Abstract
Background
Bariatric surgery is often associated with moderate to severe pain. In patients with obesity, opioids have the potential to induce ventilatory impairment; thus, opioid use needs to be limited. This study aimed to compare the novel ultrasound-guided erector spinae plane block (ESPB) technique with controls in terms of intraoperative opioid consumption and postoperative pain control.
Methods
A total of 63 patients with morbid obesity who underwent laparoscopic bariatric surgery were included in this randomized study. Patients were randomly assigned to the bilateral erector spinae plane block (ESPB) group or the control group. To evaluate perioperative pain and to adjust opioid dose, analgesia nociception index (ANI) was monitored during surgery. Total opioid dose was recorded for each patient. In addition, pain was evaluated using visual analogue scale (VAS) scores for 24 h following the operation.
Results
Total intraoperative remifentanil dose was significantly lower in the ESPB group when compared to controls (1356.3 ± 177.8 vs. 3273.3 ± 961.9 mcg, p < 0.001). In the ESPB group, none of the patients required additional analgesia during follow-up. In contrast, all control patients required analgesia. ESPB group had significantly lower VAS scores at all postoperative time points (p < 0.001 for all).
Conclusion
Bilateral ultrasound-guided ESPB appears to be a simple and effective technique to improve perioperative pain control and reduce intraoperative opioid need in patients with morbid obesity undergoing bariatric surgery.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity represents a major disease burden and healthcare challenge globally due to its increasing prevalence over the last decades. From 1980 to 2013, the worldwide prevalence of overweight/obesity increased by 27.5% for adults and 47.1% for children, resulting in an estimated global total number of obese/overweight individuals slightly more than two billion [1]. Obesity is associated with serious conditions including cardiovascular disease, diabetes, some cancers, kidney disease, sleep apnea, gout, osteoarthritis, and hepatobiliary disease, resulting in increased mortality and decreased life expectancy among individuals with obesity [2]. On the other hand, weight loss leads to improvement of these conditions and outcomes [2]. Life style modifications with diet, exercise, and behavioral therapy, pharmacotherapy, and surgery are used for the management of obesity [2, 3].
Bariatric surgery represents an effective and a sustainable treatment modality in patients with morbid obesity [3,4,5], laparoscopic sleeve gastrectomy and gastric bypass being the most common procedures [2]. However, these procedures are mostly associated with moderate to severe postoperative pain, which is often a combination of abdominal wall pain and visceral pain.
Postoperative pain, when not adequately controlled, has unfavorable consequences such as impaired quality of life and recovery as well as increased risk of surgical complications and persistent postsurgical pain [6]. Patients with morbid obesity represent a special group when it comes to perioperative pain management. These patients have a higher incidence of sleep-disordered breathing; therefore, opioids have the potential to induce ventilatory impairment resulting in increased morbidity/mortality. Stepwise- and severity-based multi-modal analgesia with opioid-sparing approach can improve safety; thus, the Enhanced Recovery After Surgery (ERAS) guidelines for bariatric surgery currently recommend opioid reduction in bariatric surgery [7]. Both systemic analgesics and regional approaches such as transversus abdominis plane (TAP) block, intraperitoneal and wound modalities, and neuraxial techniques are being used for postoperative pain control in bariatric surgery with some success [8].
Ultrasound-guided erector spinae plane block (ESPB) is a novel regional anesthesia technique where local anesthetic agent is injected deep into the erector spinae muscle to the fascial plane and allowed to diffuse in caudal and cranial direction. It has been shown effective in a number of conditions such as thoracic surgery/trauma, painful conditions of the limbs, cardiac surgery, breast surgery, and abdominal surgery [9]. To date, only few studies examined the use of ESPB in bariatric surgery [10,11,12], all indicating potential benefits of the technique in terms of opioid sparing and pain control.
This study aimed to compare bilateral ultrasound-guided erector spinae plane block technique with controls who received bupivacaine injection to the trocar sites, in terms of intraoperative opioid consumption, postoperative pain control in the first 24 h, and need for rescue analgesic.
