Abstract
This meta-analysis aimed at exploring the impact of intravenous ketamine on pain relief and analgesic consumption in patients undergoing bariatric surgery (BS). Literature searches identified nine eligible trials with 458 participants. Forest plot revealed a significantly lower pain score [mean difference (MD) = − 1.06, p = 0.005; 390 patients) and morphine consumption (MD = − 3.85 mg, p = 0.01; 212 patients) immediately after BS in patients with intravenous ketamine than in those without. In contrast, pooled analysis showed comparable pain score (p = 0.28), morphine consumption (p = 0.45) within 24 h, and risk of postoperative nausea/vomiting (p = 0.67) between the two groups. In conclusion, the meta-analysis demonstrated improvements in pain outcomes immediately after surgery through perioperative intravenous ketamine administration despite the absence of analgesic benefit in the late postoperative period and a positive impact on postoperative nausea/vomiting.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery (BS) has been reported to achieve sustainable weight loss and improve obesity-associated comorbidities (e.g., type 2 diabetes) and mortality [1, 2]. Although increased availability of BS with laparoscopic approach may be associated with less surgical pain and requirements for postoperative opioid compared to open laparotomy [3], optimal pain control in this patient population remains a challenge. Compared with patients without obesity, pain sensitivity and analgesic requirement have been found to increase significantly in patients with obesity [4, 5]. On the other hand, the high prevalence of obstructive sleep apnea (OSA) [6] may increase opioid-associated respiratory complications [7, 8] that restrict opioid use according to perioperative guidelines for this population [9, 10]. Therefore, the elevated pain sensitivity together with the restriction in perioperative opioids could compromise postoperative pain control in patients with obesity. The origins of postoperative pain in patients with abdominal surgeries are multiple, involving not only pain from the abdominal wall but also that from the abdominal organs, which are partly innervated by vagal fibers that induce sensitization in the brainstem [11]. Accordingly, previous studies have advocated multimodal pain management strategies as the optimal regimen for patients undergoing major abdominal surgery [12] and bariatric procedures [13]. Non-opioid analgesics, such as nonsteroidal anti-inflammatory drugs [14], lidocaine [15], dexmedetomidine [16], and pregabalin [17], are commonly included in the combined regimens to reduce opioid-related adverse events.
Ketamine, which exerts its physiologic effects primarily through antagonism of voltage-gated N-methyl-D-aspartate (NMDA) receptors [18], is widely used for induction and maintenance in general anesthesia [19] as well as for analgesia and procedural sedation in the emergency department [20] and in the intensive care unit (ICU) [21]. In addition, ketamine has been shown to have the clinical advantages of not only controlling postoperative pain [22] but also prolonging the time to the first requirement of narcotic and reducing narcotic-related side effects [23]. An attempt to minimize narcotic use in surgical patients has led to an increased utilization of ketamine in subanesthetic doses in the acute pain control setting [24]. Consistently, a number of review articles have demonstrated an opioid-sparing effect of ketamine through its administration at subanesthetic doses as a perioperative adjunct [25,26,27].
Despite availability of randomized controlled trials (RCTs) [28, 29] to support the use of intravenous ketamine at subanesthetic doses in patients undergoing BS, there were still inconsistent findings [30] in current literature. Therefore, examination of cumulative evidence is warranted to guide its use in this patient population. The aim of the current meta-analysis was to assess the efficacy of ketamine for pain relief as well as reductions in analgesic consumption and incidence of postoperative nausea/vomiting (PONV) in patients undergoing BS. Potential side effects with the use of ketamine were also explored.
Materials and methods
This meta-analysis was reported according to the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and was registered with the International Prospective Register of Systematic Reviews (CRD42021268730).
Eligibility criteria
Eligibility of RCTs for the current study was based on their fulfillment of the PICO (i.e., patient, intervention, comparison, outcomes) criteria:
-
(a)
Patient population: adult patients undergoing BS
-
(b)
Intervention: perioperative use of intravenous ketamine (bolus with or without infusion, alone, or with other analgesics) as the intervention treatment regardless of the timing of administration (e.g., intraoperatively or postoperatively)
-
(c)
Comparison: the use of placebo (e.g., normal saline) or conventional analgesic regimens as a control
-
(d)
Outcomes: postoperative pain assessment such as pain score or opioid requirements
There were no restrictions on ketamine dose, patient age, language, and the date of publication. On encountering missing information, we contacted the authors for original data. Exclusion criteria were (1) studies in which information regarding pain-related outcomes was unavailable, (2) observational designs (i.e., cohort studies, cross-sectional studies, case–control studies), (3) studies published only as either letters or abstracts, and (4) those using any form of regional anesthesia or non-intravenous route (e.g., intraperitoneal route).
