Abstract
There is of great interest in promotion of anti-thermal aging properties of natural rubber (NR) to improve the applicability. In this study, two novel Schiff base antioxidants (SBAOs) for NR were synthesized utilizing 4-aminodiphenylamine with 3,5-Di-tert-butyl-2-hydroxybenzaldehyde or cinnamic aldehyde in an ethanol medium. IR, 13C-NMR, and 1H-NMR confirmed the structures of SBAOs. Addition of SBAOs improved the rheometric properties, mechanical properties and thermal oxidative stability of NR vulcanizates. Introduction of SBAOs in NR increased the apparent activation energy of thermal oxidative degradation according to Kissinger and FWO methods. Anti-thermal aging performance of SBAOs for NR is related to the structures. The C=N double bonds in SBAOs improve the electron density of Ar–OH and/or Ar–NH–Ar structures, benefiting the release of active hydrogen. The active hydrogen could capture free radicals initiated during the thermal oxidative aging process. The lone pair electrons on nitrogen atom are also beneficial to delay or terminate free radical reaction. NR with SBAOs showed high mechanical properties of the tensile strength, tensile stress at 100% elongation and Shore A hardness compared to commercial BHT and 4010 during aging 96 h. It indicates potential applications of SBAOs as efficient antioxidants for NR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural rubbers (NR) are easily attacked by heat, light, and oxygen/ozone initiated free radicals (Mcdonel and Shelton 1959; Li and Koening 2003), causing deteriorated mechanical properties during the processing or application (Mcdonel and Shelton 1959; Li and Koening 2003). Like other radical reaction, the thermal oxidative degradation of NR proceeds by a free radical chain mechanism, where alkyl radical (R·) and oxygen centered radicals (ROO·, RO·) cause chain initiation and growth. It is necessary to decrease or inhibit degradation by introducing antioxidants during the processing. The role of antioxidants lies in their reaction with oxygen centered radicals or alkyl radical to break the normal radical chain propagation. The hindered phenol and hindered amine antioxidants (HPAOs and HAAOs) are two major categories of conventional antioxidants (Al-Ghonamy et al. 2010; Herdan et al. 1995; Ismail et al. 1999). The HPAOs, such as styrenated phenol, 2,2′-methylenebis (6-tert-butyl-4-methylphenol) and 2,6-Di-tert-butyl-4-methylphenol, have been commercially applied in NR (Wadelin 1956; Howard and Ingold 1962; Malshe et al. 2006). HPAOs break chain radical reactions by the active hydrogen in phenol (Ar–OH) reacting with ROO· to aryloxy radical (Ar–O·) and peroxy compounds (ROOH), and Ar-O· radical continuously capturing ROO· to form non-free radical products (ROO–O–Ar). In many instances, the HAAOs, including N-phenyl-N′-dimethyl-butyl-p-phenylenediamine, poly (2-amino pyridine), N-isopropy1-N′-pheny1-p-phenylenediamine and N,N′-substituted p-phenylenediamines, showed high antioxidant activity in comparison with HPAOs (Cataldo 2001, 2002; Sparks 1967; Cibulková et al. 2005a, 2005b). Abdel-Bary et al. found that the antioxidant efficiency of p-phenylenediamine derivatives increased with the increasing inductive effect of the substituents (Abdel-Bary et al. 1997). Hydrogen free radical produced by homolysis of –NH– structure in secondary amine antioxidants reacts with ROO· or RO· forming stable products. Tertiary amine antioxidant has a pair of lone pairs of electrons of nitrogen atom itself. Once free ROO· meets it, and the transfer of electrons delays or terminates the reaction of free ROO·.
Development of more types and high-efficient antioxidants for NR has still been focused on (Wu et al. 2015; Pan et al. 2013; Černá et al. 2012; Ahmed et al. 2012). Ismail et al. reported that synthesized seven arylphosphites compounds are good antioxidants and antifatigue agents and their antioxidant efficiency is higher than that of the commercial 4-methyl-2,6 di-tert-butyl phenol (Ismail et al. 2001). Silica-supported 2-mercaptobenzimidazole was proved to be an efficient and “green” antioxidant for styrene-butadiene rubber (Zhong et al. 2014). Wu et al. synthesized two novel macromolecular hindered phenol antioxidants containing thioether and urethane groups by the combination of thiol-acrylate Michael addition and nucleophilic addition (Wu et al. 2015). The thioether and urethane groups played an important role in improving antioxidative efficiency, and the urethane group connected with benzene ring had better antioxidative ability than that connected with alicyclic ring.
