Abstract
Objective
To explore insulin secretion patterns, β-cell function, and serum prolactin (PRL) concentrations in Chinese morbidly obese patients with Acanthosis nigricans (AN) and their alterations after laparoscopic sleeve gastrectomy (LSG).
Methods
A total of 138 morbidly obese subjects undergoing LSG were categorized as simple obesity without AN (OB group, n = 55) and obesity with AN (AN group, n = 83). Oral glucose tolerance test (OGTT), PRL, and related metabolic indices were performed pre- and 12 months post-LSG. Insulin secretion patterns were derived from insulin secretion peak time during OGTT: type I (peak at 30 or 60 min) and type II (peak at 120 or 180 min).
Results
Preoperatively, AN group showed significantly higher proportions of type II insulin secretion pattern, fasting insulin (FINS), and homeostatic model assessment of insulin resistance (HOMA-IR) whereas lower oral glucose insulin sensitivity (OGIS), insulinogenic index (IGI), and disposition index (DI) than OB group, which were improved significantly at 12 months postoperatively in both groups, more pronounced in AN group. Intriguingly, serum PRL declined substantially in AN group than OB group at baseline whereas elevated only in the AN group post-LSG. After adjusting for confounding factors, elevated PRL correlated significantly with increased IGI and DI, and decreased HOMA-IR in both genders, as well as increased OGIS in females, which was detected only in the AN group
Conclusion
Morbidly obese patients with AN presented delayed insulin secretion response, impaired insulin secretion, and β-cell dysfunction, which were significantly improved by LSG and might benefit from elevated PRL.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has currently reached a worldwide epidemic [1, 2] and is often accompanied by multiple adverse health outcomes, such as type 2 diabetes (T2DM), cardiovascular diseases, impaired insulin secretion, hormonal imbalance, and acanthosis nigricans (AN) [3,4,5,6,7]. Notably, obesity-associated AN is increasing in its prevalence and is the most prevalent cutaneous manifestation in individuals with obesity [8]. It clinically presents as symmetric hyperpigmented, brownish-black, papillomatous, velvety thickening plaques on intertriginous areas like the posterior neck fold, axillae, groin, and inframammary folds [9]. Considering that obesity and AN commonly coexists in relation with multiple complications, such as T2DM, insulin resistance (IR), hypogonadism, malignancies, and even depressive symptom [7, 10,11,12], thus, identifying the possible pathogenesis of obesity-associated AN is crucial for early detection and management of these conditions.
Accumulating evidence highlights that IR or hyperinsulinemia is the main pathophysiological mechanism of obesity-associated AN [13]. Our previous studies also revealed that obese patients with AN had more severe IR compared to their controls [7, 14]. Nonetheless, not all obese patients with significant IR have the cutaneous manifestation of AN, suggesting that some other risk factors might involve in the development of AN. Indeed, insulin secretion from pancreatic β-cell is enhanced progressively in an attempt to maintain normal glucose levels under normal conditions. Undoubtedly, impaired insulin secretion and β-cell dysfunction are observed in morbidly obese individuals and mouse models [15, 16]. Additionally, delayed insulin secretion response during the oral glucose tolerance test (OGTT) can serve as a powerful predictor of T2DM [17]. Given that AN is in a significant relation with T2DM and obesity, it can be presumed that there might be disorganized β-cell function and insulin secretion in morbidly obese subjects with AN, which has not been explored to date.
Prolactin (PRL), as a multifunctional pituitary hormone, has been known to involve in over 300 biological functions including reproduction and lactation, osmoregulation, immune modulation, and metabolic modulation [18]. In fact, more recent studies have focused on the impact of PRL signaling on glucolipid metabolism [19]. According to experimental studies, moderately elevated PRL levels can ameliorate hepatic steatosis in FFA-induced HepG2 cells [20], improve adipose tissue fitness and insulin sensitivity in obese rats [21], promote β-cell proliferation, and increase glucose-stimulated insulin secretion (GSIS) in diabetic mice [22]. The population-based study by Manshaei et al. [23] revealed that serum PRL levels could protect against T2DM and impaired glucose regulation. Therefore, we hypothesize that PRL plays an important role in improving β-cell function and stimulating insulin secretion in obese patients with AN, but the relevant data is lacking.
Bariatric surgery has currently become the most effective therapy for morbid obesity, evidenced by substantial weight reduction and resolution of obesity-related comorbidities, such as T2DM, hypertension, dyslipidemia, obstructive sleep apnea syndrome, and polycystic ovary syndrome (PCOS) [24,25,26]. Laparoscopic sleeve gastrectomy (LSG), as the predominant bariatric procedure, has been documented to improve the skin condition of AN in addition to other metabolic abnormalities in obese patients with AN [7]. Moreover, Wang et al. [27] revealed that LSG resulted in significantly elevated levels of serum PRL in obese patients. Inconsistently, Pellitero et al. [28] reported remarkably declined serum PRL levels in patients with morbid obesity during 1-year visit after operation (25 Roux-en-Y gastric bypasses and 7 sleeve gastrectomy).
However, few studies focused on the patterns of insulin secretion, β-cell function, and levels of serum PRL in morbidly obese subjects with AN, as well as their clinical outcomes following LSG. Therefore, the present study aimed to investigate the baseline insulin secretion patterns, β-cell function, and serum PRL levels in such a population and the influence of LSG on these conditions over a 12-month period.
Materials and Methods
Study Design and Participants
A retrospective observational study was conducted and 216 morbidly obese patients who underwent the standard LSG at the Department of Endocrinology and Metabolism, Shanghai Tenth People’s Hospital in China from June 2017 to October 2022 were selected in this study. Among whom, those who met the following criteria were enrolled: (1) aged 18 to 65 years, (2) BMI equal or greater than 35 kg/m2, (3) completed a 75-g oral glucose tolerance test (OGTT) and insulin release assay, and (4) eligible for the 12-month follow-up. The exclusion criteria were as follows: (1) severe liver and renal dysfunction, heart failure, cancer, or endocrine diseases such as pituitary adenoma, hypothyroidism, hyperthyroidism, or hypogonadism; (2) current or previous treatment that might affect the PRL levels, insulin secretion, or β-cell function; (3) mental illness; (4) genetic disease; (5) gestation or lactation; (6) loss to follow-up, or withdrawal from the study; and (7) unable to understand and comply with the study protocol. Finally, 138 subjects (55.8% female; age 31.3 ± 10.1 years; BMI 40.0 ± 5.9 kg/m2) were analyzed in this study and categorized as simple obesity without AN (OB group, n = 55) and obesity with AN (AN group, n = 83) according to the previously described criteria [7], as shown in Fig. 1. The severity scale for AN was described by Burke et al. [29].
