Abstract
Background
Metabolic and Bariatric surgery (MBS) leads to significant weight loss and improvements in obesity-related comorbidities. However, the impact of MBS on Apolipoprotein B100 (Apo-B100) regulation is unclear. Apo-B100 is essential for the assembly and secretion of serum lipoprotein particles. Elevated levels of these factors can accelerate the development of atherosclerotic plaques in blood vessels. This study aimed to evaluate changes in Apo-B100 levels following MBS.
Methods
121 participants from the Iranian National Obesity and Metabolic Surgery Database (INOSD) underwent Laparoscopic Sleeve Gastrectomy (LSG) (n = 43), One-Anastomosis Gastric Bypass (OAGB) (n = 70) or Roux-en-Y Gastric Bypass (RYGB) (n = 8). Serum Apo-B100, lipid profiles, liver enzymes, and fasting glucose were measured preoperatively and six months postoperatively.
Results
Apo-B100 levels significantly decreased from 94.63 ± 14.35 mg/dL preoperatively to 62.97 ± 19.97 mg/dL after six months (p < 0.01), alongside reductions in total cholesterol, triglycerides, LDL, VLDL, AST, and ALT (p < 0.05). Greater Apo-B100 reductions occurred in non-diabetics versus people with diabetes (p = 0.012) and strongly correlated with baseline Apo-B100 (r = 0.455, p < 0.01) and LDL levels (r = 0.413, p < 0.01). However, surgery type did not impact Apo-B100 changes in multivariate analysis (p > 0.05).
Conclusion
Bariatric surgery leads to a significant reduction in Apo-B100 levels and improvements in lipid profiles and liver enzymes, indicating a positive impact on dyslipidemia and cardiovascular risk in individuals with high BMI.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity currently represents one of the main global health issues. The prevalence of obesity worldwide is rising across populations [1]. Obesity leads to increased risks of various comorbidities, including elevated cardiovascular morbidity [2], increased incidence of type 2 diabetes mellitus (T2DM) [3, 4], increased risk of various types of cancer [5,6,7], elevated risk of non-alcoholic fatty liver disease (NAFLD) [8], greater susceptibility to osteoarthritis [9], and higher rates of mental health disorders [10]. Among the extensive systemic effects of obesity, one of the most pressing is the development of dyslipidemia and associated cardiovascular complications mediated by disruptions in lipid regulation [11, 12]. Obesity leads to disruptions in lipid regulation through several key mechanisms. Excess adipose tissue increases free fatty acid flux to the liver, driving increased hepatic secretion of triglyceride-rich VLDL particles [13]. Obesity also impairs VLDL clearance by reducing lipoprotein lipase activity in peripheral tissues [14]. Additionally, insulin resistance caused by obesity inhibits hepatic LDL receptor expression, further raising LDL levels [15].
Apolipoproteins play a critical role in lipid metabolism by stabilizing the structure of lipoprotein particles and regulating their transport between tissues. Specifically, Apolipoprotein B100 (Apo-B100) is essential for the assembly and secretion of very low-density lipoprotein (VLDL) particles, as well as the subsequent metabolism of VLDL to intermediate-density lipoprotein (IDL) and low-density lipoprotein (LDL) particles [16]. Elevated Apo-B100 promotes atherosclerosis through several mechanisms. The binding of Apo-B100 to arterial wall proteoglycans triggers the retention and accumulation of LDL particles in the subendothelial space [17, 18]. This aggregated LDL can then undergo oxidation and be taken up by macrophages, forming foam cells that constitute a key step in plaque formation. Additionally, Apo-B100 has direct pro-inflammatory effects on endothelial cells, stimulating the expression of adhesion molecules like VCAM-1 [19]. Through these pathways, increased Apo-B100 accelerates multiple stages of atherogenesis from subendothelial retention of lipoproteins to arterial inflammation and plaque progression. However, plasma levels of Apo-B100 represent the total number of circulating mentioned lipoproteins, and elevated levels of each of these factors can accelerate the development of atherosclerotic plaques in the blood vessels [20]. Therefore, measures of Apo-B100 consistently display superior predictive capacity for myocardial infarction, stroke, and cardiovascular death [21, 22].
Clinical attention has increasingly focused on Metabolic and Bariatric Surgery (MBS) as the most viable and enduringly effective means to generate sustained weight loss in individuals with severe obesity [23, 24]. Compared to non-surgical approaches, guidelines consistently recognize MBS – including laparoscopic sleeve gastrectomy (LSG), Roux-en-Y gastric bypass (RYGB), and one anastomosis gastric bypass (OAGB) – as interventions to improve obesity-related mortality, such as dyslipidemia in obese individuals [25]. However, no studies have quantitatively assessed the longitudinal trajectory of Apo-B100 levels following different types of MBS.
Amid the metabolic effects of MBS, a significant knowledge gap persists regarding these procedures’ influence on the regulation of Apo-B100. Quantifying Apo-B100 levels before and longitudinally after MBS would provide important insights into the impact of these procedures on MBS cardio-protective effects. While substantial evidence demonstrates the efficacy of MBS for generating enduring improvements in cardiovascular outcomes, the specific influence of these procedures on the regulation of Apo-B100 remains poorly characterized.
This study directly addresses this gap by evaluating the impact of MBS on plasma Apo-B100 concentrations in individuals with obesity over the postoperative period. The primary objective is to study the changes in plasma Apo-B100 levels after MBS. This study aims to determine if the weight loss and metabolic improvements resulting from the surgery positively impact the regulation of this apolipoprotein.
Materials and methods
This study has a prospective cohort design in which individuals with obesity were recruited before MBS and subsequently followed longitudinally for six months postoperatively. Utilizing a prospective cohort approach, in which participants serve as their controls for comparing longitudinal changes, provides distinct advantages for characterizing the trajectory of metabolic factors in response to surgical intervention over time.