Developing the antioxidants composing multifunctional reactive groups of the structures is a reasonable orientation. Actually, the Schiff base antioxidants (SBAOs), which generally are synthesized by condensation of amines with an active carbonyl group, have shown great potential in promoting aging resistance of NR during early research (George et al. 1993). However there is lack of further investigation on SBAOs.
In the present study, two novel Schiff base antioxidants SBAOs were synthesized utilizing an efficient hindered amine and different hindered phenols. The hindered phenol and hindered amine in the SBAO were bonded by carbon–nitrogen (C=N) double bonds. The advantages of HPAOs and HAAOs were expected to coexist in the SBAOs. The influence of SBAOs on the rheometric characteristics, mechanical properties and thermal oxidative stability of NR vulcanizates was investigated. Thermal oxidative degradation kinetics were further studied to analyze the anti-thermal aging performance of SBAOs.
Experimental
Materials
The 4-aminodiphenylamine (98 wt %) was purchased from Aladdin Industries, USA. The 3,5-Di-tert-butyl-2-hydroxybenzaldehyde (95 wt%) and cinnamic aldehyde were obtained from Wuhan Xinhuayuan Fine Chemicals Co., Ltd., China. The anhydrous ethanol (Analytical grade) was provided by Guangzhou Chemical Reagent Co, Ltd., China. Typical commercial antioxidants of 2,6-Di-tert-butyl-4-methylphenol (BHT) and N-isopropyl-N′-phenyl-1,4-phenylenediamine (4010NA) were purchased from Shanghai Feige Chemicals Co., Ltd, China and Guangzhou Institute of Rubber Industry Co., Ltd., China, respectively. The NR (SVR, Vietnam), vulcanization accelerators CZ and DM (80 wt%) were provided by Guangzhou Liben Rubber Materials Co., Ltd., China. The zinc oxide (technical grade) and sulfur (80 wt%) were purchased from West Long Chemical Co., Ltd., China. The stearic acid was obtained from Winner Functional Materials Company, China.
Synthesis
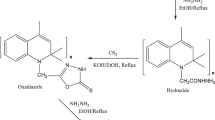
3.760 g of 4-aminodiphenylamine was entirely dissolved in 30 mL anhydrous ethanol in a 100 mL beaker under magnetic stirring. The solution was named as Solution-A. Meanwhile, 4.933 g of 3,5-Di-tert-butyl-2-hydroxybenzaldehyde or 2.5 mL cinnamic aldehyde was, respectively, dissolved in 30 mL of anhydrous ethanol in a 250 mL three-neck flask under a reflux condenser at a water-bath temperature of 78 °C. The resulting solutions were named as Solution-1 and Solution-2, respectively. The Solution-A was then slowly dropped into Solution-1 or Solution-2 in the three-neck flask and reacted for 6 h. The appeared crystals were separated out when the reacted solution was cooled to room temperature. After filtered and washed with anhydrous ethanol, the target SBAOs were obtained and named as SBAO-1 and SBAO-2, respectively (Fig. 1). The yields of SBAO-1 and SBAO-2 were 70.9 and 68.3%, respectively. The commercial antioxidants of BHT and 4010 NA were simultaneously investigated as comparative studies. The Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR) were used to analyze the structure of SBAOs on a TENSOR 27 FTIR spectroscope (Bruker, German) and an AVANCE III NMR spectrometer (Bruker, German), respectively.
Measurement methods
The anti-thermal aging properties of SBAOs were evaluated by measuring the rheometric properties, mechanical properties and thermal oxidative stability of the SBAOs added NR vulcanizates. The prescription of NR vulcanizates was given below: 100 g of NR, 1.875 g of accelerator CZ, 0.625 g of accelerator DM, 1 g of stearic acid, 5 g of zinc oxide, 1.875 g sulfur and 2.0 g of antioxidants. After shaping on an S(X) K-160A rubber open two-roll mill (Wuxi Wantailong, China), the rubber compounds were vulcanized on a XL13-DXS flat vulcanizing machine (Shanghai Yayue, China). The prepared NR vulcanizates by adding SBAO-1, SBAO-2, BHT and 4010NA were denoted as NRV-1, NRV-2, NRV-3 and NRV-4, respectively. No antioxidant added NR vulcanizate was NRV-0. Another prescription of NR vulcanizates containing additional 50 g hard carbon black in above mixing process was tested to further investigate the properties of SBAOs. Similarly, they were named as: CNRV-1, CNRV-2, CNRV-3 and CNRV-4, with SBAO-1, SBAO-2, BHT and 4010NA antioxidants, respectively.