The protocol for this research was followed in accordance with Helsinki Declaration and approved by the ethics committee of Shanghai Tenth People’s Hospital (Clinical Trials Registration Number is NCT05529563). All patients signed a written informed consent prior to the survey.
Measurements
Anthropometric Assessment
Demographic and clinical data including age, gender, and medical history were recorded. Anthropometric parameters were measured at baseline and during a 12-month follow-up visit. The height, weight, waist circumference (WC), systolic blood pressure (SBP), diastolic blood pressure (DBP), and assessment of AN were directly measured by trained physicians. Herein, BMI was calculated as weight (kg) divided by squared height (meters). Changes in BMI (ΔBMI) = Initial BMI (kg/m2) − current BMI (kg/m2).
Laboratory Measurements
Morning venous blood was drawn from all participants after an overnight 12-h fast at baseline and 12 months after LSG to measure fasting plasma glucose (FPG), fasting insulin (FINS), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), alanine transaminase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and serum creatinine. Moreover, plasma PRL levels were measured by electro-chemiluminescent immunoassay as previously described [6]. All baseline and follow-up evaluations were conducted in our institution.
Oral Glucose Tolerance Test and Insulin Secretion Patterns
To evaluate the following possible patterns of insulin secretion, a 2-h 75-g oral glucose tolerance test (OGTT) was performed after an overnight fasting in each patient pre- and 12 months post-LSG, and blood samples were taken at 0, 30, 60, 120, and 180 min to determine the concentrations of plasma glucose and insulin, which was assayed by glucose oxidase method and radioimmunoassay, respectively. Based on the peak time of the insulin release assay during the OGTT, insulin secretion was grouped into the following two types of patterns: type I and type II. Type I referred to the early peak time of insulin secretion at 30 or 60 min, and type II referred to the later peak time at 120 or 180 min. If two equal peaks occurred during the OGTT, the earlier occurrence was designated as the peak time.
Evaluation of Insulin Resistance, Insulin Sensitivity, and Beta-cell Function
Insulin resistance (IR) was estimated by using homeostasis assessment model of insulin resistance (HOMA-IR), calculated as FPG (mmol/L)×FINS (mU/L)/22.5 [30]. Insulin sensitivity derived from the OGTT was estimated by the oral glucose insulin sensitivity (OGIS) index [31]. Early-phase insulin secretion response during an OGTT was assessed by the insulinogenic index (IGI): (insulin30min-insulin0min)/(glucose30min-glucose0min). Correspondingly, β-cell function, measured as the disposition index (DI) of the early phase during an OGTT, was calculated as IGI/HOMA-IR [17].
Statistical Analysis
Data were presented as means ± standard deviation (SD) or, in case of skewed distribution, as medians (interquartile range) for continuous variables, or as proportions for categorical variables. Abnormally distributed data were log-transformed before analysis. Group differences were tested using Student’s t-test for normal distributed data, non-parametric test for non-normally distributed data, or chi-squared test for categorical variables. Paired samples t-test was performed to compare the variables before and after LSG. Δ was calculated as changes of metabolic variables before and after surgery. Pearson’s correlation analysis was used to investigate the relation between ΔPRL and changes in metabolic related indices. Multivariate regression analysis was applied to assess the contribution of ΔPRL to changing the insulin secretion and β-cell function after operation. Statistical analyses were made with SPSS software (Version 23.0 for Windows; SPSS, Chicago, IL) and figures were produced by GraphPad Prism 6 XML Project. Two-tailed P value < 0.05 was considered statistically significant.
Results
Baseline Clinical Characteristics of Study Participants
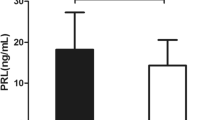
A total of 138 morbidly obese patients (77 female and 61 males) with average age 31.3 ± 10.1 years and mean preoperative BMI 40.0 ± 5.9 kg/m2 were selected for analysis. Baseline clinical characteristics of the study population are summarized in Table 1. At baseline, AN group showed significantly decreased serum PRL levels in comparison with OB group in both females (357.44 ± 16.99pmol/L vs. 530.55 ± 32.07pmol/L, P < 0.001) and males (431.22 ± 22.89pmol/L vs. 514.73 ± 28.28pmol/L, P = 0.027). In addition, AN group tended to be younger and had significantly higher weight, BMI, WC, SBP, DBP, FINS, and HOMA-IR, but lower OGIS, IGI, and DI than OB group in both genders (all P < 0.05). However, there were no differences between groups with respect to TC, TG, HDL-C, LDL-C, and FPG.
Baseline Patterns of Insulin Secretion During OGTT
As depicted in Fig. 2, no significant differences were observed in the plasma glucose levels at any point between AN and OB groups in either gender (Fig. 2A, B). The peak time of insulin secretion during OGTT was 120 min in AN group and 60 min in OB group regardless of gender (Fig. 2C, D). Additionally, AN group showed significantly higher insulin levels at 0, 120, and 180 min in both genders, as well as higher insulin levels at 60 min only in females. Based on the peak time of insulin release assay during OGTT, the patterns of insulin secretion were grouped into type I (peak time at 30 or 60 min) and type II (peak time at 120 or 180 min). As a result, AN group had significantly higher proportions of type II pattern of insulin secretion as opposed to OB group without gender difference (females: 51.3% vs. 26.3%, P = 0.025; males: 59.1% vs. 29.4%, P = 0.038) (Fig. 2E, F).