Search strategies for databases
We searched the EMBASE, Cochrane Library, Medline, and Google scholar databases from their inception dates till July 22, 2021. We used the search terms below to screen for eligible records: (“Ketalar” or “Ketamine” or “NMDA receptor antagonist”) and (“(Metabolic or bariatric or malabsorptive or restrictive or obesity) adj4 (operation* or surgery* or procedure* or technique*)” or “Gastric Bypass” or “Stomach Stapling” or “Sleeve gastrectomy” or “Gastroplasty” or “Jejunoileal Bypass” or “Roux-en-Y Gastric Bypass” or “RYGB” or “gastric banding” or “weight loss” or “vertical banded gastroplasty” or “biliopancreatic diversion”). The search strategy is demonstrated in Supplemental Table 1. Additional records were retrieved by scrutinizing the reference lists of the relevant studies.
Studies selection and data extraction
Two independent reviewers examined all abstracts for possible inclusion and exclusion based on the PICO criteria, with identification of duplicates. Full text articles were further reviewed for eligibility. Discrepancies in decision between the independent reviewers were settled through discussion with an independent third party. The same process was applied for data extraction and bias assessment. The following data were retrieved from each RCT based on a predesigned form: authors, year of publication, patient characteristics (e.g., age), study setting, dosage of ketamine, number of patients, timing (intraoperative or postoperative), pain score, opioid requirement, type of surgery, incidence of postoperative nausea/vomiting (PONV), side effects reported (e.g., hallucinations), and country.
Outcomes and definitions
Taking into consideration that the majority of postoperative opioid-related morbidity occurs within the first postoperative 24 h [31], the primary outcome of the current meta-analysis was opioid requirement within postoperative 24 h. The secondary outcomes were pain score or analgesic requirement immediately after surgery (e.g., postoperative 1 h) and risk of PONV. Other adverse events associated with ketamine use were also reviewed if they were reported in individual studies. Subgroup analysis was performed based on the type of surgery (i.e., laparotomy vs. laparoscopy) to explore whether this factor might influence the efficacy of ketamine in postoperative pain management and contribute to heterogeneity. The risk of PONV was computed as the relative risk (RR) with the random effects model according to the incidence of postoperative nausea or vomiting without considering the time of occurrence. If nausea and vomiting were described as separate outcomes, the higher of the two numbers (i.e., number of patients with either symptom) was recorded. For a unified assessment of the outcome on pain, we transformed all data from visual analog scale (VAS) 0–10 cm, VAS 0–100 mm, numerical rating scale (NRS) 0–10, and verbal rating scale (VRS) 0–10 into VAS 0–10 cm.
Risks of bias assessment
The risk of bias was ranked as “low,” “unclear,” or “high” in accordance with the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. We considered the risk of “selective outcome reporting” bias to be “unclear” for not previously registered protocols of the included trials. In addition, the sources of funding were scrutinized for the potentials of other biases. Disagreements were solved through discussion. The overall risk of bias of all the included studies and the risk of bias of individual studies were investigated.
Data synthesis
We conducted data synthesis with the Cochrane Review Manager (RevMan 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Data from at least two trials were pooled for an outcome. If dichotomous data could be pooled, we performed a meta-analysis using the Mantel–Haenszel random-effects model assuming clinical heterogeneity across the included studies followed by the calculation of a relative risk (RR) with a 95% confidence interval (CI). Outcomes of continuous data were presented as a weighted mean difference (MD) with 95% CI after weighting in accordance with the inverse variance method and pooling using a random-effects model [32]. For data reported as only medians and interquartile ranges (IQR), we assumed a normal distribution and calculated the standard deviation (SD) using SD = IQR/1.349 as described in previous meta-analyses [33]. The potential impact of the findings from an individual trial on the overall outcome was evaluated with sensitivity analysis using a leave-one-out approach. Heterogeneity was statistically assessed with I2 statistic, and substantial heterogeneity was predefined as an I2 over 50%, for which the effect of the contributing study on the overall result of that particular outcome was assessed with sensitivity analysis to determine the impact of heterogeneity on that outcome. A potential publication bias was visually assessed with a funnel plot after identifying 10 or more trials reporting on a specific outcome. A probability value less than 0.05 was considered statistically significant for all analyses.