Participants and inclusion criteria
Using the Iranian National Obesity and Metabolic Surgery Database (INOSD) [26], participants included in this study were men and women older than 18 years old with a body mass index ≥ 40 kg/m2 or ≥ 35 kg/m2 with at least one obesity-related comorbidity who were scheduled to undergo MBS from January 1, 2022, to December 31, 2022. Exclusion criteria include prior MBS, pregnancy, cardiovascular events within the past six months, advanced heart failure or renal disease, and active cancer treatment. Also, individuals using lipid-lowering drugs, including statins, fibrates, ezetimibe, PCSK9 inhibitors, niacin, and omega-3 fatty acid-containing products, were excluded from the study due to the impact of these medications on serum lipoprotein and Apo-B100 levels.
Considering an estimated effect size of 0.47 based on the study by Kjellmo et al. [27] for the reduction in Apo-B100 serum levels before and after MBS, with a margin of error of 5%, and 80% power, the sample size was estimated to be 144 cases. Finally, we decided to recruit 157 participants who met the eligibility criteria to enhance the study's power.
Study setting
Board-certified and International Federation for the Surgery of Obesity (IFSO) fellow bariatric surgeons performed surgeries at three high-volume bariatric centers in Tehran, Iran. All three hospitals possess dedicated MBS departments staffed by multidisciplinary teams equipped to provide consistent perioperative education, medical optimization before surgery, and postsurgical follow-up aligned with IFSO guidelines for bariatric surgery centers [28,29,30]. The team at each site includes bariatric physicians, dieticians, psychiatrists, and specialized nursing staff trained in caring for patients undergoing MBS. Standard surgical protocols, including a 5-trocar laparoscopic approach, and similar operative equipment specifications were utilized at all three hospitals.
LSG involves the removal of a significant portion of the stomach, resulting in a tubular-shaped gastric sleeve. Key technical details of LSG included the utilization of a bougie number 36 to guide the creation of the gastric sleeve, the removal of approximately 75–80% of the stomach, and leaving behind a narrow gastric tube. Measurement of the distance from the pylorus to be approximately 2–4 cm and from the fundus to be approximately 0.5–1 cm. The surgeon performed the closure of the gastric sleeve using a 60 mm linear cutter stapler.
RYGB involved the creation of a small gastric pouch and rerouting of the gastrointestinal tract to promote malabsorption and reduced food intake. The pouch volume typically ranges from 30–45 cc, tailored to individual patient characteristics. The limb length varied between 100–150 cm based on the patient's body mass index (BMI). The anastomosis was made using a 45 mm linear cutter stapler, ensuring a diameter of 2–3 cm to facilitate food passage. Furthermore, suturing of the anastomotic orifices using 2/0 PDS ensures secure closure and reduces the risk of postoperative complications.
OAGB also involved the creation of a larger gastric pouch (approximately 60–75 cc) and longer limb lengths (150–200 cm). Similar to RYGB, the anastomosis was made using a 45 mm linear cutter stapler, maintaining a diameter of 2–3 cm. Suturing of the anastomotic orifices with 2/0 PDS ensures adequate closure and minimizes the risk of postoperative complications.
Ethical considerations
Before participant recruitment, this study received full ethical approval from the ethics committee of the Iran University of Medical Sciences (IUMS) (Ethics code: IR.IUMS.FMD.REC.1401.422). All procedures comply with the Declaration of Helsinki Protocols on human research. During the screening visits, eligible participants were provided with a detailed information sheet explaining the purpose, procedures, potential risks, and benefits of the study. The surgeons then allowed sufficient time for the participants to read the information sheet, ask any questions they might have, and make an informed decision. After addressing all concerns and queries, the surgeons obtained written informed consent from those willing to participate in the study. The informed consent process ensured that participants understood the study details and voluntarily agreed to participate.
Data collection and analysis
The primary data collected focused on the longitudinal measurement of Apo-B100 concentrations. Apo-B100 levels in serum were quantified using commercially available enzymatic ELISA kits (Gesan Production, Italy – Ref. code 9700150; Reference values considered between 69–105 mg/dL) at designated time points before and six months after surgery. Additionally, standard lipid profiles assessing total cholesterol, triglycerides, VLDL cholesterol, LDL cholesterol, and HDL cholesterol utilizing enzymatic assays as well as liver function tests (aspartate/alanine aminotransferase) and fasting blood sugar (FBS) through automated clinical chemistry analytics were obtained at same time intervals. All blood biochemistry tests were conducted the day before the procedures to establish baseline (preoperative) values. Subsequently, follow-up laboratory assessments were conducted six months after surgery. Standard protocols for phlebotomy techniques and serum isolation via centrifugation were followed by trained clinical staff until the final sample analysis. In addition to laboratory testing, demographic details of study participants, including age, gender, and body mass index were extracted from medical records held within the hospitals’ centralized database. Furthermore, at the 6-month postoperative follow-up interval, body mass index values were retrieved from the Iranian National Obesity and Metabolic Surgery Database (INOSD), where ongoing anthropometric data from follow-up visits are captured.
All statistical analyses were conducted using SPSS Statistics software version 27 (IBM Corp). Continuous variables were presented as mean and standard deviation. Categorical variables were presented as numbers and percentages. We first assessed the normality of all the continuous data using Smirnov-Kolmogorov test. For the primary outcome, continuous variables (serum levels of Apo-B100, fasting blood sugar, total cholesterol concentration, triglyceride, HDL, LDL, VLDL, AST, and ALT) were measured before and six months after surgery. A paired t-test was used to compare means before and after surgery for normally distributed data, while a Wilcoxon signed-rank test was used for non-normal data.