Methods
Patients
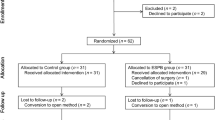
Sixty-two patients with morbid obesity who underwent laparoscopic bariatric/metabolic surgery (sleeve gastrectomy or gastric bypass) at Marmara University Pendik Training and Research Hospital were included in this randomized study. Patients were required to have a BMI ≥ 40 kg/m2 and ASA physical status class 2 or 3. Exclusion/drop-out criteria were as follows: any complication (allergic reaction or local anesthesia-related complication), failure, or patient refusal during the block procedure; switch to open surgery or a change in the planned surgical protocol; analgesia nociception index (ANI) and/or bispectral index (BIS) monitoring not possible; or patient does not provide consent or withdraws consent at any point after inclusion. The study protocol was approved by the local ethics committee (Ethics Committee for Clinical Studies, Marmara University, Medical Faculty; date, January 3, 2020; number, 09.2020.128). All patients provided informed consent prior to study entry, and the study was conducted in accordance with the Declaration of Helsinki. The study was registered to ClinicalTrials.gov with identifier NCT04915521.
Randomization for the Study Groups
Patients were randomly assigned to the erector spinae plane block (ESPB) group or the control group using sealed envelopes with 1:1 ratio. ESPB group received ultrasound-guided bupivacaine and lidocaine injection at T9 vertebral level before anesthesia induction. Control group received 5 ml 0.5% bupivacaine injection to each trocar site (total of 25 ml) at the beginning of the operation.
Erector Spinae Plane Block Technique
An experienced anesthesiologist performed ESPB in all patients in sitting position bilaterally. A linear ultrasound probe (6–13 MHz) was used for ultrasound guidance. Injection was done using in-plane technique. A 22G block needle (100 mm, B-Braun, Germany) was inserted 3 cm lateral to the T9 spinous process and advanced in cranio-caudal direction. To separate erector spinae muscle from the transverse process, initially 1–2 ml saline was injected; and then 20 ml 0.5% bupivacaine and 5 ml 0.2% lidocaine were injected following separation. Diffusion of the drugs in erector spinae plane at cranio-caudal line was ensured. No analgesic or sedative was used during the procedure.
Intraoperative Anesthesia Management
For anesthesia management, total intravenous anesthesia and a short-acting opioid were used. Based on the recommendations of recent guidelines [7], a short-acting agent (remifentanil) was preferred. In addition, titration of the opioid dose would be easier with a short-acting agent since opioid dose was titrated using ANI to administer the lowest intraoperative dose possible [7, 13]. No volatile anesthetics were used since total intravenous anesthesia offers advantages in terms of postoperative nausea and vomiting, particularly in patients prone to this complication [7, 14]. Anesthesia was induced with propofol 2.5–3.5 mg/kg and 1 mcg/kg bolus dose of remifentanil, which was followed by rocuronium 0.6 mg/kg to aid intubation. Corrected body weight (CBW) was used for the dose adjustment of propofol, and ideal body weight (IBW) was used for the dose adjustments of remifentanil and rocuronium. Total intravenous anesthesia with propofol and remifentanil was used for maintenance, and the initial doses were as follows: propofol 6–10 mg/kg/h and remifentanil 0.5 mcg/kg/h. Propofol and remifentanil dose was adjusted to keep BIS between 40 and 45 and ANI between 50 and 70, respectively. Electrocardiography, non-invasive blood pressure, bispectral index (BIS, Medtronic, Minneapolis), and ANI were continuously monitored and recorded at every 30 min.
To evaluate perioperative pain and to adjust opioid dose, ANI was continuously monitored throughout the surgical procedure. Two ANI electrodes were placed on the sternum and at the level of left nipple (to the same places with V1 and V5 ECG electrodes, respectively). Total remifentanil dose was recorded for each patient. The depth of anesthesia was monitored to adjust propofol dose using BIS (Aspect Medical Systems, Natick, Mass, ABD).
Fifteen minutes before the completion of the surgical procedure, both groups received 1 g paracetamol, and the control group received 150 mg tramadol. Tramadol use before the completion of surgery is the standard care in our institution to prevent early postoperative pain, where ESPB is not used. For ESPB patients, we prefer not to administer tramadol, in an attempt to minimize total opioid administration.