Results
Study selection
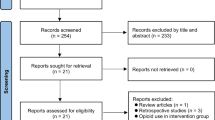
The study selection process is shown in Fig. 1. A total of 130 records were available from database search. After removing duplicates and records that did not meet the inclusion criteria for PICO, we identified 18 potentially eligible trials for a more detailed review. After analyzing the full text, eight studies were excluded because of non-RCTs (case report, n = 2; letter, n = 1), only abstract available (n = 4), non-relevant publication (n = 1), and intraperitoneal ketamine as the intervention (n = 1). Finally, nine RCTs met the inclusion criteria for our analysis [28,29,30, 34,35,36,37,38,39].
Study characteristics
A total of nine RCTs involving 458 participants published between 2003 and 2021 were included. The characteristics of the included RCTs are demonstrated in Table 1. The mean or median age ranged from 27 to 46 years [28,29,30, 34,35,36, 38, 39], while one study did not provide information on age [37]. The mean or median body mass index (BMI) ranged from 40 to 56 kg/m2 across the studies. Laparoscopic approach was performed in six RCTs [29, 30, 35,36,37, 39], while open laparotomy was performed in the other three trials [28, 34, 38]. The surgical time varied among the included studies, ranging from 47 to 198 min. Intravenous ketamine was administered intraoperatively in eight studies [28,29,30, 34,35,36,37,38] and in the post-anesthetic care unit in one trial [39]. Racemic ketamine was used in eight RCTs [28,29,30, 34,35,36,37, 39], and S-ketamine was administered in one trial [38]. Ketamine was used as a single agent in five trials [30, 34, 36, 37, 39] and as a component of a combined regimen in four studies [28, 29, 35, 38]. Normal saline was used in the control group in three trials [30, 34, 39] and opioid-based regimens served as the control group in six studies [28, 29, 35,36,37,38]. For maintenance in general anesthesia, seven studies used inhalation agents [28, 30, 34,35,36, 38, 39] and one chose total intravenous anesthesia [29]. However, one study did not specify this information [37].
Although psychotomimetic adverse events were lacking in six studies [28,29,30, 34, 35, 37](supplemental table 2), two studies reported the occurrence of hallucinations in 7.14% [36] and 4.5% [39] of patients receiving ketamine, respectively. In contrast, one study on patients undergoing open gastric bypass mentioned that two of the 15 patients in the control group remained intubated during their stay in the postanesthesia care unit (PACU), while no patient required prolonged intubation in the ketamine group [28].
Risk of bias assessment
Results of the risk of bias assessment are shown in Fig. 2. The sequence generation for randomization and allocation concealment were adequate in eight and five studies, respectively, while the former was unavailable in one study [35] and the latter was unavailable in four studies [29, 34,35,36]. Performance bias and detection bias were unclear in four [28, 29, 35, 38] and two [35, 38] studies, respectively, as these studies did not provide relevant information. Attrition bias was considered to be low in all studies (supplement table 3). Reporting bias was unclear in six studies [28, 29, 34,35,36, 38] that did not provide detail regarding trial registration. The risk of other bias of two studies that did not give information regarding conflict of interest [28, 29] was considered to be unclear. Detailed information on bias assessment of the included studies is shown in supplemental Table 3 and Fig. 3.
Synthesis of results
One-hour postoperative pain score
Pooled results revealed a significantly lower pain score in the ketamine group than that in the control group (MD = − 1.06, 95% CI: − 1.81 to − 0.31, p = 0.005; I2 = 87%; 7 RCTs; n = 390) (Fig. 4)[28,29,30, 35, 37,38,39]. However, sensitivity analysis revealed a loss of the significant therapeutic benefit when one study was removed [38]. The heterogeneity was high for this outcome. Exclusion of an outlier study [29] from the summary estimate still revealed a lower pain score (MD = − 0.61, 95% CI: − 1.0 to − 0.22, p = 0.002) with a reduction of heterogeneity down to 48%, suggesting that heterogeneity did not significantly impact the result of this particular outcome. Subgroup analysis of the impact of surgical procedures (e.g., laparotomy vs. laparoscopy) on pain score showed no significant difference (p = 0.92), indicating that the benefits of intravenous ketamine were comparable between the two surgical populations.
