Abstract
Background
Obesity is a well-known risk factor for gastroesophageal reflux disease (GERD). Even though symptoms may be mitigated or resolved with the weight loss caused by sleeve gastrectomy (SG), it may be associated with higher incidences of postoperative GERD. Ligamentum teres cardiopexy (LTC) is an alternative to Roux-en-Y gastric bypass, the gold standard treatment for GERD.
Methods
This study was a retrospective single-center chart review, all patients in this cohort underwent LTC to treat refractory GERD at our institution. The option for LTC was presented after patients’ refusal to undergo RYGB conversion. We collected baseline characteristics, standard demographics, pre-operative tests and imaging, and SG information, as well as intraoperative and perioperative data regarding LTC, and postoperative complications.
Results
Our cohort included 29 patients; most were Caucasian (44.8%) females (86.2%). The mean weight and BMI before LTC were 216.5 ± 39.3 lb and 36.1 ± 5.4 kg/m2, respectively. Mean total body-weight loss (TBWL) at 12 and 24 months were 28.7% ± 9.5% and 28.4% ± 12.4%, respectively. The mean interval between the index bariatric surgery and LTC was 59.9 ± 34.9 months, mean operative time was 67 ± 18.2 min, and median length of stay (LOS) was 1 day (IQR = 1–2 days). Twelve patients (57.1%) were able to discontinue antisecretory medications, while 9 (42.9%) still required them to remain asymptomatic. Mortality and reoperation rates were 0% and the incidence of complication was 19.4% (n = 6).
Conclusions
LTC is a safe and effective surgical alternative to treat refractory GERD symptoms after SG.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastroesophageal reflux disease (GERD) is associated with obesity; it is estimated that its prevalence reaches up to 15.4% of the population in North America, and that the annual health care costs reach up to $10 billion in the USA alone [1]. Even though lifestyle modifications and antisecretory medications may be sufficient to manage symptoms, in a subset of patients, Roux-en-Y gastric bypass may be the last alternative, remaining the gold standard and considered the ideal anti-reflux procedure for obese patients [2]. The mechanisms that cause obesity-related GERD are not completely understood. It is postulated that an increased intra-abdominal pressure may induce greater reflux of gastric content through an incompetent lower esophageal sphincter (LES). Another explanation includes reduced parasympathetic activity in the bariatric population, the possible missing link between obesity and GERD [3].
In the context of increasing popularity of sleeve gastrectomy (SG) for weight loss and obesity-related comorbidity management, the issue of reflux gains new importance. It is estimated that half of all bariatric surgical patients exhibit GERD symptoms at baseline, with 25% meeting the criteria for severe GERD [4]. Paradoxically, according to the recent literature, despite weight loss, an increase in reflux symptoms can occur in the first year after SG, with a gradual improvement in the subsequent years [5]. According to recent data, new onset of GERD after SG is detected in approximately 10% of patients; however, the incidence of worsening pre-existing GERD or the development of novel symptoms may exceed that [4].
The pathophysiological mechanisms involved in GERD after SG have been studied and include the modification of the angle of His, increased intraluminal pressure associated to lower LES tone, progression of a hiatal hernia, and narrowing at the junction of the vertical and horizontal portions of the sleeve [6]. Regardless of the mechanism, a parcel of patients may have their symptoms controlled pharmacologically. When they fail medical management, endoscopic and surgical approaches present as alternatives. Part of the routine follow-up after bariatric surgery includes detecting symptoms of obesity-related intractable reflux as well as de novo reflux after SG, for which conversion to RYGB is the ultimate resource. Nonetheless, the intestinal rearrangement inevitably increases surgical risk and contributes to the ongoing pursuit of safe and effective alternatives. One alternative includes using the ligamentum teres hepatis (round ligament of the liver), the remnant of the umbilical vein, as a “sling” around the LES to calibrate the pressure and mitigate symptoms of reflux, as initially described by Rampal et al. in 1964 [7]. Our experience on ligamentum teres cardiopexy (LTC) is described in a retrospective review, exploring the safety and effectiveness entailed by this approach.