Assessments
The primary outcome measure was total intraoperative opioid consumption, and the secondary outcome measure was the change in postoperative pain as assessed by 10-point visual analogue scale (VAS). A blinded investigator recorded self-assessed VAS scores of the patients upon awakening and at 6, 12, and 24 h. In case VAS ≥ 4, rescue analgesic was given (100 mg tramadol). Timing of first analgesic requirement was recorded.
Statistical Analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS) version 21 software. Mean ± standard deviation or median (range) was used to express descriptive data. Both hypothesis tests and graphical method were used to test the distribution of continuous variables. Student t test for independent samples or Mann–Whitney U test was used to test between-group differences. Fisher’s exact test was used to compare the two groups in terms of complication frequencies. Two-way ANOVA test for repeated measurements was used to examine the significance of differences between groups in postoperative VAS scores and intraoperative measurements over time. All data were recorded by dedicated investigators, and there were no missing values of intraoperative measurements or VAS scores. In addition, there were not significant time point deviations from the prespecified time points. Therefore, two-way ANOVA test for repeated measurements was considered suitable. Two-sided p values < 0.05 were considered indication of statistical significance.
Results
Patients
Sixty-two patients were randomized. One patient in the ESPB group was excluded since adequate ultrasound image could not be obtained due to fatty tissue in the back, and a control patient was excluded for switching to open surgery due to intraoperative bleeding. Thus, thirty patients were assigned to each of the groups (ESPB group, 30; controls, 30). The two groups were similar in terms of mean age: 40.2 ± 12.2 versus 39.4 ± 10.5 years for the ESPB groups and controls, respectively (p = 0.803).
Intraoperative Measurements
Figure 1 shows intraoperative changes in heart rate, mean arterial pressure, ANI, and BIS scores at 15-min intervals. There was a significant difference between the ESPB group and the controls in terms of intraoperative heart rate (p < 0.001), mean arterial pressure (p < 0.001), and ANI (p < 0.001) changes over time (Fig. 1a, b, and c). Heart rate was lower in controls at baseline (p < 0.001), and no difference was evident at 15 and 30 min (p = 0.077 and 0.182, respectively); however, ESPB group had significantly lower heart rate in all other time points (p < 0.001 for all) (Fig. 1a). ESPB group had lower mean arterial pressure starting from 15 min (p = 0.025) and thereafter (p < 0.001 for all) (Fig. 1b). ANI score was lower in the ESPB group at all time points (p < 0.01 for all) (Fig. 1c). However, the two groups did not differ in terms of changes in BIS scores (p = 0.916) (Fig. 1d).
Intraoperative changes in heart rate (a), mean arterial pressure (b), analgesia nociception index (ANI) (c), and bispectral index (BIS) score (d) over time at 15-min intervals. Dotted lines indicate control group, and straight lines indicate ESPB group. Error bars indicate 95% confidence intervals for the mean
Intraoperative Medications
Total intraoperative remifentanil dose was significantly lower in the ESPB group when compared to controls (1356.3 ± 177.8 vs. 3273.3 ± 961.9 mcg, p < 0.001). However, the two groups did not differ regarding total propofol dose (1790.0 ± 323.1 vs. 1867.4 ± 447.1 mg, p = 0.446).
Postoperative Analgesia Requirement
In the ESPB group, none of the patients required additional analgesia during the 24-h postoperative follow-up period. In contrast, all control patients required analgesia within postoperative 24 h after a mean duration of 3.5 ± 0.6 h (median, 4; range, 2–4) from awakening. At the time when first analgesic was given, the mean VAS score was 7.0 ± 1.0 (median, 7; range, 5–8).
Changes in Postoperative VAS Scores
Figure 2 shows changes in VAS scores within 24 h after awakening, where a significant difference in VAS scores was evident over time between the two groups (p < 0.001). ESPB group had significantly lower VAS scores at all time points (p < 0.001 for all).
Complications
Four patients (13.3%) in the control group developed atelectasis, whereas none of the patients in the ESPB group developed atelectasis; however, the difference did not reach statistical significance (p = 0.112).