Morphine consumption within 6 postoperative hours
Regarding analgesic requirement within a 6-h postoperative period, our finding revealed a significantly lower morphine consumption in the ketamine group than that in the control group (MD = − 3.85 mg, 95% CI: − 6.77 to − 0.92, p = 0.01; I2 = 85%; 5 RCTs; n = 212) (Fig. 5)[28, 29, 34, 35, 38]. Omitting certain trials had no significant impact on this outcome on sensitivity analysis. The heterogeneity was high for this outcome. Exclusion of an outlier study [38] from the summary estimate showed a consistent finding (MD = − 1.73, 95% CI: − 2.82 to − 0.63, p = 0.002) with a statistical heterogeneity down to 9%, indicating a non-significant contribution of heterogeneity to the overall result. Subgroup analysis showed no significant difference (p = 0.43), suggesting that the positive impact of intravenous ketamine on postoperative immediate analgesic requirement was not affected by different surgical populations.
Pain outcomes at postoperative 12–24 h
Although forest plot demonstrated no significant difference between the ketamine group and the control group in the impact on pain score (MD = − 0.24, 95% CI: − 0.69 to 0.2, p = 0.28; I2 = 66%; 4 RCTs; n = 244) (Fig. 6)[28, 30, 38, 39], sensitivity analysis revealed a significant reduction in pain score associated with ketamine administration compared to that in the control group when one study was removed [38]. The heterogeneity was high for this outcome. The finding was consistent after exclusion of an outlier study [39] from the summary estimate (MD = − 0.01, 95% CI: − 0.34 to 0.35, p = 0.96) with a statistical heterogeneity being reduced to 0%, denoting a non-significant contribution of heterogeneity to the overall finding. Subgroup analysis showed no significant difference (p = 0.24), implying that the impact of intravenous ketamine on this outcome was not influenced by either laparoscopic or laparotomy BS.
Morphine consumption at postoperative 16–24 h
Despite the association of perioperative intravenous ketamine with a lower morphine consumption (i.e., MD = − 4.98 mg) at postoperative 16–24 h compared to that in the control group according to the pooled results, this difference was not significant (95% CI: − 17.8 to 7.84, p = 0.45; I2 = 90%; 5 RCTs; n = 258) (Fig. 7)[28,29,30, 34, 37]. Sensitivity analysis showed no significant influence on this outcome by omitting certain trials. The heterogeneity was high for this outcome. Exclusion of an outlier study [29] from the summary estimate demonstrated a consistent finding (MD = 3.2, 95% CI: − 2.56 to 8.95, p = 0.28) with a statistical heterogeneity being reduced to 47%, implying a non-significant role of heterogeneity in the overall result. Subgroup analysis demonstrated no significant difference (p = 0.11), suggesting that different surgical procedures had no impact on this outcome.
Postoperative nausea and vomiting
Analysis of the five RCTs with information on PONV showed no significant difference in risk between the ketamine and control groups (RR = 1.13, 95% CI: 0.64 to 2, p = 0.67, I2 = 0%; n = 264) (Fig. 8)[29, 35,36,37, 39]. Omitting certain trials had no significant impact on this outcome on sensitivity analysis.
Discussion
Acute pain management is an essential component of perioperative anesthetic care. Although opioids remain the mainstay strategy for acute pain control, opioid-based regimens are associated with the clinical concerns of potential side effects, opioid tolerance, and drug interactions [25]. Current practice guidelines advocate the use of multimodal analgesia to reduce opioid-related complications [13]. Intravenous ketamine has gained popularity in postoperative pain care; therefore, clarification of its efficacy in patients undergoing BS is warranted. The current meta-analysis demonstrated an association of perioperative ketamine use with a significantly lower opioid consumption within postoperative 6 h and reduced pain score within 1 h after BS compared to those in the control group. However, ketamine administration had no impact on postoperative morphine consumption at 16–24 h, pain score at 12–24 h, or risk of PONV. Subgroup analysis indicated no significant impact of the type of surgery (i.e., laparotomy vs. laparoscopy) on these pain-related outcomes. Nevertheless, the evidence supporting the beneficial effects of ketamine against acute postoperative pain that occurred immediately (i.e., within 1 h) and at 12–24 h was weak due to inconsistent findings on sensitivity analysis.