Materials and Methods
The Committee for Research and Ethics approved the protocol. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee together with the 1964 Helsinki Declaration and its later amendments. This study was a retrospective single-center chart review; all data was obtained from prospectively maintained databases. Inclusion criteria encompassed previous SG, and persistent symptoms after pharmacological management failure; patients who did not return for follow-up visits were excluded. All patients in this cohort were adults who underwent LTC to treat refractory GERD at our institution from January 2018 to December 2021.
Refractory cases were defined by pharmacological treatment failure despite optimal proton pump inhibitor (PPI) and/or H2 blocker dosage. Surgical alternatives were discussed between patients and surgeons in preoperative office visits; the option for LTC was presented after patients’ refusal to undergo RYGB conversion, with their full comprehension and informed consent. Preoperative workup included upper gastrointestinal series (UGI) with barium, esophagogastroduodenoscopy (EGD), and/or CT imaging. We collected baseline characteristics, standard demographics, pre-operative tests and imaging, and SG information, as well as intraoperative and perioperative data regarding LTC, and postoperative complications. After surgery, patients were scheduled for follow-up visits, when anthropometric and clinical data were gathered, at 1 week; 1, 3, and 6 months; and annually thereafter. Statistical analysis was performed using means/median and standard deviations/IQR, using IBM Statistical Package for Social Science (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp).
Operative Details
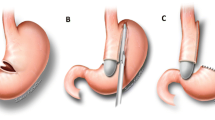
Access to the abdominal cavity was achieved using five trocars located at the midline oriented horizontally and spaced 6–8 cm apart as follows: above the umbilicus, midclavicular line in the bilateral lower quadrants, subcostal region midaxillary line bilaterally, and in the subxiphoid region. Adhesions from previous surgery were lysed and the left and right crus were exposed posterior to the sleeve. After the phreno-esophageal ligament was identified, a mediastinal circumferential dissection of the esophagus was carried out, allowing 3–4 cm of intraabdominal esophagus without tension. Special care in this step was taken to avoid a posterior vagus nerve injury. The ligamentum teres was carefully dissected from the abdominal wall towards the interlobar fissure to preserve its blood supply (Fig. 1a), passed through a window created behind the esophagus, wrapped around the gastro-esophageal junction (Fig. 1b), and finally sutured to itself in a 360-degree fashion; the ligament was also secured to the right crus and to the stomach to prevent herniation of the stomach (Fig. 1c).
Results
Our cohort included 29 patients; of those, 27 underwent SG and 2 underwent SG plus second-stage duodenal switch. All of them received antisecretory medication prior to LTC. Patients’ demographics and mean interval between SG and LTC can be seen in Table 1. Most patients in our cohort were Caucasian (44.8%) females (86.2%). The mean weight and BMI before LTC were 216.5 ± 39.3 lb and 36.1 ± 5.4 kg/m2, respectively.
Mean total body-weight loss (TBWL) at 12 and 24 months were 28.7% ± 9.5 and 28.4% ± 12.4 respectively. At the time of SG, 44.8% (n = 13) of patients were taking PPI and/or H2 blocker for a previously diagnosed GERD; the remaining patients had a median of 3 months (IQR = 47) between SG and the initial diagnosis of GERD; 63.6% of those patients started with symptoms in the first 12 months. Before LTC, patients with GERD diagnosed post SG underwent a pharmacological treatment for a mean period of 42.85 ± 25.3 months. Most patients (51.7%; n = 15) underwent preoperative upper gastrointestinal (UGI) series that achieved inconsistent results, ranging from mild to severe reflux; 26.3% of our cohort underwent an esophagogastroduodenoscopy (EGD) that also evidenced inconsistent findings that encompassed findings that went from no esophagitis and no spontaneous reflux to small volume gastroesophageal reflux. Postoperatively, 68% (n = 13) of patients underwent either EGD or UGI series; 2 patients (10.5%) still presented severe reflux and 1 (5.25%) mild reflux. The remaining of the cohort (32%) was clinically assessed for GERD resolution.
The mean interval between the index bariatric surgery and LTC was 59.9 ± 34.9 months, mean operative time was 67 ± 18.2 min, and median length of stay (LOS) was 1 day (IQR = 1–2 days). Follow-up was possible in all 29 cases, with a mean period of 17.3 ± 11.1 months. At their last visit, 21 patients (72.4%) stated that their symptoms completely subsided. Twelve patients (57.1%) were able to discontinue antisecretory medications, while 9 (42.9%) still required them to remain asymptomatic. Eight patients (31.6%) remained symptomatic despite surgical and pharmacological treatments, 7 of which endorsed symptom improvement, while one had persistent and severe symptoms (Fig. 2).