The impact of surgery type and other categorical background variables on the changes in Apo-B100 levels was assessed by the independent t-test and One-way ANOVA test. We used Pearson correlation analysis to assess the correlation between continuous variables such as age, pre-operative BMI, pre-operative blood tests, and post-operative Apo-B100 reduction. Using a multivariate analysis of covariance (MANCOVA), we assessed the effect of surgery type on Apo-B100 reduction and improvement in lipid profile, FBS, and liver function tests adjusting for potential covariates (with a p-value < 0.1). P-value < 0.05 was considered statistically significant. To address false discovery rates in multiple tests, the study employed false discovery rate corrections to ensure result reliability. A biostatistician reviewed analytic plans to ensure accuracy.
Results
Demographic characteristics
A total of 157 participants were recruited to participate in the study. We excluded 36 participants due to a lack of post-operative laboratory data, resulting in 121 participants being successfully enrolled. (Fig. 1).
The difference in baseline characteristics of the excluded cohort (n = 36) and the cohort that completed the follow-up (n = 121), such as age, frequency of each sex, and different co-morbidities was not statistically significant. (Table 1).
Enrolled participants
Of the 121 individuals who completed the six-month follow-up period, Eighty-nine were female (73.55%). The mean age was 41.48 ± 11.11 years, and the pre-operative BMI was 44.59 ± 6.60 kg/m2. Baseline demographics are shown in Table 2- Baseline demographic characteristics.
Primary outcome: Apo-B100 levels, metabolic parameters, and weight loss
The pre- and post-operative Apo-B100 levels were 94.63 ± 14.35 mg/dl and 62.97 ± 19.97 mg/dl, respectively. The distribution of pre- and post-operative serum Apo-B100 levels was normal. The serum level of Apo-B100 significantly decreased after surgery (Mean difference = 31.66 ± 22.55; 95% CI [27.60, 35.72]; p < 0.01). Specific results of changes in laboratory data post-operatively are shown in Table 3. All results obtained from blood tests after the surgery showed a decreasing trend, statistically significant, except for HDL.
The pre-operative and post-operative BMIs were also 44.59 ± 6.60 kg/m2 and 33.12 ± 4.41 kg/m2, respectively. Table 4 provides additional details of weight loss parameters.
Impact of background variables on Apo-B100 reduction
The impact of variables such as gender, T2DM status, history of hypertension, and dyslipidemia on Apo-B100 reduction was investigated. Apo-B100 reduction was not statistically different between genders. History of dyslipidemia and hypertension was not associated with changes in Apo-B100 levels. However, the difference in Apo-B100 reduction was statistically significant between groups with different T2DM status (Table 5). Using a post hoc Tukey test with pairwise comparisons, we observed a significant difference between the “no T2DM” and “T2DM” groups (Table 6). Additionally, the detailed diabetes status of participants in each surgery type is shown in Table 7.
We also assessed the impact of continuous variables such as age, pre-operative blood tests, and weight loss outcomes on the reduction of Apo-B100. (Table 8). Analysis showed that age, pre-operative BMI, FBS, Triglyceride, Cholesterol, HDL, VLDL, AST, ALT, %TWL, and %EWL did not have a significant impact on Apo-B100 reduction. Still, pre-operative Apo-B100 and LDL had a statistically significant correlation with Apo-B100 reduction.
Impact of surgery type on Apo-B100 reduction
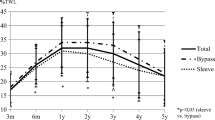
Apo-B100 reduction was not influenced by the type of surgery, even after adjusting for diabetes status, baseline APO-B100, baseline FBS, baseline HDL, and baseline LDL. Details of the relationship between the type of surgery and serum Apo-B100 and other blood tests are presented in Table 9 and Fig. 2.
Discussion
The findings from our multi-centric prospective cohort study indicate a significant decrease in Apo-B100, FBS, cholesterol, triglyceride, LDL, VLDL, AST, and ALT levels six months after MBS. In our study, we opted for a 6-month follow-up period because most substantial changes in weight, BMI, and lipid profile parameters predominantly occur within the first six months post-surgery, after which these changes tend to stabilize [31]. The decrease in Apo-B100 is a crucial indicator of the improvement in dyslipidemia and the potential reduction of atherosclerotic cardiovascular disease (ASCVD) risk [32].
It is widely recognized that obesity is a significant risk factor for developing ASCVD [33]. While MBS is considered one of the most effective treatment options for obesity. The comprehensive metabolic changes induced by MBS extend beyond weight loss, significantly impacting diabetes, hypertension, and inflammatory markers. Notably, dyslipidemia, a significant modifiable risk factor for ASCVD, shows substantial improvement or even resolution following such procedures. This surgical intervention leads to a marked reduction in visceral fat, which is closely associated with dyslipidemia and other ASCVD risk factors [34]. Moreover, MBS reduces the incidence rates of cardiovascular disease events, but not all benefits are explained by improvements in traditional ASCVD risk factors [35, 36].
Apo-B100 has emerged as a promising biomarker for ASCVD risk [37]. Unlike the standard LDL, Apo-B100 provides a direct measure of the number of atherogenic particles (including VLDL, LDL, and lipoprotein A), as each atherogenic particle contains a single molecule of Apo-B100 [32, 38]. Previous studies have shown that MBS can reduce the concentration of different ASCVD inflammatory and metabolic biomarkers (e.g., C-reactive protein, interleukin 6, monocyte chemotactic protein-1, lipoprotein A, LDL, HDL, and Apo-B100) [27, 31, 39,40,41,42].