Previous animal experimental studies have demonstrated that ketamine acts on opioid receptors [40] and may exert its analgesic/anti-hyperalgesic action through immunomodulation [41]. Clinically, ketamine has been shown to improve the efficacy of opioids, prevent the development of opioid tolerance and spinal sensitization, as well as reduce the risk of chronic pain syndromes [41, 42]. When used at low doses (e.g., 0.05–0.3 mcg/kg/min), ketamine shows specific affinity for the postsynaptic NMDA receptor in the dorsal horn of the spinal cord [43]. A previous review article has attributed the postoperative morphine-sparing and anti-hyperalgesic effects of ketamine to its “low-dose” regimen and highlighted the additive effect of combining ketamine with opioids in the treatment of postoperative pain [41]. In addition to the opioid-induced reduction in overall neurotransmitter release through enhancing presynaptic inhibition at the first and second sensory neurons of the spinal cord, NMDA receptor inhibitors (e.g., ketamine) further blunt the metabotropic response to activation in the second sensory neuron and minimize the postsynaptic effects of glutamate release [41]. Therefore, taking into account the potential additive effects of combining ketamine and opioids in the treatment of postoperative pain [41], the pros and cons of such combinations have been widely investigated [44, 45]. In contrast, the use of ketamine at higher doses is less desirable due to adverse effects, such as hallucinations, nightmares, nausea, dizziness, and blurred vision [41].
Adequate pain control immediately following BS is important as inadequate pain management may prevent deep breathing, leading to basal lung atelectasis in this patient population [3]. A previous study has demonstrated a positive correlation between the effectiveness of postoperative analgesia with improvements in postoperative pulmonary function tests among patients with morbid obesity receiving abdominal surgery [3]. Besides, pain has also been linked to other undesirable outcomes, including an increased myocardial oxygen consumption [46]. Therefore, current evidence supports the prevention of pulmonary morbidity (e.g., atelectasis and pneumonia) and the optimization of hemodynamics through optimal pain control [47]. The established positive association of obesity with risk of cardiovascular diseases and associated mortality [48] further underscores the importance of optimal pain control in this patient population. Our results demonstrated that the use of ketamine significantly decreased the pain score during the immediate postoperative period (i.e., within 1 h) and opioid requirement within postoperative 6 h despite weak evidence on the former, suggesting that perioperative ketamine may enhance the actions of conventional analgesics in the acute postoperative setting. Our results were partly consistent with those in a recent meta-analysis [27] that showed the effectiveness of intravenous ketamine for reducing the resting pain scores at 4, 12, and 24 h as well as opioid requirements at 4 and 12 h after surgery in the general population.
Optimal pain management is one of the important components of the Enhanced Recovery after Surgery (ERAS) program [49] that aims at improving short-term recovery and reducing consumption of hospital-related resources. In addition, optimal pain management may also shorten the time to full ambulation, reducing the risk of venous thromboembolism (VTE) after BS [50, 51]. The current meta-analysis showed that perioperative intravenous ketamine did not significantly reduce the postoperative pain score and opioid consumption at 12–24 h and 16–24 h, respectively. Our findings were consistent with those in a previous meta-analysis involving non-bariatric surgeries that failed to demonstrate significant postoperative ketamine-related reductions in opioid dosage and pain scores after the first 24 h [27]. Despite its limited benefit at postoperative 12–24 h in the current study, the analgesic effect of intravenous ketamine immediately after surgery still supports its use in this patient population. Taking into consideration its limited efficacy at postoperative 24 h, intravenous ketamine and other non-opioid analgesics (e.g., nonsteroidal anti-inflammatory drugs [14] may be used in combination for multimodal analgesia to enhance analgesic efficacy. Because intravenous ketamine was administered intraoperatively in eight of the nine studies included in the current meta-analysis, further investigations are warranted to explore its efficacy when administered in the postoperative setting.