Most procedures (96.8%) were performed robotically; only one case was performed laparoscopically. Mortality and reoperation rates were 0%, and the incidence of complication was 19.4% (n = 6), as shown in Table 2. In our cohort, one patient had early complications on the second postoperative day: the patient was seen in the emergency department due to chest pain and shortness of breath and was discharged 3 h later after symptoms spontaneously subsided. Four patients presented with dysphagia between 1 and 12 months after surgery and underwent EGD and balloon dilation due to stricture at the GE junction. One patient underwent RYGB conversion after 10 months due to refractory and severe reflux. Two patients were excluded from the study, one for loss of follow-up, and the second for death unrelated to LTC.
Discussion
Obesity is a well-known risk factor for GERD, with a reported incidence of up to 73% of bariatric surgery candidates. The pathophysiological mechanisms encompass increased intra-abdominal pressure, impaired gastric emptying, decreased LES pressure, and more frequent transient LES relaxation [8]. Obesity-related GERD symptoms may be mitigated or resolved with the weight loss caused bariatric surgery; nevertheless, patients who undergo SG deserve further investigation as it may be associated with higher incidences of postoperative GERD, as well as de novo symptoms, up to 78% and 22%, respectively, according to Hawasli et al. [9]. Sleeve gastrectomy currently stands as the most-performed procedure in the bariatric surgery armamentarium, accounting for 61% of all surgeries [10]. The short- and mid-term outcomes associated with a lower technical complexity and excellent safety profile may explain its growing popularity [11, 12]. The presence of preoperative GERD symptoms can serve as a predictor of greater need for PPI usage after SG [13]. Bou Daher et al. explored the association between the severity of preoperative GERD symptoms and their postoperative resolution, concluding that those with severe reflux and erosive disease appear to have a higher probability of persistent GERD [8]. Even though patients’ symptoms were the most important variable in GERD management, 78% of our cohort underwent either EGD or UGI series prior to LTC as suggested by a recent expert’s consensus. In the same study specialists agree that, in spite of test results, a conservative treatment option based on patient symptoms and severity of GERD plays an important role prior to surgery [14].
The gold standard surgical option to mitigate post-sleeve reflux has been conversion to RYGB, with a resolution rate close to 80% [15]. Due to its additional surgical risks and added malabsorptive component, other alternatives such as LTC were pursued. Since its first description, the laparoscopic and robotic LTC became a surgical option to manage reflux and decrease the risk of Barrett’s esophagus in patients who previously underwent SG. The procedure works like a floating anchor that moves along with the respiratory cycle below the level of the esophageal hiatus. This system maintains the angle of His, pulling the GEJ forward, downward, and toward the right, reproducing the physiological work of the gastroesophageal sphincter [7, 16].
In the present study, symptom resolution was achieved in 57.1% of patients in the absence of antisecretory medication; this is lower than the 86.6% and 80% resolution rates published by Gálvez-Valdovinos et al. and by Hakwasli et al., respectively. Although those rates are comparable to the resolution rates achieved by RYGB, authors believe that the size of the cohorts and the severity of esophagitis prior to LTC may partially explain these encouraging results [17]. In a similar study, Mackey et al. achieved results comparable to ours, with 56% of patients discontinuing medications altogether. The similarity in symptom resolution was paired with similar reoperation rates; while we achieved 0% reoperation with a 360-degree wrap, they recorded a 6.7% rate, leading their authors to adjust their technique to a 270-degree wrap to avoid new reoperations. The rationale for this decision includes adequate reflux control, with less risk of excessive restriction or esophageal obstruction provided by a 270-degree wrap, while a 360-degree wrap was perceived as too restrictive, not allowing for GEJ distension with food boluses [18].
Our cohort included patients that, despite a mean TBWL of 28.7% and 28.45% at 12- and 24-month assessments, remained symptomatic. The higher BMI of our patients prior to LTC when compared to other cohorts may have been a contributing factor for our lower resolution rates [9, 16]. While 57.1% of our cohort remained asymptomatic in the absence of antisecretory medication after a mean follow-up period of 17.3 months, relevant data has been published on symptom recurrence after initial improvement [19]. The etiology of this finding is multifactorial; it is postulated that it may be greatly impacted by a reduction in the LES pressure, possibly caused by the division of the phrenoesophageal ligaments and blunting of the angle of His [20].