MBS improves lipid and lipoprotein profiles through several mechanisms, but they are not fully understood. The improvements are partly due to weight loss, which enhances insulin sensitivity and glucose metabolism, subsequently affecting lipid-lipoprotein metabolism, redistributing adipose tissue from visceral to subcutaneous compartments, reducing hepatic lipid accumulation, complex endocrine and metabolic changes that affect various organs and related metabolic conditions, decreasing systemic inflammation, improving sensitivity towards lipolysis, and adipose tissue fatty acid handling [42, 43].
Heneghan et al. found that RYGB significantly lowers Apo-B100 levels and the Apo-B100/ApoA-1 ratio, potentially due to a decrease in ceramide production. These changes are associated with reduced atherosclerotic ASCVD risk. The study also indicates that the effects of RYGB on lipid metabolism may be partly independent of weight loss, possibly due to its malabsorptive aspects, highlighting the complex interplay between weight loss, lipid regulation, and ASCVD risk reduction [44]. However, another prospective study demonstrated that lifestyle modifications significantly reduced Apo-B100 levels before MBS. Although MBS did not further decrease Apo-B100, it effectively maintained these levels within the normal range. Post-surgery, the Apo-B100 levels remained lower than in the control group and after lifestyle modifications, but this difference was not statistically significant [27].
In our study, the observed significant reductions in Apo-B100 levels and subsequent improvements in lipid profiles after surgery can be attributed to a multifaceted approach encompassing the surgical procedure itself, coupled with rigorous post-operative lifestyle modifications. It is important to highlight that none of the participants were on lipid-lowering medications before or after the surgery, ensuring that the changes in lipid profiles were directly related to the intervention and not confounded by pharmacological effects. All individuals were enrolled in a comprehensive lifestyle modification program, which included nutritional consultations leading to a dietary plan with limited caloric intake, high protein, low fats and carbohydrates, and protein and vitamin supplementation if needed. Additionally, the protocol for all patients included recommendations for daily physical activity.
Generally, when comparing the effect of MBS with lifestyle interventions or pharmacotherapy on lipid-lipoprotein profile, MBS is more effective or equally effective in ameliorating lipid and lipoprotein risk factors for ASCVD [27, 45,46,47,48,49,50,51]. However, a meta-analysis by Hasan et al. elucidated that MBS, while effective, showed the least changes in lipid levels per kilogram of weight loss compared to the other methods [52]. Still, as the authors of this meta-analysis mentioned, the sustainability of weight loss is critical to achieving improvements in risk factors for ASCVD. It has been shown that MBS leads to more sustainable and durable long-term weight loss compared to lifestyle modifications and pharmacotherapy [53,54,55]. Ultimately, while MBS significantly reduces lipid-lipoprotein risk factors, thereby lowering the risk of ASCVD, the benefits of it go beyond simply managing lipids and losing weight. Studies have demonstrated that MBS also contributes to hypertension and diabetes management, decreasing systemic inflammation, and beneficial changes in cardiac function—all of which collectively contribute to a substantial decrease in the risk of cardiovascular disease and events [56,57,58,59,60].
The reduction in Apo-B100 levels was more pronounced in non-diabetics compared to individuals with diabetes. This finding underscores the complex interplay between diabetes and lipid metabolism and highlights the need for a nuanced understanding of MBS's metabolic impacts. Our finding aligns with the results reported by Iqbal et al. in their study. However, this does not mean that ASCVD risk reduction is also more pronounced in non-diabetic patients. Rather, previous reports have highlighted that reduction in overall cardiovascular risk and increase in life expectancy may be greater in patients with type 2 diabetes [36, 61, 62].
The effect of the type of surgery on Apo-B100
In our study, the type of surgery had no significant impact on the reduction of Apo-B100 levels, independent of diabetes status and other variables. Although, RYGB surgery reduced Apo-B100 levels more than OAGB and LSG, however, the difference was not statistically significant. Previous studies have reported that gastric bypass procedures are generally more effective than LSG in lowering lipid-lipoprotein profiles, and overall improvement of dyslipidemia [63,64,65,66,67,68,69,70,71]. This could be related to the malabsorption effects of gastric bypass procedures [72]. Yet, major limitations including lack of randomization, heterogeneous population, small sample sizes, and lack of long-term assessment of the Apo-B100 remain unsolved in this study and previous studies. Future research should investigate the impact of different types of MBS on the lipid-lipoprotein profile and dyslipidemia, as well as its influence on other ASCVD biomarkers.
Clinical implications
Apo-B100 is emerging as a valuable marker in cardiovascular risk assessment and management, potentially offering a more nuanced and effective approach to predicting and reducing the risk of MACE, particularly in patients with recent acute coronary syndrome and elevated atherogenic lipoproteins [73].
Ongoing concerns persist regarding the standardization of Apo-B measurements, casting doubts on its widespread clinical application. Despite these reservations, accumulating evidence has strongly established Apo-B as a superior indicator of ASCVD risk and the effectiveness of lipid-lowering interventions over traditional markers such as LDL-cholesterol (LDL-C) and non-HDL-cholesterol (non-HDL-C). Esteemed bodies, including the European Atherosclerosis Society, the European Society of Cardiology, and the American Association of Clinical Chemistry have collectively endorsed the enhanced accuracy of Apo-B measurement, especially at lower concentrations, compared to LDL-C and non-HDL-C [74]. However, the commercial availability of Apo-B testing remains limited in several regions, including Iran, which hampers its clinical utility.
Apo-B's superior association with atherosclerosis, over cholesterol concentration, is underscored by clinical trials involving statins, ezetimibe, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors [75]. These studies reveal that Apo-B levels more reliably predict coronary heart disease risk than LDL-C or non-HDL-C levels. Approximately 20% of patients exhibit discordance between Apo-B and LDL-C levels, a phenomenon observed in conditions like hypertriglyceridemia, T2DM, or obesity [76]. In such cases, LDL-C levels may appear within acceptable ranges, yet atherogenic particle concentration remains elevated, suggesting a higher cardiovascular disease risk. This observation advocates for the consideration of lipid-lowering treatments in patients with elevated Apo-B levels [77].