The thermal oxidative aging of NR vulcanizates was carried out on a UA-2071A aging oven tester (U-CAN, Taiwan, China) at 100 °C and different lengths of time based on GB/T3512-2001 (China national standard). The rheometric properties were measured using a UR-2010SD-A rheometer (U-CAN, Taiwan, China) at 150 °C according to GB/T9869-1997 (China national standard). The mechanical properties were analyzed on a UT-2080 electronic tensile testing machine (U-CAN, Taiwan, China) at a stretching rate of 500 mm/min in accordance with GB/T528-1998 (China national standard). The Shore A hardness was recorded on an LX-A shore′s durometer (Shanghai Liuling, China) according to GB/T531-1999 (China national standard). The thermal gravimetric analysis (TGA) was used to analyze the thermal oxidative stability on a TG209 F3 thermal gravimetric analyzer (NETZSCH, German) in an air flow of 50 mL/min. Consequently, the thermal oxidative degradation kinetics was calculated according to the Flynn–Wall–Ozawa (FWO) method (Núñez, et al. 2000).
Results and discussion
IR spectra of SBAO-1 showed the typical absorption peaks of –OH (3406.17 cm−1), CH3 (2962.56 cm−1), symmetric CH2 stretching vibration (2906.62 cm−1), asymmetric CH2 stretching vibration (2868.05 cm−1) and C=N (1604 cm−1) (Fig. 2a). 13C-NMR spectra of SBAO-1 exhibited 19 carbon lines (Fig. 2b). The chemical shifts (δ) at 29.56 and 31.63 ppm were assigned to two kinds of primary carbon peaks, while those at 34.30 and 35.20 ppm were ascribed to two types of tertiary carbon peaks. The δ values at 118.18–158.19 ppm, 161.15 and 77.2 ppm were attributed to 14 types of carbons on the benzene ring, the carbon of C=N and solvent CDCl3 carbon peaks, respectively. Besides, the distortionless enhancement by polarization transfer (DEPT) spectra (135°) of SBAO-1 displayed CH3 peaks (δ = 29.56 and 31.63 ppm), CH peak in C=N (δ = 161.15 ppm) and 7 types of CH on the benzene ring (δ = 118.2687–129.6339 ppm), respectively (Fig. 2c). The typical 1H-NMR spectra of SBAO-1 (Fig. 2d) showed proton peaks in tert-butyl carbon (δ = 1.48 and 1.38 ppm), –NH– (δ = 5.76 ppm), –N = CH– (δ = 8.64 ppm), benzene ring (δ = 6.90, 7.09, 7.24 and 7.45 ppm), solvent CDCl3 (δ = 7.25 ppm) and the associated –OH (δ = 13.91 ppm). Integral hydrogen peak areas (S) in tert-butyl carbon, –NH–, –N=CH– and benzene ring at (δ = 6.90, 7.09, 7.24 and 7.45 ppm) were 9, 1, 1 and 11 (1, 4, 5 and 1), respectively. Similarly, the characterization results of SBAO-2 are also summarized in Table 1. The SBAOs were successfully synthesized according to the results of IR, 13C-NMR, DEPT (135°) and 1H-NMR spectra.
The rheometric properties of NR vulcanizates by adding various antioxidants are listed in Table 2. The NRV-1 and NRV-2 showed low minimum torque (M L ) and maximum torque (M H ) compared to NRV-0 and NRV-3 and NRV-4. The decreased M L by adding SBAOs suggests the improved fluidity. The reduced M H is related to weakened crosslinking density of NRV samples before aging. The addition of SBAOs caused long vulcanization time (t C90) and scorching time (t S1 and t S2), thus improving the safety during manufacturing of NRV samples. The newly synthesized SBAOs exhibited better performance on promoting rheometric properties of NRV samples relative to commercial BHT and 4010 NA. As for CNRV samples, increase of M L and decrease of t C90 reveals weakened mobility and shortened the vulcanization time, respectively. Introduction of SBAO-1 improves operational safety relative to that of SBAO-2 in CNRV samples. For various prescription of NR vulcanizates (NRV and CNRV), SBAOs show their advantages on the improvement of rheometric properties.