Discussion
In this study, ultrasound-guided bilateral ESPB provided significant relief of postoperative pain throughout the 24-h period as well as a significant reduction in intraoperative opioid consumption, thus representing a promising pain management modality for patients with morbid obesity undergoing laparoscopic bariatric surgery. To the best of our knowledge, only a few studies have examined so for the potential role of ESPB in perioperative pain management in patients with obesity undergoing bariatric surgery.
ESPB has gained popularity as a regional anesthetic technique in a variety of surgical [15,16,17,18,19,20,21] and non-surgical painful conditions [22,23,24,25,26] since its first definition by Forero et al. in two patients with neuropathic pain [27]. Initial data on its use in patients with morbid obesity came from a case series of three patients [11]. In that study, bilateral ESPBs were performed at T7 transverse process level to relieve postoperative pain after bariatric surgery with encouraging results. This was followed by two recent randomized controlled trials [10, 12]. The randomized study by Abdelhamid et al. compared three techniques in terms of perioperative pain control in 66 patients who received laparoscopic sleeve gastrectomy: bilateral ESPB at T9 level, bilateral subcostal transversus abdominis block, and opioid analgesia. The patients who received bilateral ESPB had lower pain scores when compared to other techniques, particularly within the first 12 h. In the ESPB group, total postoperative 24-h opioid (pethidine) consumption and total postoperative 24-h paracetamol consumption were significantly lower than only controls (opioid analgesia group) but not different than transversus abdominis block group, and total intraoperative opioid (fentanyl) consumption was significantly lower than both other groups. The randomized study by Mostafa et al. compared ESPB at T7 level and controls (sham block) in terms of postoperative pain control and perioperative opioid consumption [12]. That study also evaluated the postoperative pulmonary function. ESPB provided an advantage over controls in terms of postoperative pain management during the first 8 h; however, the pain scores were similar between 12 and 24 h. ESPB was associated with less intraoperative and postoperative (within 24 h) morphine consumption. However, the two groups did not differ in terms of pulmonary function impairment. In the present study, ESPB was associated with less intraoperative remifentanil consumption, better postoperative pain control, and no need for rescue analgesia, which are in line with previous evidence including the study by Mostafa et al. Higher intraoperative remifentanil may be partly responsible for the higher postoperative VAS scores in the control group when compared to the ESPB group, possibly through hyperalgesia induced by remifentanil, in addition to the beneficial effect of ESPB in the study group. However, it is of note to emphasize that advantage of the ESPB in terms of postoperative pain control lasted 24 h in the present study and none of the patients required rescue analgesia. Regarding the similarities and differences between our findings and the study by Mostafa et al., in both studies, ESPB resulted in lower intraoperative opioid need and less postoperative analgesia; however, in that study, lower VAS scores were seen in the ESPB group only during the first 8 h. In our study on the other hand, the benefit on VAS scores was maintained for 24 h. One explanation may be the higher dose of blocking agent used in our study (20 ml 0.5% bupivacaine plus 5 ml 0.2% lidocaine versus 20 ml 0.25% bupivacaine). Another explanation may be the differences in the level of the block, which was lower in our (T9 versus T7) technique. And finally, opioids administered through PCA might have masked the additional benefit of ESPB beyond 8 h in Mostafa study, during which the patient is well familiarized with the PCA procedure. Thus, such a difference in the duration of analgesic effect may be attributed to the differences in the techniques used (i.e., thoracic level, anesthetic dose/concentration, postoperative analgesia management), which may be subject to future research.
ESPB has several advantages in terms of pain control when it comes to abdominal surgery in general, particularly in bariatric surgery. When anesthetic solution is injected into the deep fascial plane of erector spinae muscle, it spreads several levels upwards and downwards; thus, when injected to the lower vertebral levels (such as T7 to T9), it has the potential to block lower thoracoabdominal nerves that innervate the abdomen [9]. In addition, anesthetic agent penetrates to the thoracic intervertebral space and has blocking effect for both ventral branches and communicating branches, thus providing both somatic and visceral blockade [9].