Ligamentum teres cardiopexy is a safe alternative for patients with a prior SG and refractory GERD. Before considering, TLC patients received pharmacological treatment for mean period of 44.27 ± 26.8 months, aligned with experts that believe in a prior minimum of a 12-month medical and supportive management [14]. The median LOS (1 day) in our cohort was similar to the results published by Mackey et al. (1.3 days), with the same mortality rate of 0%, and lower reoperation rate (3.4% vs 0%) [17]. The mean operative time of 67 min was comparable to the results published by a similar study, where it was also associated with low early complication rates [16]. The cause of the early complication included in this study was thoroughly investigated in the emergency department; after 3 h, symptoms subsided, and the patient was discharged without a diagnosis (acute myocardial infarction, GE obstruction and perforation, hemorrhage, and sepsis were ruled out). The remaining five patients presented with late complications; four of them underwent EGD and balloon dilation due to dysphagia caused by GE stricture/increased GE pressure, after which symptoms resolved. Only one patient in our cohort underwent conversion to RYGB due to refractory and severe GERD despite LTC plus optimized antisecretory regimen, the outcome also published in a recent systematic search [21]. Although EGD or UGI series have been performed in 68% of patients to assess GERD resolution, due to the inconsistency of results, remission was evaluated mainly using symptomatology.
One limitation of this study, despite being the second largest cohort available in the current literature, is the small sample size. The descriptive nature of the study does not allow any group comparisons and does not permit definitive conclusions. Pre- and post-operative tests were not standardized to confirm or reject LTC effectiveness, and authors relied on symptom resolution and antisecretory medication discontinuation to determine improvement. The insufficient data on this topic magnifies the importance of our contribution while encouraging further studies with greater samples and control groups.
Conclusion
Ligamentum teres cardiopexy is an underutilized surgical alternative to treat refractory GERD symptoms in patients who decline conversion to RYGB. The symptom improvement and resolution rates point to an effective procedure, while the complication and mortality rates attest to its safety. The laparoscopic and robotic approaches allow a reduced operative time and length of stay. The lack of alternatives to treat refractory GERD after SG shines a new light on this old procedure, paving the way for surgeons to strengthen the level of evidence and to seek new therapeutic options.
References
Fass R. Gastroesophageal reflux disease. N Engl J Med. 2022;387(13):1207–16. https://doi.org/10.1056/NEJMcp2114026.
Mahawar KK, Jennings N, Balupuri S, et al. Sleeve gastrectomy and gastro-oesophageal reflux disease: a complex relationship. Obes Surg. 2013;23(7):987–91. https://doi.org/10.1007/s11695-013-0899-x.
Melissas J, Braghetto I, Molina JC, et al. Gastroesophageal reflux disease and sleeve gastrectomy. Obes Surg. 2015;25(12):2430–5. https://doi.org/10.1007/s11695-015-1906-1.
DuPree CE, Blair K, Steele SR, et al. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: a national analysis. JAMA Surg. 2014;149(4):328–34. https://doi.org/10.1001/jamasurg.2013.4323.
Lazoura O, Zacharoulis D, Triantafyllidis G, et al. Symptoms of gastroesophageal reflux following laparoscopic sleeve gastrectomy are related to the final shape of the sleeve as depicted by radiology. Obes Surg. 2011;21(3):295–9. https://doi.org/10.1007/s11695-010-0339-0.
Popescu AL, Ioniţa-Radu F, Jinga M, et al. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. Rom J Intern Med. 2018;56(4):227–32. https://doi.org/10.2478/rjim-2018-0019.
Rampal M, Perillat P, Rougaud R. Notes preliminaires sur une nouvelle tech- nique de cure chirurgicale des hernies hiatales: la cardiopexie par le ligament rond. Marseille Chir. 1964;16:488.
Bou Daher H, Sharara AI. Gastroesophageal reflux disease, obesity and laparoscopic sleeve gastrectomy: the burning questions. World J Gastroenterol. 2019;25(33):4805–13. https://doi.org/10.3748/wjg.v25.i33.4805.