With evolving clinical evidence, various guidelines have converged on the significance of Apo-B in cardiovascular risk assessment and management. The American Heart Association (AHA) has highlighted Apo-B as a critical marker for ASCVD risk and considers Apo-B levels greater than or equal to 130mg/dL as an ASCVD risk enhancer. This association becomes particularly relevant for individuals with triglyceride levels ≥ 200 mg/dL [78]. Similarly, the 2021 Canadian Cardiovascular Society guidelines on dyslipidemia advocate for the use of either non-HDL-C or Apo-B levels over LDL-C for both screening and therapeutic targeting [79]. The 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for dyslipidemia management recommend Apo-B analysis for risk assessment, especially in individuals with high triglyceride levels, diabetes, obesity, or very low LDL-C. These guidelines further articulate treatment goals for Apo-B, setting targets of < 65 mg/dL for very high-risk individuals, < 80 mg/dL for those at high risk, and < 100 mg/dL for patients at moderate risk [80].
Although Apo-B100 levels play a critical role in atherosclerosis, they are rarely reported in clinical trials for MBS [81]. Further research is necessary to investigate the impact of MBS on Apo-B100 and its relation to ASCVD risk and cardiovascular events.
Limitations
This study has several key limitations. Firstly, the lack of randomization and a control group limits the ability to establish causality and compare the surgery's effects against other weight loss methods. Secondly, the short-term follow-up period of six months may not adequately capture the long-term outcomes of the surgery on Apo-B100 levels and ASCVD risk factors. Thirdly, the final sample size of 121 participants, reduced from the initial 157 due to dropouts, and the small size of RYGB group (n = 8) may affect the generalizability of the results and indicate potential selection bias. Lastly, there could be unmeasured confounding variables not accounted for in the study, which could influence the outcomes and the interpretation of the impact of MBS on Apo-B100 levels. Given the current design and scope of our study, quantifying the respective contributions of MBS and lifestyle modifications to the reduction in Apo-B100 levels remains challenging. Both elements are likely to have played a role in the metabolic improvements; however, without a control group undergoing lifestyle changes without surgery, delineating their impacts is not feasible. Future research is necessary to distinguish the effects of surgical intervention from lifestyle modifications on lipid profiles, specifically Apo-B100 levels.
Conclusion
In conclusion, the study demonstrates a significant decrease in Apo-B100 levels, alongside improvements in lipid profiles and liver enzymes, six months post-MBS. These findings highlight the potential of MBS in positively impacting dyslipidemia and reducing cardiovascular disease risk in individuals with high BMI.
Data Availability
The data that supports the findings of this study are available on request. Contact the corresponding author in case of necessity.
References
Li Q, Blume SW, Huang JC, Hammer M, Ganz ML. Prevalence and healthcare costs of obesity-related comorbidities: evidence from an electronic medical records system in the United States. J Med Econ. 2015;18:1020–8.
Powell-Wiley TM, Poirier P, Burke LE, Després J-P, Gordon-Larsen P, Lavie CJ, et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation [Internet]. 2021 [cited 2023 Dec 11];143. Available from: https://www.ahajournals.org/doi/https://doi.org/10.1161/CIR.0000000000000973.
Maggio CA, Pi-Sunyer FX. Obesity and type 2 diabetes. Endocrinol Metab Clin North Am. 2003;32:805–22.
Antza C, Kostopoulos G, Mostafa S, Nirantharakumar K, Tahrani A. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2022;252:125–41.
Yücel KB, Aydos U, Sütcüoglu O, Kılıç ACK, Özdemir N, Özet A, et al. Visceral obesity and sarcopenia as predictors of efficacy and hematological toxicity in patients with metastatic breast cancer treated with CDK 4/6 inhibitors. Cancer Chemother Pharmacol [Internet]. 2024 [cited 2024 Mar 12]; Available from: https://springerlink.bibliotecabuap.elogim.com/https://doi.org/10.1007/s00280-024-04641-z
Gałązka JK, Czeczelewski M, Kucharczyk T, Szklener K, Mańdziuk S. Obesity and lung cancer – is programmed death ligand-1 (PD-1L) expression a connection? Arch Med Sci. 2024;20:313–6.
Behrooz AB, Cordani M, Fiore A, Donadelli M, Gordon JW, Klionsky DJ, et al. The obesity-autophagy-cancer axis: Mechanistic insights and therapeutic perspectives. Semin Cancer Biol. 2024;99:24–44.
Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22:63.
Jang S, Lee K, Ju JH. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. IJMS. 2021;22:2619.
Morandini HAE, Watson P, Stewart RM, Wong JWY, Rao P, Zepf FD. Implication of saturated fats in the aetiology of childhood attention deficit/hyperactivity disorder – A narrative review. Clinical Nutr ESPEN. 2022;52:78–85.
Vekic J, Zeljkovic A, Stefanovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. Obesity and dyslipidemia. Metabolism. 2019;92:71–81.
Drozdz D, Alvarez-Pitti J, Wójcik M, Borghi C, Gabbianelli R, Mazur A, et al. Obesity and Cardiometabolic Risk Factors: From Childhood to Adulthood. Nutrients. 2021;13:4176.
Franssen R, Monajemi H, Stroes ESG, Kastelein JJP. Obesity and Dyslipidemia. Med Clin North Am. 2011;95:893–902.
Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol-Endocrinol Metab. 2009;297:E271–88.
Au DT, Strickland DK, Muratoglu SC. The LDL Receptor-Related Protein 1: At the Crossroads of Lipoprotein Metabolism and Insulin Signaling. J Diabetes Res. 2017;2017:1–10.