The mechanical properties of NRV and CNRV samples by adding various antioxidants under thermal oxidative aging are illustrated in Figs. 3 and 4, respectively. Tensile strength of VNR-0 and CNRV-0 gradually decreased at aging from 0 to 96 h. Tensile strength of VNR samples with antioxidants firstly increased at aging 24 h and 48 h, and then gradually decreased at aging times (Fig. 3a). The increased tensile strength at aging 24 and 48 h may be probably ascribed to the promoted internal crosslinking density of rubber induced by antioxidants during the aging process. Internal crosslinking of molecules of NR continuously changes with various aging time and the maximization happened at aging 24 h for NRV with antioxidants according to the previous investigation (Li et al. 2015). Differently, tensile strength of CNRV samples gradually decreased at aging from 0 to 96 h (Fig. 4a). The hard carbon black in CNRV blocks internal crosslinking of rubber and weakens its influence on tensile strength. The elongation at break declined with the increase of aging time for VNR and CNRV (Figs. 3b, 4b). The tensile stress at 100% elongation and shore A hardness increased as aging time increased for VNR (Fig. 3c, d) and CNRV (Fig. 4c, d). After aging 96, NRV-1 (CNRV-1) and NRV-2 (CNRV-2) displayed higher tensile strength, tensile stress at 100% elongation and Shore A hardness than NRV-0 (CNRV-0), revealing anti-aging effects of SBAO-1 and SBAO-2 presence. Besides the tensile strength, tensile stress at 100% elongation and Shore A hardness of NRV-3 (CNRV-3) and NRV-4 (CNRV-4) were lower than those of NRV-1(CNRV-1) and NRV-2 (CNRV-2) at aging 96 h. The respective advantages of SBAO-1 and SBAO-2 antioxidants are revealed compared to commercial BHT and 4010NA antioxidants.
Further investigation on thermal oxidative stability and dynamics was performed according to NRV samples. The TGA curves (in air) of NR vulcanizates by adding various antioxidants are illustrated in Fig. 5 and temperatures at various weight losses are listed in Table 3. The TGA curves of NR vulcanizates showed two stages. The stage of 250–410 °C probably represented the degradation of organic components in NR, and the stage of 410–540 °C presumably signified the pyrolysis of NR vulcanizates. The addition of four antioxidants significantly promoted the initial degradation temperatures (T 0), maximum degradation temperature (T max), and degradation temperatures (T 0.1, T 0.15, T 0.2, and T 0.3) at various weight losses of 10, 15, 20 and 30% for NR vulcanizates, respectively. It reveals that the function of antioxidants is in delaying degradation of NR vulcanizates in thermal oxidative process. Besides, degradation temperatures gradually increased from T0 to Tmax for NRV-0. However, for NRV-1, NRV-2, NRV-3, and NRV-4 vulcanizates, degradation temperatures displayed two stages: a little increase or decrease from T 0 to T 0.1, and subsequent quick increase. T 0 (T max) increased by 8.7 °C (6 °C), 18.6 °C (12.8 °C), 4.8 °C (2.3 °C) and 12.4 °C (26 °C) for NRV-1, NRV-2, NRV-3, and NRV-4, respectively, relative to that for NRV-0 (Table S1). The degradation temperature of T 0.1, T 0.15, T 0.2, and T 0.3 increased by 4.9–6.8 °C, 6.9–10.8 °C, 0.1–3.5 °C, and 4.7–8.1 °C for NRV-1, NRV-2, NRV-3, and NRV-4, respectively, compared to that for NRV-0. In contrast to the degradation temperatures of NRV-0 at various weight losses, the largest increase of degradation temperature of NRV-1, NRV-2, and NRV-3 occurred at T0, whereas that of NRV-4 happened at T max (Table S1). For NRV-1, NRV-2, and NRV-3 with SBAO-1, SBAO-2, and BHT antioxidants, its degradation is difficult at initial stage, and comparatively easy at subsequent stage. For NRV-4 with 4010NA antioxidant, its degradation is as follows: difficult at initial stage, comparatively easy at intermediate stage, and harder at later stage. Probably, the divergence is due to different mechanism of action of various antioxidants.
NRV-1 and NRV-2 showed higher degradation temperatures at various weight losses than NRV-3 vulcanizate. It reveals that SBAO-1 and SBAO-2 significantly delay the degradation of NR vulcanizate in thermal oxidative process compared to BHT. Besides, SBAOs and 4010NA antioxidants show their respective advantages. Anti-thermal-oxidative aging of SBAO-1 is likely ascribed to the structures of both Ar–OH and Ar–NH–Ar. Both structures provide more active hydrogen under the steric effect of benzene rings. Anti-thermal-oxidative aging of SBAO-2 probably benefits from the structures of Ar–NH–Ar, C=N double bonds, and the interaction between them despite the lack of Ar–OH structure. As an electron donating group, the C=N double bonds improved the electron density of Ar–NH–Ar structures, promoting the release of active hydrogen to capture more free radicals. Besides, the lone pair electrons on nitrogen atom are also beneficial to delay or terminate free radical reaction.