Baseline insulin secretion patterns and plasma glucose levels during an OGTT. Insulin secretion patterns were derived based on peak time of insulin secretion during a 2-h OGTT: type I (peak time at 30 or 60min), type II (peak time at 120 or 180 min). A, B In either gender, the plasma glucose levels did not differ between OB and AN groups at any point. C, D Compared to OB group, AN group had significantly higher insulin levels at 0, 120, and 180 min in both genders, as well as higher insulin levels at 60 min only in females. E, F As opposed to OB group, AN group had significantly higher proportions of Type II insulin secretion pattern in both genders. One asterisk (*) P < 0.05, two asterisks (**) P < 0.01, three asterisks (***) P < 0.001. P < 0.05 was considered to be statistically significant
Changes in Serum PRL and Other Metabolic Variables at 12 Months Following LSG
Twelve months following LSG, we observed substantial weight reduction and metabolic adaptation in both AN and OB groups. As shown in Fig.3, a noticeable improvement of skin condition was observed in patients with AN at 12 months post-LSG (Fig. 3A). The AN score declined significantly from 3.21 ± 0.86 to 1.10 ± 0.68 in females (P < 0.001) and from 3.34 ± 0.83 to 1.11 ± 0.57 in males (P < 0.001) (Fig. 3B). Intriguingly, the levels of serum PRL in AN group were significantly elevated after surgery, changing from 350.66 ± 16.99 to 524.54 ± 41.47pmol/L (P = 0.001) among females and from 431.22 ± 22.83 to 515.06 ± 44.48pmol/L among males (P = 0.004), which was not observed in OB group (P > 0.05) (Table 2). In addition, we observed significantly decreased BMI, WC, TC, TG, and FPG and elevated HDL-C levels in both AN and OB groups regardless of gender, as well as decreased LDL-C only in OB group among males after LSG.
Variations in Insulin Sensitivity and Beta-cell Function at 12 Months Following LSG
Among females, we observed a significant reduction in FINS and HOMA-IR (all P < 0.001), as well as increase in OGIS, IGI, and DI (all P < 0.05) at 12 months after LSG in both groups (Table 2). Likewise, the alterations of these variables remained significant among males. Furthermore, the absolute values of alterations (Δ) in FINS, HOMA-IR, OGIS, IGI, and DI before and after surgery were remarkably higher in AN group than those in OB group in both genders (Fig. 4C–G), although ΔBMI and ΔFPG did not differ between groups (Fig. 4A, B).
Comparison of changes in metabolic related indices at 12 months post-LSG from baseline between AN and OB group. Δ was calculated as changes of metabolic variables before and after LSG. The absolute values of ΔFINS (C), ΔHOMA-IR (D), ΔOGIS (E), ΔIGI (F), and ΔDI (G) were significantly higher in AN group than OB group regardless of gender, whereas ΔBMI (A) and ΔFPG (B) did not differ between groups. BMI body mass index, FPG fasting plasma glucose, FINS fasting insulin, HOMA-IR homeostasis assessment model of insulin resistance, OGIS oral glucose insulin sensitivity, IGI insulinogenic index, DI disposition index. One asterisk (*) P < 0.05, two asterisks (**) P < 0.01, three asterisks (***) P < 0.001. ns, no statistical significance in variables between AN and OB group
Alterations in Insulin Secretion Patterns at 12 Months Following LSG
As depicted in Fig. 5, the plasma glucose levels declined remarkably postoperatively at 5 points in females and at 0, 60, 120, and 180 min in males in both groups (Fig. 5A, B). The insulin levels at 0, 120, and 180 min declined significantly in both groups regardless of gender (all P < 0.01), whereas the insulin levels at 60min were decreased significantly in females with AN and males without AN (all P < 0.05) (Fig. 5C, D). In addition, the insulin peak time changed from 120 to 60 min in AN group and from 60 to 30min in OB group without gender difference (Fig. 5C, D). Also, the proportion of type II pattern of insulin secretion in AN group declined from 51.3 to 8.1% among females and from 59.1 to 9.1% among males (all P < 0.01), which was more pronounced than that in OB group (females: 26.3% vs. 5.3%; males: 29.4% vs. 11.8%, all P <0.05) (Fig. 5E, F).
Changes in insulin secretion pattern and plasma glucose levels during an OGTT at 12 months post-LSG from baseline. A, B After LSG, the plasma glucose levels declined remarkably at 5 points in females and at 0, 60, 120, and 180 min in males in both groups. C, D In both genders, the insulin peak time changed from 120 to 60 min in AN group and from 60 to 30 min in OB group. In females, the insulin levels declined significantly at 0, 120, and 180 min in both groups and 60 min only in the AN group after LSG. In males, the insulin levels declined significantly at 0, 120, and 180 min in both groups and 60 min only in the OB group after LSG. E, F After LSG, the proportions of type II insulin secretion pattern declined significantly in both groups, especially in AN group. Compared to baseline values, one asterisk (*) P < 0.05, two asterisks (**) P < 0.01, three asterisks (***) P < 0.001
Relationship Between Elevated PRL and Changes in Insulin Secretion and Beta-cell Function at 12 Months Following LSG
To explore the correlation between PRL variations and changes in insulin secretion and β-cell function after LSG, the Pearson’s correlation analysis was applied (Table 3). Among females, ΔPRL correlated significantly with ΔFINS (r = −0.485, P = 0.002), ΔHOMA-IR (r = −0.442, P = 0.006), ΔOGIS (r = 0.481, P = 0.003), ΔIGI (r = 0.352, P = 0.029), and ΔDI (r = 0.613, P < 0.001) in AN group but ΔFPG (r = −0.409, P = 0.011) in OB group. Among males, ΔPRL correlated significantly with ΔHOMA-IR (r = −0.744, P < 0.001), ΔOGIS (r = 0.436, P = 0.003), ΔIGI (r = 0.394, P = 0.009), and ΔDI (r = 0.378, P = 0.013) in AN group, which was not observed in OB group. No significant associations were observed between ΔPRL and ΔBMI, ΔTC, ΔTG, ΔHDL-C, and ΔLDL-C in both groups. To examine the contribution of PRL variations to the improved insulin secretion and β-cell function after surgery, multivariate linear regression analysis was performed (Table 4). After adjusting for age, baseline BMI, and ΔBMI, the elevated PRL correlated remarkably with ΔHOMA-IR (β = −0.433, P = 0.041), ΔOGIS (β = 0.467, P = 0.035), ΔIGI (β = 0.492, P = 0.031), and ΔDI (β = 0.484, P = 0.029) among females, as well as ΔHOMA-IR (β = −0.699, P < 0.001), ΔIGI (β = 0.380, P = 0.017), and ΔDI (β = 0.333, P = 0.019) among males, which was observed only in the AN group but not OB group. In contrast, there were no significant associations of ΔPRL with ΔFPG and ΔFINS in either group (all P > 0.05).