Nilsson J, Björkbacka H, Fredrikson GN. Apolipoprotein B100 autoimmunity and atherosclerosis – disease mechanisms and therapeutic potential. Curr Opin Lipidol. 2012;23:422–8.
Skålén K, Gustafsson M, Rydberg EK, Hultén LM, Wiklund O, Innerarity TL, et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–4.
Morita S. Metabolism and Modification of Apolipoprotein B-Containing Lipoproteins Involved in Dyslipidemia and Atherosclerosis. Biol Pharm Bull. 2016;39:1–24.
LaFramboise WA, Dhir R, Kelly LA, Petrosko P, Krill-Burger JM, Sciulli CM, et al. Serum protein profiles predict coronary artery disease in symptomatic patients referred for coronary angiography. BMC Med. 2012;10:157.
Rutledge AC, Su Q, Adeli K. Apolipoprotein B100 biogenesis: a complex array of intracellular mechanisms regulating folding, stability, and lipoprotein assemblyThis paper is one of a selection of papers published in this special issue entitled “Canadian Society of Biochemistry, Molecular & Cellular Biology 52nd Annual Meeting — Protein Folding: Principles and Diseases” and has undergone the Journal’s usual peer review process. Biochem Cell Biol. 2010;88:251–67.
Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res. 2016;57:1339–59.
Matveyenko A, Seid H, Kim K, Ramakrishnan R, Thomas T, Matienzo N, et al. Association of free-living diet composition with plasma lipoprotein(a) levels in healthy adults. Lipids Health Dis. 2023;22:144.
Hsu JL, Farrell TM. Updates in bariatric surgery. Am Surg. 2024;90(5):925–33. https://doi.org/10.1177/00031348231220576.
Železnik U, Kokol P, Starc J, Železnik D, Završnik J, Vošner HB. Research Trends in Motivation and Weight Loss: A Bibliometric-Based Review. Healthcare. 2023;11:3086.
Esparham A, Roohi S, Ahmadyar S, Dalili A, Moghadam HA, Torres AJ, et al. The Efficacy and Safety of Laparoscopic Single-Anastomosis Duodeno-ileostomy with Sleeve Gastrectomy (SADI-S) in Mid- and Long-Term Follow-Up: a Systematic Review. Obes Surg. 2023;33:4070–9.
Kermansaravi M, Shahmiri SS, Khalaj A, Jalali SM, Amini M, Alamdari NM, Mahmoudieh M, Jangjoo A, Abbas SI, Naeini SMM, Sayadishahraki M, Eghbali F, Mirhashemi SH, Mokhber S, Jazi AD, Pazouki A. The First Web-Based Iranian National Obesity and Metabolic Surgery Database (INOSD). Obes Surg. 2022;32(6):2083–6. https://doi.org/10.1007/s11695-022-06014-y.
Kjellmo CA, Karlsson H, Nestvold TK, Ljunggren S, Cederbrant K, Marcusson-Ståhl M, et al. Bariatric surgery improves lipoprotein profile in morbidly obese patients by reducing LDL cholesterol, apoB, and SAA/PON1 ratio, increasing HDL cholesterol, but has no effect on cholesterol efflux capacity. J Clin Lipidol. 2018;12:193–202.
Angrisani L, Santonicola A, Iovino P, Palma R, Kow L, Prager G, et al. IFSO Worldwide Survey 2020–2021: Current Trends for Bariatric and Metabolic Procedures. OBES SURG [Internet]. 2024 [cited 2024 Mar 7]; Available from: https://springerlink.bibliotecabuap.elogim.com/https://doi.org/10.1007/s11695-024-07118-3.
Salminen P, Kow L, Aminian A, Kaplan LM, Nimeri A, Prager G, et al. IFSO Consensus on Definitions and Clinical Practice Guidelines for Obesity Management—an International Delphi Study. Obes Surg. 2024;34:30–42.
Sharaiha RZ, Shikora S, White KP, Macedo G, Toouli J, Kow L. Summarizing Consensus Guidelines on Obesity Management: A Joint, Multidisciplinary Venture of the International Federation for the Surgery of Obesity & Metabolic Disorders (IFSO) and World Gastroenterology Organisation (WGO). J Clin Gastroenterol. 2023;57:967–76.
Yadav R, Hama S, Liu Y, Siahmansur T, Schofield J, Syed AA, et al. Effect of Roux-en-Y Bariatric Surgery on Lipoproteins, Insulin Resistance, and Systemic and Vascular Inflammation in Obesity and Diabetes. Front Immunol. 2017;8:1512.
Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, et al. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019;4:1287.
Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77.
English WJ, Spann MD, Aher CV, Williams DB. Cardiovascular risk reduction following metabolic and bariatric surgery. Ann Transl Med. 2020;8:S12–S12.
Sjöström L. Review of the key results from the Swedish Obese Subjects ( SOS ) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–34.
Iqbal Z, Bashir B, Adam S, Ho JH, Dhage S, Azmi S, et al. Glycated apolipoprotein B decreases after bariatric surgery in people with and without diabetes: A potential contribution to reduction in cardiovascular risk. Atherosclerosis. 2022;346:10–7.
Dhingra R, Vasan RS. Biomarkers in cardiovascular disease: Statistical assessment and section on key novel heart failure biomarkers. Trends Cardiovasc Med. 2017;27:123–33.
Behbodikhah J, Ahmed S, Elyasi A, Kasselman LJ, De Leon J, Glass AD, et al. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites. 2021;11:690.
Illán-Gómez F, Gonzálvez-Ortega M, Orea-Soler I, MaS Alcaraz-Tafalla, Aragón-Alonso A, Pascual-Díaz M, et al. Obesity and Inflammation: Change in Adiponectin, C-Reactive Protein, Tumour Necrosis Factor-Alpha and Interleukin-6 After Bariatric Surgery. Obes Surg. 2012;22:950–5.