The thermal oxidative degradation kinetics were studied to analyze the anti-thermal oxidative aging of SBAOs. The addition of four antioxidants in NRV samples all increased the apparent activation energy according to the Kissinger and FWO methods (Fig. 6). Actually, the apparent activation energy by Kissinger is calculated by differential calculation at the maximum degradation temperature. According to the Kissinger method, the apparent activation energy of NRV samples increased as follows: NRV-0 (97.11 kJ/mol) < NRV-3 (107.84 kJ/mol) < NRV-1 (108.93 kJ/mol) < NRV-4 (154.69 kJ/mol) < NRV-2 (156.99 kJ/mol) (Fig. 6a). The difference of apparent activation energy among NRV-1, NRV-2, NRV-3, and NRV-4 is probably related to different mechanisms of action of antioxidants. By FWO method, apparent activation energy decreased and subsequently increased for NRV samples. The former probably corresponds to the auto-acceleration of thermal oxidation process, and the latter is likely due to decomposition of thermal oxidation products combining with intensified thermal oxidation process. NRV-1 and NRV-2 showed higher apparent activation energy than NRV-3 and NRV-4 at various weight loss, suggesting the better thermal oxidative stability of SBAOs added NRV samples. It also indicates the potential applications of SBAOs as efficient antioxidants for NR.
Conclusions
Two novel Schiff base antioxidants (SBAOs) for NR were synthesized utilizing 4-aminodiphenylamine with 3,5-Di-tert-butyl-2-hydroxybenzaldehyde or cinnamic aldehyde in an ethanol medium. The structures of SBAOs were characterized by IR, 13C-NMR, and 1H-NMR Rheometric properties, mechanical properties and thermal oxidative stability of NR vulcanizates were improved due to the addition of SBAOs antioxidants. Introduction of SBAOs in NR increased the apparent activation energy of thermal oxidative degradation according to the Kissinger and FWO methods. The anti-thermal aging performance of SBAOs for NR vulcanizates lies in the structures. The C=N double bonds in SBAOs improve the electron density of Ar–OH and/or Ar–NH–Ar structures, promoting the release of active hydrogen. The active hydrogen could capture free radicals initiated during the thermal oxidative aging process. The lone pair electrons on nitrogen atom are also beneficial to delay or terminate the free radical reaction. NR with SBAOs showed high mechanical properties of the tensile strength, tensile stress at 100% elongation and Shore A hardness compared to commercial BHT and 4010 during aging 96 h. This study indicated potential applications of SBAOs as efficient antioxidants for NR.
References
Abdel-Bary EM, Moawad EB, Helaly FM, Abdelaal MY, Rushed WF (1997) Evaluation of some organic compounds as antioxidants in rubber. Polym Degrad Stab 57:283–292. doi:10.1016/S0141-3910(97)00008-6
Ahmed FS, Shafy M, Abd El-megeed AA, Hegazi EM (2012) The effect of γ-irradiation on acrylonitrile–butadiene rubber NBR seal materials with different antioxidants. Mater Des 36:823–828. doi:10.1016/j.matdes.2011.02.066
Al-Ghonamy AI, El-Wakil AA, Ramadan M (2010) Enhancement the thermal stability and the mechanical properties of acrylonitrile-butadiene copolymer by grafting antioxidant. Int J Polym Sci 2010:1–7. doi:10.1155/2010/981690
Cataldo F (2001) On the ozone protection of polymers having non-conjugated unsaturation. Polym Degrad Stab 72:287–296. doi:10.1016/S0141-3910(01)00017-9
Cataldo F (2002) A study on the reaction between N-substituted p-phenylenediamines and ozone: experimental results and theoretical aspects in relation to their antiozonant activity. Eur Polym J 38:85–93. doi:10.1016/S0014-3057(01)00248-8
Černá A, Cibulková Z, Šimon P, Uhlár J, Lehocký P (2012) DSC study of selected antioxidants and their binary mixtures in styrene-butadiene rubber. Polym Degrad Stab 97:1724–1729. doi:10.1016/j.polymdegradstab.2012.06.012