Hawasli A, Foster R, Lew D, et al. Laparoscopic ligamentum teres cardiopexy to the rescue; an old procedure with a new use in managing reflux after sleeve gastrectomy. Am J Surg. 2021;221(3):602–5. https://doi.org/10.1016/j.amjsurg.2020.12.036.
Lind R, Hage K, Ghanem M, et al. Long-term outcomes of sleeve gastrectomy: weight recurrence and surgical non-responders. Obes Surg. 2023;33(10):3028–34. https://doi.org/10.1007/s11695-023-06730-z.
Hayes K, Eid G. Laparoscopic sleeve gastrectomy: surgical technique and perioperative care. Surg Clin North Am. 2016;96(4):763–71. https://doi.org/10.1016/j.suc.2016.03.015.
Maroun J, Li M, Oyefule O, et al. Ten year comparative analysis of sleeve gastrectomy, Roux-en-Y gastric bypass, and biliopancreatic diversion with duodenal switch in patients with BMI ≥ 50 kg/m2. Surg Endosc. 2022;36(7):4946–55. https://doi.org/10.1007/s00464-021-08850-y.
Sharara AI, Rustom LBO, Bou Daher H, et al. Prevalence of gastroesophageal reflux and risk factors for erosive esophagitis in obese patients considered for bariatric surgery. Dig Liver Dis. 2019;51(10):1375–9. https://doi.org/10.1016/j.dld.2019.04.010.
Kermansaravi M, Parmar C, Chiappetta S, et al. Best practice approach for redo-surgeries after sleeve gastrectomy, an expert’s modified Delphi consensus, 37. Surg Endosc. 2023 Mar;(3):1617–28. https://doi.org/10.1007/s00464-023-09879-x.
Parmar CD, Mahawar KK, Boyle M, et al. Conversion of sleeve gastrectomy to roux-en-Y gastric bypass is effective for gastro-oesophageal reflux disease but not for further weight loss. Obes Surg. 2017;27:1651–8.
Qumseya BJ, Qumsiyeh Y, Ponniah SA, et al. Barrett’s esophagus after sleeve gastrectomy: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93(2):343–352.e2.
Gálvez-Valdovinos R, Cruz-Vigo JL, Marín-Santillán E, et al. Cardiopexy with ligamentum teres in patients with hiatal hernia and previous sleeve gastrectomy: an alternative treatment for gastroesophageal reflux disease. Obes Surg. 2015;25(8):1539–43. https://doi.org/10.1007/s11695-015-1740-5.
Mackey EE, Dore FJ, Kelly JF, et al. Ligamentum teres cardiopexy for post vertical sleeve gastrectomy gastroesophageal reflux. Surg Endosc. 2023;37(9):7247–53. https://doi.org/10.1007/s00464-023-10239-y.
Martínez Caballero J, de la Cruz VF, Gómez Rodríguez P, et al. Ligamentum teres cardiopexy might not prevent gastro-esophageal reflux after laparoscopic sleeve gastrectomy: case series. Obes Surg. 2023;33(3):965–8. https://doi.org/10.1007/s11695-022-06413-1.
Chiu S, Birch DW, Shi X, et al. Effect of sleeve gastrectomy on gastroesophageal reflux disease: a systematic review. Surg Obes Relat Dis. 2011;7(4):510–5. https://doi.org/10.1016/j.soard.2010.09.011.
Runkel A, Scheffel O, Marjanovic G, et al. The new interest of bariatric surgeons in the old ligamentum teres hepatis. Obes Surg. 2020;30(11):4592–8. https://doi.org/10.1007/s11695-020-04918-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
For this type of study formal consent is not required. Informed consent does not apply. The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
•GERD is a burden to a parcel of patients who underwent SG.
•A total of 72.4% of patients stated that their symptoms completely subsided.
•LTC is a safe and effective surgical alternative to treat refractory GERD.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lind, R.P., Antunes, J., Ghanem, M. et al. Reflux After Sleeve Gastrectomy: Safety and Effectiveness of Laparoscopic Ligamentum Teres Cardiopexy, a Single-Center Experience. OBES SURG 34, 1232–1237 (2024). https://doi.org/10.1007/s11695-024-07103-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-024-07103-w