Discussion
Obesity-associated AN is currently the most common skin lesion in obesity [8] and poses a great health burden to the affected individuals and therapeutic challenge for physicians; however, the mechanism underlying the pathogenesis of obesity-associated AN has not been fully elucidated. In the present study, we revealed a significant delayed insulin secretion response, impaired β-cell function, and declined serum PRL levels in morbidly obese patients with AN compared to those without AN, which was remarkably improved at 12 months following LSG. Additionally, the changes of insulin secretion and β-cell function after LSG were more pronounced and correlated significantly with elevated serum PRL levels in AN group as opposed to OB group, indicating that the alleviated insulin secretion and β-cell function in AN group after LSG might be mediated by the increased serum PRL. Our findings provide a novel insight in the pathogenesis of obesity-associated AN and the important role of serum PRL in the regulation of insulin secretion and β-cell function.
Obesity-associated AN is proved to be often accompanied by more severe metabolic abnormalities than simple obesity, such as abdominal obesity, hypertension, and hyperinsulinemia [7, 12, 32]. In agreement with this, the present study showed that patients in AN group had significantly higher weight, BMI, WC, SBP, DBP, and FINS than those in OB group. As is commonly known, IR is the crucial mechanism in the pathogenesis of AN in obesity [13, 33]. It can be explained by the direct and indirect activation of the insulin-like growth factor receptor by hyperinsulinemia, which triggers the dermal fibroblast, and thus leads to the proliferation of epidermal keratinocyte [34]. Consistently, the present study and our previous studies demonstrated significantly higher FINS and more severe IR in obese patients with AN than their counterparts [7, 14, 32]. Notably, in some cases, obese individuals who have severe IR do not have the cutaneous feature of AN, implying that there must be other essential factors involving in the occurrence of AN in obesity. Intriguingly, recent studies have reported significantly impaired insulin secretion and β-cell dysfunction in morbidly obesity [15, 16]. Using both diet-and genetically-induced obese rodent models, Ying and colleagues [35, 36] found significantly increased accumulation of pancreatic macrophages in obese mice, thereby leading to the development of obesity-associated islet inflammation, which mechanistically involved in β-cell hyperplasia and impairing insulin secretory capacity in a PDGFR signaling-dependent manner. Moreover, Zhang et al. [16] revealed that miR-802 was significantly increased in islets of both dietary and genetic obese mouse models, which caused impaired insulin transcription and secretion, and β-cell dysfunction mediated by upregulation of transcription factor Foxo1 and repression of NeuroD1 and Fzd5. In a population-based prospective cohort study including 94,952 participants in Chinese adults, Wang et al. [37] found a significant association of β-cell dysfunction with incident T2DM. Hayashi et al. [17] also revealed that the delayed insulin secretion response could independently predict the incidence of T2DM. In view of that obesity-associated AN and T2DM often have the common pathophysiological basis such as IR, we hypothesize that there might be β-cell dysfunction and delayed insulin secretion in morbidly obese patients with AN, which has not been explored to date. As expected, the present study showed that AN group had significantly higher proportions of type II pattern of insulin secretion (peak time at 120 or 180 min) during OGTT than OB group in both males and females. That was to say, the pattern of delayed insulin secretion response was mainly found in AN group. Furthermore, we demonstrated a marked reduction in OGIS, IGI, and DI in AN group as opposed to OB group in both genders. These findings suggest that AN group had significantly delayed insulin secretion pattern, impaired insulin secretion, as well as β-cell dysfunction as compared to OB group without gender difference. The reason for this phenomenon may be that insulin sensitivity and insulin secretion in the early phase were decreased and the β-cell function was impaired in those with delayed insulin secretion patterns [17, 38]. Therefore, more attention should be given to those with delayed pattern of insulin secretion in the AN group. To our best knowledge, this is the first study to examine the insulin secretion response and β-cell function in morbidly obese patients with AN, which can broaden our understanding in the mechanism of obesity-associated AN.
PRL is a multifunctional pituitary hormone that plays a crucial role in diverse physiological functions broadly classified as reproduction and lactation, immune modulation, osmoregulation, and metabolic homeostasis [18, 19]. Lately, research related to the influence of PRL signaling on metabolic modulation have attracted more attention, and disorganized PRL and its receptor signaling in metabolic tissues can result into multiple pathological conditions in host supported by multiple preclinical and clinical studies. In population-based studies, Glintborg et al. [39] demonstrated that serum PRL levels correlated inversely with TC, TG, and LDL-C and positively with HDL-C in premenopausal women with or without PCOS. Additionally, serum PRL levels were significantly lower in individuals with T2DM [23] or non-alcoholic fatty liver disease (NAFLD) [20] than their controls. In experimental animal studies, PRL administration significantly downregulated hepatic TG accumulation in female mice and protected male mice from liver steatosis induced by HFD [40]. Conversely, PRLR-deficient mice fed with HFD developed more severe IR, glucose intolerance, and adipocyte hypertrophy in comparison with control mice [21]. Therefore, we assumed that there might be certain association between serum PRL and the incidence of obesity-associated AN; however, data with regard to their relationship in such a population is limited. Interestingly, our study found that morbidly obese patients with AN had significantly declined levels of serum PRL as compared to those without AN in both males and females, indicating that serum PRL may play an important role in the pathogenesis of obesity-associated AN. The possible explanation may be that low PRL levels are associated with obesity, glucose intolerance, T2DM, metabolic syndrome, and fatty liver [20, 41]. These metabolic disorders have been validated to be closely linked to hyperinsulinemia and IR [42, 43], which is commonly known to be the crucial contributing factor to the development of AN in obesity [13].
