02.060
Sánchez MT, Flores-Rojas K, Guerrero JE, Garrido-Varo A, Pérez-Marín D (2010) Measurement of pesticide residues in peppers by near-infrared reflectance spectroscopy. Pest Manag Sci 66:580–586. https://doi.org/10.1002/ps.1910
Schettgen T, Koch HM, Drexler H, Angerer J (2002) New gas chromatographic-mass spectrometric method for the determination of urinary pyrethroid metabolites in environmental medicine. J Chromatogr B 778:121–130. https://doi.org/10.1016/S0378-4347(01)00452-2
Sobhanzadeh E, Bakar NKA, Abas MRB, Nemati K (2012) A simple and efficient multi-residue method based on QuEChERS for pesticides determination in palm oil by liquid chromatography time-of-flight mass spectrometry. Environ Monit Assess 184:5821–5828. https://doi.org/10.1007/s10661-011-2384-0
Tsoutsi C, Konstantinou I, Hela D, Albanis T (2006) Screening method for organophosphorus insecticides and their metabolites in olive oil samples based on headspace solid-phase microextraction coupled with gas chromatography. Anal Chim Acta 573:216–222. https://doi.org/10.1016/j.aca.2006.04.075
Wang GS, Gao LH, Sun JH, Cui JM, Liu W (2012) Matrix effects on analysis of organochlorine and pyrethroid pesticides in grain by GC-MS. Chn Brw 31(11):169–172
Wang H, Zhao L, Yang HM, Guo Q, Shi H, Pan HY, Zhao LP, Qian C (2014) Determination of benzo (a) pyrene and aflatoxins (B1, B2, G1, G2) in vegetable oil by GPC–HPLC–FLD. Anal Methods 6:1545–1549. https://doi.org/10.1039/C3AY41955J
Wu CC (2017) Multiresidue method for the determination of pesticides in Oolong tea using QuEChERS by gas chromatography-triple quadrupole tandem mass spectrometry. Food Chem 229:580–587. https://doi.org/10.1016/j.foodchem.2017.02.081
Acknowledgements
The work was supported by the Science and Technology Projects of Zhejiang Province of China (2013C37037). We thank International Science Editing for the English language editing service.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, XY., Mou, RX., Cao, ZY. et al. Analysis of pyrethroid pesticides in Chinese vegetables and fruits by GC–MS/MS. Chem. Pap. 72, 1953–1962 (2018). https://doi.org/10.1007/s11696-018-0447-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0447-1