Casimiro I, Hanlon EC, White J, De Leon A, Ross R, Moise K, et al. Reduction of IL-6 gene expression in human adipose tissue after sleeve gastrectomy surgery. Obes Sci Pract. 2020;6:215–24.
Jamialahmadi T, Abbasifard M, Reiner Ž, Kesharwani P, Sahebkar A. The Effect of Bariatric Surgery on Circulating Levels of Monocyte Chemoattractant Protein-1: A Systematic Review and Meta-Analysis. JCM. 2022;11:7021.
Piché M-E, Tardif I, Auclair A, Poirier P. Effects of bariatric surgery on lipid-lipoprotein profile. Metabolism. 2021;115: 154441.
Bays HE, Jones PH, Jacobson TA, Cohen DE, Orringer CE, Kothari S, et al. Lipids and bariatric procedures part 1 of 2: Scientific statement from the National Lipid Association, American Society for Metabolic and Bariatric Surgery, and Obesity Medicine Association: FULL REPORT. J Clin Lipidol. 2016;10:33–57.
Heneghan HM, Huang H, Kashyap SR, Gornik HL, McCullough AJ, Schauer PR, et al. Reduced cardiovascular risk after bariatric surgery is linked to plasma ceramides, apolipoprotein-B100, and ApoB100/A1 ratio. Surg Obes Relat Dis. 2013;9:100–7.
Ikramuddin S, Korner J, Lee W-J, Thomas AJ, Connett JE, Bantle JP, et al. Lifestyle Intervention and Medical Management With vs Without Roux-en-Y Gastric Bypass and Control of Hemoglobin A 1c, LDL Cholesterol, and Systolic Blood Pressure at 5 Years in the Diabetes Surgery Study. JAMA. 2018;319:266.
Berk KA, Borgeraas H, Narverud I, Mulder MT, Øyri LKL, Verhoeven AJM, et al. Differential effects of bariatric surgery and lifestyle interventions on plasma levels of Lp(a) and fatty acids. Lipids Health Dis. 2022;21:145.
Courcoulas AP, Gallagher JW, Neiberg RH, Eagleton EB, DeLany JP, Lang W, et al. Bariatric Surgery vs Lifestyle Intervention for Diabetes Treatment: 5-Year Outcomes From a Randomized Trial. J Clin Endocrinol Metab. 2020;105:866–76.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes — 5-Year Outcomes. N Engl J Med. 2017;376:641–51.
Cummings DE, Arterburn DE, Westbrook EO, Kuzma JN, Stewart SD, Chan CP, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia. 2016;59:945–53.
Courcoulas AP, Belle SH, Neiberg RH, Pierson SK, Eagleton JK, Kalarchian MA, et al. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. JAMA Surg. 2015;150:931.
Ho JH, Adam S, Liu Y, Azmi S, Dhage S, Syed AA, et al. Effect of bariatric surgery on plasma levels of oxidised phospholipids, biomarkers of oxidised LDL and lipoprotein(a). J Clin Lipidol. 2021;15:320–31.
Hasan B, Nayfeh T, Alzuabi M, Wang Z, Kuchkuntla AR, Prokop LJ, et al. Weight Loss and Serum Lipids in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2020;105:3695–703.
Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS, Weidenbacher HJ, et al. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg. 2016;151:1046.
Arterburn DE, Johnson E, Coleman KJ, Herrinton LJ, Courcoulas AP, Fisher D, et al. Weight Outcomes of Sleeve Gastrectomy and Gastric Bypass Compared to Nonsurgical Treatment. Ann Surg. 2021;274:e1269–76.
Slomski A. Weight Loss Is Still Substantial a Decade After Bariatric Surgery. JAMA. 2022;328:415.
Van Veldhuisen SL, Gorter TM, Van Woerden G, De Boer RA, Rienstra M, Hazebroek EJ, et al. Bariatric surgery and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2022;43:1955–69.
Benraouane F, Litwin SE. Reductions in cardiovascular risk after bariatric surgery. Curr Opin Cardiol. 2011;26:555–61.
Doumouras AG, Wong JA, Paterson JM, Lee Y, Sivapathasundaram B, Tarride J-E, et al. Bariatric Surgery and Cardiovascular Outcomes in Patients With Obesity and Cardiovascular Disease: A Population-Based Retrospective Cohort Study. Circulation. 2021;143:1468–80.
Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, et al. Bariatric Surgery and Long-term Cardiovascular Events. JAMA. 2012;307:56.
Bottino R, Carbone A, Formisano T, D’Elia S, Orlandi M, Sperlongano S, et al. Cardiovascular Effects of Weight Loss in Obese Patients with Diabetes: Is Bariatric Surgery the Additional Arrow in the Quiver? Life. 2023;13:1552.
Wei J-H, Lee M-H, Lee W-J, Chen S-C, Almalki OM, Chen J-C, et al. Change of cardiovascular risk associated serologic biomarkers after gastric bypass: A comparison of diabetic and non-diabetic Asian patients. Asian J Surg. 2022;45:2253–8.
Syn NL, Cummings DE, Wang LZ, Lin DJ, Zhao JJ, Loh M, et al. Association of metabolic–bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021;397:1830–41.
Maraninchi M, Padilla N, Béliard S, Berthet B, Nogueira J-P, Dupont-Roussel J, et al. Impact of bariatric surgery on apolipoprotein C-III levels and lipoprotein distribution in obese human subjects. J Clin Lipidol. 2017;11:495-506.e3.