LSG, as a widely used bariatric surgery around the world, has been demonstrated to be safe and effective for achieving substantial weight loss and metabolic control of obesity and its comorbidities [7, 44, 45], which was in accordance with our results. More importantly, we detected significantly improved insulin secretion response in AN group, determined by the peak time of insulin secretion during OGTT changing from 120min to 60min in AN group in both genders. Furthermore, we observed a significant increase in OGIS, IGI, and DI, and reduction in FINS and HOMA-IR in both groups, whereas the variations of these items were more pronounced in AN group as opposed to OB group. In line with some of these findings, our previous studies showed that substantial weight loss induced by LSG could result in a much more significant reduction in FINS and HOMA-IR in obese patients with AN compared to their controls [7, 14]. OGIS, a model-based method for assessing insulin sensitivity from the OGTT [31, 38], has been reported in several studies. In the study by Pontiroli et al. [46], OGIS was significantly improved at 7 days after biliopancreatic diversion (BPD) in 10 subjects with baseline BMI 54.5±3.75kg/m2. Also, Mari et al. [47] revealed a significant restoration in OGIS within 10 days following BPD in 32 morbidly obese patients (BMI 52.0±7.0 kg/m2). Another study by Samaras et al. [48] showed a marked improvement in OGIS at 2 and 12 weeks among 15 obese participants (BMI 43.4±4.9 kg/m2) who underwent laparoscopic adjustable gastric banding surgery. These results support an early benefit effect of bariatric surgery on insulin sensitivity. Moreover, IGI is a widely accepted index evaluating the early-phase insulin secretion response during an OGTT [49]. In a prospective study including 98 obese participants who underwent LSG, Sista and colleagues [50] divided them into two groups based on resected gastric volume (RGV) (group A, RGV <1200 mL; group B, RGV >1200 mL). The results demonstrated that IGI was significantly increased postoperatively from baseline, with Group B performing better than group A at the 3rd day and at the 6th, 12th, and 24th months. Their further study detected a noticeable increase in IGI at the first 3 days, and 6, 12, and 24 months after LSG among 91 obese patients [51]. Furthermore, DI is a common index measuring β-cell function and defined as the product of insulin sensitivity and insulin secretion [17]. In a retrospective study by Salinari et al. [52], the results reported an about 10-fold increase in the DI derived from OGTT at 1 month after BPD in obese subjects with T2DM, which was similar to the values found in normal-weight control subjects. In contrast, Kashyap et al. [53] revealed a 5.8-fold increase in the DI at 12 months after gastric bypass among patients with moderate obesity and T2DM. These findings support that bariatric surgery provides marked restoration of insulin secretion and β-cell function. However, evidence regarding the impact of LSG on those items related to insulin secretion and β-cell function is limited in morbidly obese patients with AN. Unlike in previous studies, we observed significantly increased OGIS, IGI, and DI at 12-month post-LSG from baseline in both AN ands OB groups, with their more prominent variations in AN group than in OB group. These results indicate that LSG is also effective in improving insulin secretion and β-cell function among morbidly obese patients with AN, but the exact mechanism needs to be furtherly investigated and warranted.
Lately, evidence has accumulated that serum PRL levels are significantly lower in obese individuals accompanied by multiple comorbidities such as T2DM, IR, or NAFLD than their controls [20, 21, 23, 54], and metabolic improvement induced by bariatric surgery has produced controversial impact on the levels of serum PRL during follow-up [27]. In a retrospective study by Wang et al. [27], the results showed that serum PRL levels were significantly decreased in the high PRL (HP) group and increased in the normal PRL (NP) group at 12 months following LSG. In contrast, Mingrone et al. [55] demonstrated a significant reduction in 24-h plasma PRL concentrations and normalization of its secretion rhythm in severely obese fertile women at 1 year after BPD. In addition, Chen et al. [56] reported a marked reduction in serum PRL levels in obese men at least 1 year postoperatively (28 patients undergoing LSG and 31 undergoing LRYGB). Notably from our study, we investigated the serum PRL levels in morbidly obese patients with or without AN according to gender before and 12 months post-LSG, and detected that serum PRL levels were significantly decreased in AN group as opposed to OB group without gender difference at baseline, which were significantly elevated at 1 year postoperatively observed only in AN but not OB group. Inconsistently, Emami et al. [57] found that serum PRL levels showed no significant changes in patients who had undergone bariatric surgery. In view of this fact, it is important to underline that this discrepancy among these studies might result from different sample size, study populations, surgical procedures, and follow-up duration.
Noticeably, multiple observational studies have reported the close association of serum PRL with insulin secretion and β-cell function in humans and rodents [58, 59]. Yang et al. [58] reported that low serum PRL levels within the physiological range were associated with increased risk of IR and β-cell dysfunction in infertile women with PCOS. Additionally, targeted knockout of PRLR signaling in β-cells led to reduced β-cell proliferation and insulin secretion, which ultimately induced the occurrence of gestational diabetes mellitus during pregnancy [59]. However, no previous study has been designed to investigate the association of PRL variation and changes in insulin secretion and β-cell function among Chinese morbidly obese patients with AN following LSG. Thus, we further evaluated the alterations of serum PRL after LSG and tried to examine whether PRL elevation correlated with improved insulin secretion and β-cell function in such a population. As a result, the correlation analysis revealed that elevated PRL levels after LSG in AN group correlated significantly with increased OGIS, IGI, and DI, and decreased HOMA-IR in both genders, as well as decreased FINS among females. To identify the contribution of PRL variations to the alleviated insulin secretion and β-cell function after operation, multivariate linear regression analysis was performed. After adjusting for age, baseline BMI, and changes in BMI after LSG, the elevated PRL in AN but not OB group was still correlated significantly with decreased HOMA-IR and increased OGIS, IGI, and DI in females whereas correlated with decreased HOMA-IR and increased IGI and DI in males. This indicates that alleviated insulin secretion and β-cell function in obese patients with AN following LSG may benefit from the elevated PRL in addition to substantial weight reduction and metabolic improvement.