A previous review article focusing on a variety of surgeries reported the highest efficacy of ketamine for opioid reduction in patients receiving thoracic, upper abdominal, and major orthopedic procedures compared to those undergoing other surgeries [25]. Although it seems reasonable to speculate that the analgesic efficacy of intravenous ketamine for reducing opioid requirement would be more conspicuous in patients receiving laparotomy compared to those choosing the laparoscopic approach as the former is believed to be more painful than the latter [3], the current meta-analysis did not show a significant impact of the type of surgical approach on the patients’ pain score or opioid consumption at all time points. Despite the unknown reasons, the findings suggested that intraoperative intravenous ketamine may still be beneficial and could be routinely used in patients undergoing laparoscopic BS.
Although the current meta-analysis did not demonstrate a correlation between the use of perioperative intravenous ketamine and the risk of PONV, another meta-analysis of 130 studies with 8341 participants undergoing different operations reported a small but significant association between intravenous ketamine and a reduction in risk of PONV compared to that in the placebo group [52]. Despite the lack of numerical data to examine evidence linking ketamine use to the incidence of psychotomimetic adverse events, relevant descriptions available in six out of the nine included studies did not report a significant association between low-dose ketamine use and hallucinations (supplement table 2). Therefore, our findings were consistent with those of a recent meta-analysis that showed no significant correlation between the administration of ketamine and an increased risk of PONV and psychotomimetic adverse events [27]. Nevertheless, because of the limited number of trials analyzed in the present meta-analysis, further studies are needed to support our findings.
There were several limitations in the current meta-analysis. First, in spite of the documented sympathomimetic (i.e., cardiovascular stimulatory) properties of ketamine [53] and the known association between obesity and cardiovascular disease [54], the present study was unable to address the safety issue regarding ketamine use. Second, heterogeneity among studies was significant (i.e., I2 > 50%) for four of the outcomes (i.e., 1-h postoperative pain score, morphine consumption within six postoperative hours, pain outcomes at postoperative 12–24 h, and morphine consumption at postoperative 16–24 h). Such heterogeneity may have resulted from variations in methodologies and clinical factors across the studies. After exclusion of the outlier study for each outcome, the degree of heterogeneity dropped below 50% without a negative impact on the outcomes, indicating heterogeneity did not significantly impact the overall results. The consistent findings supported the application our results in clinical practice. Third, despite the wide support of low-dose ketamine use in the treatment of postoperative pain from current literature, there is no consensus on the definition of “low-dose” ketamine [41, 55]. Fourth, since only short-term pain outcomes were available in the current study, investigation into the long-term effect (e.g., chronic surgical pain) of ketamine was not feasible. Fifth, outcomes regarding postoperative hyperalgesia were unavailable because not all studies provided the relevant information. Finally, the limited data in current meta-analysis warrant further studies to support our findings.
Conclusions
This meta-analytical study is the first to assess the association of perioperative ketamine use with postoperative opioid consumption and changes in pain score in patients undergoing bariatric surgeries. The results demonstrated a correlation between perioperative ketamine use and a significantly lower opioid consumption within postoperative 6 h and a reduced pain score within 1 h after surgery compared to those in the control group. Nevertheless, ketamine use had no impact on postoperative morphine consumption at 16–24 h, pain score at 12–24 h, and the risk of postoperative nausea/vomiting. The limited number of trials included in the present investigation warrants further large-scale studies to validate our findings.
References
Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324:879–87.
Wiggins T, Guidozzi N, Welbourn R, et al. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: A systematic review and meta-analysis. PLOS Medicine. 2020;17:e1003206
Joris JL, Hinque VL, Laurent PE, et al. Pulmonary function and pain after gastroplasty performed via laparotomy or laparoscopy in morbidly obese patients. Br J Anaesth. 1998;80:283–8.
Campbell AL, Yu S, Karia R, et al. The effects of body mass index on pain control with liposomal bupivacaine in hip and knee arthroplasty. J Arthroplasty. 2018;33:1033–9.
Majchrzak M, Brzecka A, Daroszewski C, et al. Increased pain sensitivity in obese patients after lung cancer surgery. Front Pharmacol. 2019;10:626.
Lopez PP, Stefan B, Schulman CI, et al. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg. 2008;74:834–8.
Kaw R, Chung F, Pasupuleti V, et al. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109:897–906.
Blake DW, Chia PH, Donnan G, et al. Preoperative assessment for obstructive sleep apnoea and the prediction of postoperative respiratory obstruction and hypoxaemia. Anaesth Intensive Care. 2008;36:379–84.
Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2014;120:268–86
Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–93; quiz 117–8
Schuligoi R, Jocic M, Heinemann A, et al. Gastric acid-evoked c-fos messenger RNA expression in rat brainstem is signaled by capsaicin-resistant vagal afferents. Gastroenterology. 1998;115:649–60.
Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048–56.
Thorell A, MacCormick AD, Awad S, et al. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg. 2016;40:2065–83.
Abou Zeid H, Kallab R, Najm MA, et al. Safety and efficacy of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) used for analgesia after bariatric surgery: a retrospective case-control study. Obes Surg. 2019;29:911–6.
De Oliveira GS, Jr., Duncan K, Fitzgerald P, et al. Systemic lidocaine to improve quality of recovery after laparoscopic bariatric surgery: a randomized double-blinded placebo-controlled trial. Obes Surg. 2014;24:212-8
Singh PM, Panwar R, Borle A, et al. Perioperative analgesic profile of dexmedetomidine infusions in morbidly obese undergoing bariatric surgery: a meta-analysis and trial sequential analysis. Surg Obes Relat Dis. 2017;13:1434–46.
Cabrera Schulmeyer MC, de la Maza J, Ovalle C, et al. Analgesic effects of a single preoperative dose of pregabalin after laparoscopic sleeve gastrectomy. Obes Surg. 2010;20:1678–81.
Suzuki M. Role of N-methyl-D-aspartate receptor antagonists in postoperative pain management. Curr Opin Anaesthesiol. 2009;22:618–22.
Peltoniemi MA, Hagelberg NM, Olkkola KT, et al. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016;55:1059–77.
Radvansky BM, Puri S, Sifonios AN, et al. Ketamine-A narrative review of its uses in medicine. Am J Ther. 2016;23:e1414–26.
Erstad BL, Patanwala AE. Ketamine for analgosedation in critically ill patients. J Crit Care. 2016;35:145–9.
Zakine J, Samarcq D, Lorne E, et al. Postoperative ketamine administration decreases morphine consumption in major abdominal surgery: a prospective, randomized, double-blind, controlled study. Anesth Analg. 2008;106:1856–61.
Radvansky BM, Shah K, Parikh A, et al. Role of ketamine in acute postoperative pain management: a narrative review. Biomed Res Int. 2015;2015:749837
Schwenk ES, Viscusi ER, Buvanendran A, et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43:456–66.
Laskowski K, Stirling A, McKay WP, et al. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58:911–23.
Wang L, Johnston B, Kaushal A, et al. Ketamine added to morphine or hydromorphone patient-controlled analgesia for acute postoperative pain in adults: a systematic review and meta-analysis of randomized trials. Can J Anaesth. 2016;63:311–25.
Wang X, Lin C, Lan L, et al. Perioperative intravenous S-ketamine for acute postoperative pain in adults: A systematic review and meta-analysis. J Clin Anesth. 2021;68:110071
Feld JM, Laurito CE, Beckerman M, et al. Non-opioid analgesia improves pain relief and decreases sedation after gastric bypass surgery. Can J Anaesth. 2003;50:336–41.
Hasanein R, El-Sayed W, Nabil N, et al. The effect of combined remifentanil and low dose ketamine infusion in patients undergoing laparoscopic gastric bypass. Egypt J Anaesth. 2011;27:255–60.
Adhikary SD, Thiruvenkatarajan V, McFadden A, et al. Analgesic efficacy of ketamine and magnesium after laparoscopic sleeve gastrectomy: A randomized, double-blind, placebo-controlled trial. J Clin Anesthesia. 2021;68:110097
Taylor S, Kirton OC, Staff I, et al. Postoperative day one: a high risk period for respiratory events. Am J Surg. 2005;190:752–6.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Seib RK, Paul JE. Preoperative gabapentin for postoperative analgesia: a meta-analysis. Can J Anaesth. 2006;53:461–9.
Jabbour H, Jabbour K, Abi Lutfallah A, et al. Magnesium and ketamine reduce early morphine consumption after open bariatric surgery: a prospective randomized double-blind study. Obes Surg. 2020;30:1452–8.
Kasputyte G, Karbonskiene A, Macas A, et al. Role of ketamine in multimodal analgesia protocol for bariatric surgery. Medicina. 2020;56:26.
Mansour MA, Mahmoud AAA, Geddawy M. Nonopioid versus opioid based general anesthesia technique for bariatric surgery: A randomized double-blind study. Saudi J Anaesth. 2013;7:387–91.