Cibulková Z, Šimon P, Lehocký P, Balko J (2005a) Antioxidant activity of p-phenylenediamines studied by DSC. Polym Degrad Stab 87:479–486. doi:10.1016/j.polymdegradstab.2004.10.004
Cibulková Z, Šimon P, Lehocký P, Balko J (2005b) Antioxidant activity of 6PPD derivatives in polyisoprene matrix studied by non-isothermal DSC measurements. J Therm Anal Calorim 80:357–361. doi:10.1007/s10973-005-0660-3
George RS, Joseph R, George KE (1993) Study of poly-Schiff”s based as a protective agent in natural rubber. Int J Polym Mater 23:17–26. doi:10.1080/00914039308009655
Herdan JM, Stan M, Giurginca M (1995) Grafting antioxidants VIII. Antixoidant activity and grafting of some N-(aryl)-2,6-di-tert-butylquinoneimines. Polym Degrad Stab 50:59–63. doi:10.1016/0141-3910(95)00105-U
Howard JA, Ingold KU (1962) The inhibited autoxidation of styrene: the deuterium isotope effect for inhibition by 2, 6-Di-tert-butyl-4-methylphenol. Can J Chem 40:1851–1864. doi:10.1139/v62-281
Ismail MN, Abd EI Ghaffar MA, Shaffei KA, Mohamed NA (1999) Some novel polyamines as antioxidants for SBR vulcanizates. Polym Degrad Stab 63:377–383. doi:10.1016/S0141-3910(98)00115-3
Ismail MN, Yehia AA, Korium AA (2001) Evaluation of some arylphosphites as antioxidants and antifatigue agents in natural rubber and styrene–butadiene rubber vulcanizates. Polym Degrad Stab 74:247–253. doi:10.1016/S0141-3910(01)00111-2
Li GY, Koening JL (2003) FT-IR imaging of oxidation of polyisoprene: the role of N-phenyl-N′-dimethyl-butyl-p-phenylenediamine antioxidant. Polym Degrad Stab 81:377–385. doi:10.1016/S0141-3910(03)00109-5
Li WP, Lv MZ, Yang ZM, Peng Z, Li SD (2015) Crosslinking strucures changes during accelerated storage aging of epoxidated natural (in chinese). Synth Mater Aging Appl 1:1–4. doi:10.16584/j.cnki.issn1671-5381.2015.01.011
Malshe VC, Elango S, Rane S (2006) Alkylated phenolic resins as antioxidants for rubber. J Appl Polym Sci 100:2649–2651. doi:10.1002/app.22736
Mcdonel ET, Shelton JR (1959) Effect of curing system on rubber oxidation and physical degradation. J Chem Eng Data 4:360–366. doi:10.1021/je60004a021
Núñez L, Fraga F, Nunez MR, Villanuev M (2000) Thermogravimetric study of the decomposition process of the system BADGE(n = 0)/1,2 DCH. Polymer 41:4635–4641. doi:10.1016/S0032-3861(99)00687-4
Pan Q, Wang B, Chen Z, Zhao J (2013) Reinforcement and antioxidation effects of antioxidant functionalized silica in styrene–butadiene rubber. Mater Des 50:558–565. doi:10.1016/j.matdes.2013.03.050
Sparks AK (1967) U.S. Patent No. 3645966A. Edwin J Latos: U.S. Patent and Trademark Office
Wadelin CW (1956) Ultraviolet determination of phenolic antioxidants in rubber. Anal Chem 28:1530–1531. doi:10.1021/ac60118a008
Wu W, Zeng X, Li H, Lai X, Xie H (2015) Synthesis and antioxidative properties in natural rubber of novel macromolecular hindered phenol antioxidants containing thioether and urethane groups. Polym Degrad Stab 111:232–238. doi:10.1016/j.polymdegradstab.2014.12.001
Zhong B, Shi Q, Jia Z, Luo Y, Chen Y, Jia D (2014) Preparation of silica-supported 2-mercaptobenzimidazole and its antioxidative behavior in styrene-butadiene rubber. Polym Degrad Stab 110:260–267. doi:10.1016/j.polymdegradstab.2014.09.008
Acknowledgements
This project was supported in part by Academic Innovation Team foundation of China University of Political Science and Law (No. 1000-10814340).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, HY., Liu, KK. The synthesis of novel Schiff base antioxidants to promote anti-thermal aging properties of natural rubber. Chem. Pap. 71, 1481–1489 (2017). https://doi.org/10.1007/s11696-017-0142-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0142-7