However, the underlying mechanism of elevated serum PRL contributing to the improved insulin secretion and β-cell function after surgery remains elusive. Available evidence has demonstrated that the moderately elevated PRL would promote β-cell mass by increasing β-cell proliferation and neogenesis through the phosphorylation of STAT-5, and potentiate GSIS though glucokinase (GK) and glucose transporter 2 (GLUT-2) induction in the diabetic rats [22]. Thus, we assumed that surgery-induced elevation of serum PRL might be responsible for the improved insulin secretion and β-cell function through potentiating STAT-5 phosphorylation and molecular expression of GK and GLUT-2. A second explanation may derive from the marked increased serum adiponectin induced by surgery [60]. Adiponectin, as an adipokine that is secreted in large quantities primarily from adipose tissue and directly sensitizes the body to insulin, has potential therapeutic targets to combat obesity-associated diseases characterized by IR [61]. Previous studies have showed that serum PRL correlated positively with circulating adiponectin levels and were significantly reduced in insulin-resistant subjects [21]. Moreover, adiponectin was positively associated with insulin secretion estimated using the index DI after adjusting IR (95%CI 0.06–0.24, P = 0.0016) [62]. We thus infer that the elevation of serum PRL may mediate the improvement of insulin secretion and β-cell function through increased circulating adiponectin after surgery. Another noteworthy explanation may result from the significant improvement of hyperinsulinemia and IR after surgery [6, 7]. Lower PRL serum levels have been revealed to be associated with a higher HOMA-IR [54], and the elevated PRL could promote adipose tissue fitness and insulin sensitivity in obese rats and human [21]. In addition, hyperinsulinemia caused by IR further results in the activation of IGF-1 receptors on keratinocytes and fibroblasts directly or indirectly, finally leading to hyperplasia and hyperpigmentation observed in AN [34]. Furthermore, infusion of IGF-1 LR3, an analog of IGF-1 with high affinity for the IGF-1 receptors, for 1 week could reduce insulin concentrations and GSIS in vivo and in vitro [63]. We speculate that alteration in the insulin/IGF-1 axis accompanied by improved IR after surgery contributes to the long-term alleviation of insulin secretion and β-cell function. Consistently, in our study, we detected significantly decreased serum PRL levels, increased FINS and HOMA-IR, as well as declined OGIS, IGI, and DI in AN group compared to OB group at baseline, which were reversed significantly at 12 months following LSG. Strikingly, we demonstrated a significant correlation of elevated PRL with decreased HOMA-IR and increased IGI and DI in both genders, which was observed only in the AN but not OB group. Taken together, the elevated PRL may make a contribution to the improvement of insulin secretion and β-cell function following LSG. Still, further mechanistic studies are needed to elucidate the exact mechanism.
Undeniably, several limitations must be acknowledged in the present study. Firstly, we used a relatively small sample size for assessing the insulin secretion, β-cell function, serum PRL, and their clinical outcomes at 12 months following LSG in morbidly obese patients with AN, which could lead to the high variability. Secondly, there exist unmeasured confounding factors including menstrual cycles, dietary, physical activity, and sleep schedule, which could affect the study results. Thirdly, our study findings concluded from the relatively short follow-up period after LSG might not represent the long-term effects of LSG on β-cell function, insulin secretion patterns, and serum PRL levels. Future studies with extended follow-up in a larger cohort are warranted to verify our findings and investigate the underlying mechanism.
Conclusion
Morbidly obese patients with AN present significant delayed insulin secretion response, impaired insulin secretion, and β-cell dysfunction, which could be significantly ameliorated by LSG and might be mediated by significant elevated serum PRL levels. Further studies are needed to validate our findings and elucidate the exact mechanism.
References
Jaacks LM, Vandevijvere S, Pan A, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7(3):231–40. https://doi.org/10.1016/S2213-8587(19)30026-9.
Di Cesare M, Soric M, Bovet P, et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 2019;17(1):212. https://doi.org/10.1186/s12916-019-1449-8.
Lu Y, Yang H, Xu Z, et al. Association between different obesity patterns and the risk of developing type 2 diabetes mellitus among adults in eastern china: a cross-sectional study. Diabetes Metab Syndr Obes. 2021;14:2631–9. https://doi.org/10.2147/DMSO.S309400.
Mouton AJ, Li X, Hall ME, et al. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ Res. 2020;126(6):789–806. https://doi.org/10.1161/CIRCRESAHA.119.312321.
van Vliet S, Koh HE, Patterson BW, et al. Obesity is associated with increased basal and postprandial beta-cell insulin secretion even in the absence of insulin resistance. Diabetes. 2020;69(10):2112–9. https://doi.org/10.2337/db20-0377.
Zhu C, Zhang Y, Zhang L, et al. Changes in sex hormones after laparoscopic sleeve gastrectomy in Chinese obese men: a 12-month follow-up. Obes Surg. 2019;29(3):869–77. https://doi.org/10.1007/s11695-018-3611-3.
Zhu C, Mei F, Gao J, et al. Changes in inflammatory markers correlated with increased testosterone after laparoscopic sleeve gastrectomy in obese Chinese men with acanthosis nigricans. J Dermatol. 2019;46(4):338–42. https://doi.org/10.1111/1346-8138.14783.
Ng HY. Acanthosis nigricans in obese adolescents: prevalence, impact, and management challenges. Adolesc Health Med Ther. 2017;8:1–10. https://doi.org/10.2147/AHMT.S103396.
Das A, Datta D, Kassir M, et al. Acanthosis nigricans: a review. J Cosmet Dermatol. 2020;19(8):1857–65. https://doi.org/10.1111/jocd.13544.
Bhagyanathan M, Dhayanithy D, Parambath VA, et al. Acanthosis nigricans: a screening test for insulin resistance - An important risk factor for diabetes mellitus type-2. J Family Med Prim Care. 2017;6(1):43–6. https://doi.org/10.4103/2249-4863.214961.
West L, Carlson M, Wallis L, et al. The sign of Leser-Trelat and malignant acanthosis nigricans associated with fallopian tube carcinoma. Obstet Gynecol. 2018;132(5):1116–9. https://doi.org/10.1097/AOG.0000000000002920.
Huang Y, Chen J, Yang J, et al. Evaluation of depressive symptoms in obese patients with or without acanthosis nigricans. Hormones (Athens). 2015;14(3):417–24. https://doi.org/10.14310/horm.2002.1575.
Koh YK, Lee JH, Kim EY, et al. Acanthosis nigricans as a clinical predictor of insulin resistance in obese children. Pediatr Gastroenterol Hepatol Nutr. 2016;19(4):251–8. https://doi.org/10.5223/pghn.2016.19.4.251.
Zhang Y, Zhu C, Wen X, et al. Laparoscopic sleeve gastrectomy improves body composition and alleviates insulin resistance in obesity related acanthosis nigricans. Lipids Health Dis. 2017;16(1):209. https://doi.org/10.1186/s12944-017-0598-z.
Garcia-Fuentes E, Garcia-Almeida JM, Garcia-Arnes J, et al. Morbidly obese individuals with impaired fasting glucose have a specific pattern of insulin secretion and sensitivity: effect of weight loss after bariatric surgery. Obes Surg. 2006;16(9):1179–88. https://doi.org/10.1381/096089206778392383.