Gómez-Martin JM, Balsa JA, Aracil E, Cuadrado-Ayuso M, Rosillo M, De La Peña G, et al. Beneficial changes on plasma apolipoproteins A and B, high density lipoproteins and oxidized low density lipoproteins in obese women after bariatric surgery: comparison between gastric bypass and sleeve gastrectomy. Lipids Health Dis. 2018;17:145.
Gomes-Rocha SR, Costa-Pinho AM, Pais-Neto CC, De Araújo PA, Nogueiro JPM, Carneiro SPR, et al. Roux-en-Y Gastric Bypass Vs Sleeve Gastrectomy in Super Obesity: a Systematic Review and Meta-Analysis. Obes Surg. 2022;32:170–85.
Coleman KJ, Basu A, Barton LJ, Fischer H, Arterburn DE, Barthold D, et al. Remission and Relapse of Dyslipidemia After Vertical Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass in a Racially and Ethnically Diverse Population. JAMA Netw Open. 2022;5: e2233843.
Lobo LM, Nogueira JP, Clos C, Masson W, Molinero G, Lavalle Cobo A, et al. Effectiveness of roux-en-Y gastric bypass vs sleeve gastrectomy on lipid levels in type 2 diabetes: a meta-analysis. Eur Heart J. 2022;43:ehac544.2345.
Han Y, Jia Y, Wang H, Cao L, Zhao Y. Comparative analysis of weight loss and resolution of comorbidities between laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: A systematic review and meta-analysis based on 18 studies. Int J Surg. 2020;76:101–10.
Aaseth JO, Rootwelt H, Retterstøl K, Hestad K, Farup PG. Circulating Lipoproteins in Subjects with Morbid Obesity Undergoing Bariatric Surgery with Gastric Bypass or Sleeve Gastrectomy. Nutrients. 2022;14:2381.
Khalaj A, Tasdighi E, Hosseinpanah F, Mahdavi M, Valizadeh M, Farahmand E, et al. Two-year outcomes of sleeve gastrectomy versus gastric bypass: first report based on Tehran obesity treatment study (TOTS). BMC Surg. 2020;20:160.
Bettini S, Segato G, Prevedello L, Fabris R, Prà CD, Zabeo E, et al. Improvement of Lipid Profile after One-Anastomosis Gastric Bypass Compared to Sleeve Gastrectomy. Nutrients. 2021;13:2770.
Al Khalifa K, Al Ansari A, Alsayed AR, Violato C. The Impact of Sleeve Gastrectomy on Hyperlipidemia: A Systematic Review. J Obes. 2013;2013:1–7.
Elshazly MB, Quispe R. The Lower the ApoB, the Better: Now, How Does ApoB Fit in the Upcoming Era of Targeted Therapeutics? Circulation. 2022;146:673–5.
Contois JH, Langlois MR, Cobbaert C, Sniderman AD. Standardization of Apolipoprotein B, LDL-Cholesterol, and Non-HDL-Cholesterol. JAHA. 2023;12: e030405.
Marston NA, Giugliano RP, Melloni GEM, Park J-G, Morrill V, Blazing MA, et al. Association of Apolipoprotein B-Containing Lipoproteins and Risk of Myocardial Infarction in Individuals With and Without Atherosclerosis: Distinguishing Between Particle Concentration, Type, and Content. JAMA Cardiol. 2022;7:250.
Lawler PR, Akinkuolie AO, Ridker PM, Sniderman AD, Buring JE, Glynn RJ, et al. Discordance between Circulating Atherogenic Cholesterol Mass and Lipoprotein Particle Concentration in Relation to Future Coronary Events in Women. Clin Chem. 2017;63:870–9.
Ahmad M, Sniderman AD, Hegele RA. Apolipoprotein B in cardiovascular risk assessment. CMAJ. 2023;195:E1124.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation [Internet]. 2019 [cited 2024 Apr 3];140. Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000677.
Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can J Cardiol. 2021;37:1129–50.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88.
Bays H, Kothari SN, Azagury DE, Morton JM, Nguyen NT, Jones PH, et al. Lipids and bariatric procedures Part 2 of 2: scientific statement from the American Society for Metabolic and Bariatric Surgery (ASMBS), the National Lipid Association (NLA), and Obesity Medicine Association (OMA). Surg Obes Related Dis. 2016;12:468–95.
Acknowledgements
The authors declare that there are no conflicts of interest related to the content of this manuscript. All authors have provided explicit disclosure of any potential conflicts, that could influence the interpretation of the data or the presentation of information. this study received no financial support from any external sources. The research was conducted independently, and the authors did not receive funding or sponsorship that could have influenced the outcomes or conclusions drawn from the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest related to the content of this manuscript. All authors have provided explicit disclosure of any potential conflicts, that could influence the interpretation of the data or the presentation of information.
Ali Jaliliyan: “No Conflict of interests”, Ahmad Madankan: “No Conflict of interests”, Hesam Mosavari: “No Conflict of interests”, Pantea Khalili: “No Conflict of interests”, Bahador Pouraskari: “No Conflict of interests”, Saeed Lotfi: “No Conflict of interests”, Andia Honarfar: “No Conflict of interests”, Elham Fakhri: “No Conflict of interests”, Foolad Eghbali: “No Conflict of interests”.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Research Ethics Committees of Iran University of Medical Sciences and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Keypoints
• Serum Apo-B100 concentration decreases significantly after metabolic and bariatric surgery.
• Apo-B100 reduction in individuals with diabetes is significantly lower than in non-diabetics.
• Surgery type does not impact the Apo-B100 reduction after metabolic and bariatric surgery.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jaliliyan, A., Madankan, A., Mosavari, H. et al. The Impact of Metabolic and Bariatric Surgery on Apo B100 Levels in Individuals with high BMI: A Multi-Centric Prospective Cohort Study. OBES SURG 34, 2454–2466 (2024). https://doi.org/10.1007/s11695-024-07258-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-024-07258-6