Mehta SD, Smyth D, Vasilopoulos T, et al. Ketamine infusion reduces narcotic requirements following gastric bypass surgery: a randomized controlled trial. Surg Obes Related Dis. 2021;17:737–43.
Sollazzi L, Modesti C, Vitale F, et al. Preinductive use of clonidine and ketamine improves recovery and reduces postoperative pain after bariatric surgery. Surg Obes Related Dis. 2009;5:67–71.
Wang J, Echevarria GC, Doan L, et al. Effects of a single subanaesthetic dose of ketamine on pain and mood after laparoscopic bariatric surgery: A randomised double-blind placebo controlled study. Eur J Anaesthesiol. 2019;36:16–24.
Sarton E, Teppema LJ, Olievier C, et al. The involvement of the mu-opioid receptor in ketamine-induced respiratory depression and antinociception. Anesth Analg. 2001;93:1495–500, table of contents
Berti M, Baciarello M, Troglio R, et al. Clinical uses of low-dose ketamine in patients undergoing surgery. Curr Drug Targets. 2009;10:707–15.
Koppert W, Schmelz M. The impact of opioid-induced hyperalgesia for postoperative pain. Best Pract Res Clin Anaesthesiol. 2007;21:65–83.
Willetts J, Rice A, Balster RL. (+)-N-Allylnormetazocine (NANM)-like discriminative stimulus effects of N-methyl-D-aspartate (NMDA) antagonists. Behav Pharmacol. 1990;1:453–8.
Bell RF, Dahl JB, Moore RA, et al. Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane review). Acta Anaesthesiol Scand. 2005;49:1405–28.
Elia N, Tramèr MR. Ketamine and postoperative pain–a quantitative systematic review of randomised trials. Pain. 2005;113:61–70.
Loick HM, Schmidt C, Van Aken H, et al. High thoracic epidural anesthesia, but not clonidine, attenuates the perioperative stress response via sympatholysis and reduces the release of troponin T in patients undergoing coronary artery bypass grafting. Anesth Analg. 1999;88:701–9.
Ballantyne JC, Carr DB, deFerranti S, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg. 1998;86:598–612.
Ortega FB, Lavie CJ, Blair SN. Obesity and Cardiovascular Disease. Circ Res. 2016;118:1752–70.
Dogan K, Kraaij L, Aarts EO, et al. Fast-track bariatric surgery improves perioperative care and logistics compared to conventional care. Obes Surg. 2015;25:28–35.
Almarshad FM, Almegren M, Alshuaibi T, et al. Thromboprophylaxis after bariatric surgery. Blood Res. 2020;55:44–8.
Soleimanpour H, Safari S, Sanaie S, et al. Anesthetic considerations in patients undergoing bariatric surgery: a review article. Anesth Pain Med. 2017;7:e57568
Brinck EC, Tiippana E, Heesen M, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev. 2018;12:Cd012033
Zhou YL, Liu WJ, Wang CY, et al. Cardiovascular effects of repeated subanaesthetic ketamine infusion in depression. J Psychopharmacol. 2021;35:159–67.
Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:98–107.
Himmelseher S, Durieux ME. Ketamine for perioperative pain management. Anesthesiology. 2005;102:211–20.
Author information
Authors and Affiliations
Contributions
Conceptualization, K.-C.H. and P.-C.C.; methodology, I.-W.C.; software, C.-H.H.; formal analysis, C.-M.L; data curation, J.-Y.C. and S.-C.W.; writing—original draft preparation, K.-C.H. and C.-C.C.; writing—review and editing, C.-K.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Institutional review board statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent statement
Informed consent does not apply.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
•Pain sensitivity and need for analgesics increased in patients with obesity.
•We studied intravenous (IV) ketamine at subanesthetic doses in patients receiving BS.
•IV ketamine reduced pain score and opioid need in immediate postoperative period.
•IV ketamine had no impact on pain in late postoperative period and nausea/vomiting
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hung, KC., Wu, SC., Chang, PC. et al. Impact of Intraoperative Ketamine on Postoperative Analgesic Requirement Following Bariatric Surgery: a Meta-analysis of Randomized Controlled Trials. OBES SURG 31, 5446–5457 (2021). https://doi.org/10.1007/s11695-021-05753-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05753-8