Zhang F, Ma D, Zhao W, et al. Obesity-induced overexpression of miR-802 impairs insulin transcription and secretion. Nat Commun. 2020;11(1):1822. https://doi.org/10.1038/s41467-020-15529-w.
Hayashi T, Boyko EJ, Sato KK, et al. Patterns of insulin concentration during the OGTT predict the risk of type 2 diabetes in Japanese Americans. Diabetes Care. 2013;36(5):1229–35. https://doi.org/10.2337/dc12-0246.
Bernard V, Young J, Binart N. Prolactin - a pleiotropic factor in health and disease. Nat Rev Endocrinol. 2019;15(6):356–65. https://doi.org/10.1038/s41574-019-0194-6.
Bernard V, Young J, Chanson P, et al. New insights in prolactin: pathological implications. Nat Rev Endocrinol. 2015;11(5):265–75. https://doi.org/10.1038/nrendo.2015.36.
Zhang P, Ge Z, Wang H, et al. Prolactin improves hepatic steatosis via CD36 pathway. J Hepatol. 2018;68(6):1247–55. https://doi.org/10.1016/j.jhep.2018.01.035.
Ruiz-Herrera X, de Los Rios EA, Diaz JM, et al. Prolactin promotes adipose tissue fitness and insulin sensitivity in obese males. Endocrinology. 2017;158(1):56–68. https://doi.org/10.1210/en.2016-1444.
Park S, Kim DS, Daily JW, et al. Serum prolactin concentrations determine whether they improve or impair beta-cell function and insulin sensitivity in diabetic rats. Diabetes Metab Res Rev. 2011;27(6):564–74. https://doi.org/10.1002/dmrr.1215.
Manshaei N, Shakibaei F, Fazilati M, et al. An investigation of the association between the level of prolactin in serum and type II diabetes. Diabetes Metab Syndr. 2019;13(5):3035–41. https://doi.org/10.1016/j.dsx.2018.07.007.
Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113–23. https://doi.org/10.1056/NEJMoa1506699.
Del Genio G, Limongelli P, Del Genio F, et al. Sleeve gastrectomy improves obstructive sleep apnea syndrome (OSAS): 5 year longitudinal study. Surg Obes Relat Dis. 2016;12(1):70–4. https://doi.org/10.1016/j.soard.2015.02.020.
Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29(1):93–8. https://doi.org/10.1007/s11695-018-3473-8.
Wang X, Ma B, Li G, et al. Glucose-lipid metabolism in obesity with elevated prolactin levels and alteration of prolactin levels after laparoscopic sleeve gastrectomy. Obes Surg. 2020;30(10):4004–13. https://doi.org/10.1007/s11695-020-04771-2.
Pellitero S, Olaizola I, Alastrue A, et al. Hypogonadotropic hypogonadism in morbidly obese males is reversed after bariatric surgery. Obes Surg. 2012;22(12):1835–42. https://doi.org/10.1007/s11695-012-0734-9.
Burke JP, Hale DE, Hazuda HP, et al. A quantitative scale of acanthosis nigricans. Diabetes Care. 1999;22(10):1655–9. https://doi.org/10.2337/diacare.22.10.1655.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. https://doi.org/10.1007/BF00280883.
Mari A, Pacini G, Murphy E, et al. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24(3):539–48. https://doi.org/10.2337/diacare.24.3.539.
Zhu C, Cui R, Gao M, et al. The associations of serum uric acid with obesity-related Acanthosis nigricans and related metabolic indices. Int J Endocrinol. 2017;2017:5438157. https://doi.org/10.1155/2017/5438157.
Hermanns-Le T, Scheen A, Pierard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol. 2004;5(3):199–203. https://doi.org/10.2165/00128071-200405030-00008.
Lee JM, Okumura MJ, Davis MM, et al. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29(11):2427–32. https://doi.org/10.2337/dc06-0709.
Ying W, Lee YS, Dong Y, et al. Expansion of islet-resident macrophages leads to inflammation affecting beta cell proliferation and function in obesity. Cell Metab. 2019;29(2):457–474 e5. https://doi.org/10.1016/j.cmet.2018.12.003.
Ying W, Fu W, Lee YS, et al. The role of macrophages in obesity-associated islet inflammation and beta-cell abnormalities. Nat Rev Endocrinol. 2020;16(2):81–90. https://doi.org/10.1038/s41574-019-0286-3.
Wang T, Lu J, Shi L, et al. Association of insulin resistance and beta-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol. 2020;8(2):115–24. https://doi.org/10.1016/S2213-8587(19)30425-5.
Sun Y, Han J, Lin Z, et al. Delayed insulin secretion response during an OGTT is associated with an increased risk for incidence of diabetes in NGT subjects. J Diabetes Complications. 2016;30(8):1537–43. https://doi.org/10.1016/j.jdiacomp.2016.07.029.
Glintborg D, Altinok M, Mumm H, et al. Prolactin is associated with metabolic risk and cortisol in 1007 women with polycystic ovary syndrome. Human reproduction. 2014;29(8):1773–9. https://doi.org/10.1093/humrep/deu133.
Shao S, Yao Z, Lu J, et al. Ablation of prolactin receptor increases hepatic triglyceride accumulation. Biochem Biophys Res Commun. 2018;498(3):693–9. https://doi.org/10.1016/j.bbrc.2018.03.048.
Macotela Y, Ruiz-Herrera X, Vazquez-Carrillo DI, et al. The beneficial metabolic actions of prolactin. Front Endocrinol (Lausanne). 2022;13:1001703. https://doi.org/10.3389/fendo.2022.1001703.
Gluvic Z, Zaric B, Resanovic I, et al. Link between metabolic syndrome and insulin resistance. Curr Vasc Pharmacol. 2017;15(1):30–9. https://doi.org/10.2174/1570161114666161007164510.
Tanase DM, Gosav EM, Costea CF, et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J Diabetes Res. 2020;2020:3920196. https://doi.org/10.1155/2020/3920196.
Zhu C, Gao J, Mei F, et al. Reduction in Thyroid-stimulating hormone correlated with improved inflammation markers in Chinese patients with morbid obesity undergoing laparoscopic sleeve gastrectomy. Obes Surg. 2019;29(12):3954–65. https://doi.org/10.1007/s11695-019-04063-4.
Elnabil-Mortada A, Elmaleh HM, Ackroyd R, et al. Effectiveness and safety of laparoscopic sleeve gastrectomy for weight loss in mild obesity: prospective cohort study with 3-year follow-up. Obes Surg. 2022;32(6):1918–25. https://doi.org/10.1007/s11695-022-05958-5.
Pontiroli AE, Gniuli D, Mingrone G. Early effects of gastric banding (LGB) and of biliopancreatic diversion (BPD) on insulin sensitivity and on glucose and insulin response after OGTT. Obes Surg. 2010;20(4):474–9. https://doi.org/10.1007/s11695-010-0076-4.
Mari A, Manco M, Guidone C, et al. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia. 2006;49(9):2136–43. https://doi.org/10.1007/s00125-006-0337-x.
Samaras K, Viardot A, Botelho NK, et al. Immune cell-mediated inflammation and the early improvements in glucose metabolism after gastric banding surgery. Diabetologia. 2013;56(12):2564–72. https://doi.org/10.1007/s00125-013-3033-7.
Potter KJ, Boudreau V, Bonhoure A, et al. Insulinogenic index and early phase insulin secretion predict increased risk of worsening glucose tolerance and of cystic fibrosis-related diabetes. J Cyst Fibros. 2022; https://doi.org/10.1016/j.jcf.2022.07.014.
Sista F, Abruzzese V, Clementi M, et al. Effect of resected gastric volume on ghrelin and GLP-1 plasma levels: a prospective study. J Gastrointest Surg. 2016;20(12):1931–41. https://doi.org/10.1007/s11605-016-3292-y.
Sista F, Abruzzese V, Clementi M, et al. Resolution of type 2 diabetes after sleeve gastrectomy: a 2-step hypothesis. Surg Obes Relat Dis. 2018;14(3):284–90. https://doi.org/10.1016/j.soard.2017.12.009.
Salinari S, Bertuzzi A, Asnaghi S, et al. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32(3):375–80. https://doi.org/10.2337/dc08-1314.
Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36(8):2175–82. https://doi.org/10.2337/dc12-1596.
Ponce AJ, Galvan-Salas T, Lerma-Alvarado RM, et al. Low prolactin levels are associated with visceral adipocyte hypertrophy and insulin resistance in humans. Endocrine. 2020;67(2):331–43. https://doi.org/10.1007/s12020-019-02170-x.
Mingrone G, Manco M, Iaconelli A, et al. Prolactin and insulin ultradian secretion and adipose tissue lipoprotein lipase expression in severely obese women after bariatric surgery. Obesity (Silver Spring). 2008;16(8):1831–7. https://doi.org/10.1038/oby.2008.297.
Chen G, Sun L, Jiang S, et al. Effects of bariatric surgery on testosterone level and sexual function in men with obesity: a retrospective study. Front Endocrinol (Lausanne). 2022;13:1036243. https://doi.org/10.3389/fendo.2022.1036243.
Emami MR, Safabakhsh M, Khorshidi M, et al. Effect of bariatric surgery on endogenous sex hormones and sex hormone-binding globulin levels: a systematic review and meta-analysis. Surg Obes Relat Dis. 2021;17(9):1621–36. https://doi.org/10.1016/j.soard.2021.05.003.
Yang H, Lin J, Li H, et al. Prolactin is associated with insulin resistance and beta-cell dysfunction in infertile women with polycystic ovary syndrome. Front Endocrinol (Lausanne). 2021;12:571229. https://doi.org/10.3389/fendo.2021.571229.
Banerjee RR, Cyphert HA, Walker EM, et al. Gestational diabetes mellitus from inactivation of prolactin receptor and MafB in islet beta-cells. Diabetes. 2016;65(8):2331–41. https://doi.org/10.2337/db15-1527.
Sebunova N, Stsepetova J, Kullisaar T, et al. Changes in adipokine levels and metabolic profiles following bariatric surgery. BMC Endocr Disord. 2022;22(1):33. https://doi.org/10.1186/s12902-022-00942-7.
Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784–92. https://doi.org/10.1172/JCI29126.
Nakamura A, Miyoshi H, Ukawa S, et al. Serum adiponectin and insulin secretion: A direct or inverse association? J Diabetes Investig. 2018;9(5):1106–9. https://doi.org/10.1111/jdi.12821.
White A, Stremming J, Boehmer BH, et al. Reduced glucose-stimulated insulin secretion following a 1-wk IGF-1 infusion in late gestation fetal sheep is due to an intrinsic islet defect. Am J Physiol Endocrinol Metab. 2021;320(6):E1138–47. https://doi.org/10.1152/ajpendo.00623.2020.
Acknowledgements
We are grateful to all the research individuals for their participation in this study. We would also like to acknowledge all the endocrinologists, surgeons, and nurses of the Multidisciplinary Group for their contribution to this study.
Funding
This project was funded by the Climbing Talent Program of the 10th People’s Hospital affiliated to Tongji University (2021SYPDRC050), Clinical Research Funds for Shanghai Municipal Health Commission (202040170), and National Natural Science Foundation of China (No. 81700752, 82201867).
Author information
Authors and Affiliations
Contributions
Cuiling Zhu participated in the study design, data analysis, and the draft of the manuscript. Xin Wen and Hui You contributed to the data collection and statistical analysis. Liesheng Lu and Lei Du performed the surgery and revised the manuscript. Cuiling Zhu and Chunhua Qian conceived and proposed the study program, interpreted the study results, and revised the manuscript. All authors approved the final version for publication.
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from each participant included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Morbid obesity with AN had delayed insulin secretion response and β-cell dysfunction.
• Serum PRL levels were significantly lower in AN group than in OB group.
• Insulin secretion and β-cell function was improved more by LSG in AN than in OB group.
• Improved insulin secretion and β-cell function may benefit from elevated PRL by LSG.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, C., Wen, X., You, H. et al. Improved Insulin Secretion Response and Beta-cell Function Correlated with Increased Prolactin Levels After Laparoscopic Sleeve Gastrectomy in Morbidly Obese Patients with Acanthosis Nigricans. OBES SURG 33, 2405–2419 (2023). https://doi.org/10.1007/s11695-023-06686-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-023-06686-0