Currently used methods for perioperative pain management have certain shortcomings. Respiratory side effects of opioids are a major concern, particularly for individuals with obesity who are more prone to respiratory impairment. Non-steroid anti-inflammatory agents have the potential to increase the risk of some complications such as anastomotic leaks [28]. Regional anesthetics have the advantage of having less systemic side effects. In laparoscopic abdominal surgery, local anesthetics are infiltrated to the trocar sites, but they may have limited efficacy and duration of action and are not effective in controlling visceral pain [29,30,31]. Epidural analgesia has the disadvantages of cost and technique-related complication risks. Transversus abdominis plane (TAP) block (either ultrasound-guided or using blind technique) has been shown to reduce opioid consumption and postoperative pain after lower abdominal surgery in a number of studies [32]. Probably since it blocks only somatic nerves, it does not seem to have advantage over systemic multi-modal analgesia in patients that underwent laparoscopic bariatric surgery [33]. ESPB on the other hand is a relatively safe and simple technique requiring less expertise, and the procedure is remote from vital structures. However, one should always keep potential complications in mind, particularly in patients with obesity, such as pneumothorax, intravascular injection, and local anesthetic systemic toxicity [34, 35]. In addition, technical difficulties in ultrasound examination may be encountered in patients with excess fat and a very deep fat layer, which may result in block failure and safety problems. However, such technical difficulties may be offset by high level of expertise, high device quality, and appropriate device setting [12]. In addition, the ability of ESPB to block both somatic and visceral nerves allows it to target two main pain sources after abdominal surgery.
In this study, initial design did not include a detailed respiratory monitoring, so no such data exist. Lack of a detailed postoperative respiratory evaluation (for example, using spirometry) represents a potential limitation of the study and precludes to identify any advantage of ESPB in terms of respiratory impairment. We believe that such an analysis should be added in related future studies.
Conclusion
Findings of this study suggest that ultrasound-guided bilateral ESPB represents a relatively simple and effective technique for the management of perioperative pain in patients undergoing laparoscopic bariatric surgery. In addition, it reduces intraoperative opioid need, which is particularly important for individuals with obesity. Further large-scale studies are required to examine its use in this setting with robust evidence.
References
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81.
Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018;39:79–132.
Alamuddin N, Bakizada Z, Wadden TA. Management of obesity. J Clin Oncol. 2016;34:4295–305.
Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96.
Nielsen S, Svane MS, Bojsen-Møller KN, et al. Effects of bariatric surgery on weight loss and quality of life. Anaplastology. 2014;3:1000136.
Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–57.
Thorell A, MacCormick AD, Awad S, et al. Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg. 2016;40:2065–83.
Budiansky AS, Margarson MP, Eipe N. Acute pain management in morbid obesity - an evidence based clinical update. Surg Obes Relat Dis. 2017;13:523–32.
Chin K, Adhikary S, Forero M. Erector spinae plane (ESP) block: a new paradigm in regional anesthesia and analgesia. Curr Anesthesiol Rep. 2019;9:271–80.
Abdelhamid BM, Khaled D, Mansour MA, et al. Comparison between the ultrasound-guided erector spinae block and the subcostal approach to the transversus abdominis plane block in obese patients undergoing sleeve gastrectomy: a randomized controlled trial. Minerva Anestesiol. 2020;86:816–26.
Chin KJ, Malhas L, Perlas A. The erector spinae plane block provides visceral abdominal analgesia in bariatric surgery: a report of 3 cases. Reg Anesth Pain Med. 2017;42:372–6.
Mostafa SF, Abdelghany MS, Abu Elyazed MM. Ultrasound-guided erector spinae plane block in patients undergoing laparoscopic bariatric surgery: a prospective randomized controlled trial. Pain Pract. 2020;4:445–53.
Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr. 2012;31:783–800.
Apfel CC, Kranke P, Katz MH, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. 2002;88:659–68.
Abu Elyazed MM, Mostafa SF, Abdelghany MS, et al. Ultrasound-guided erector spinae plane block in patients undergoing open epigastric hernia repair: a prospective randomized controlled study. Anesth Analg. 2019;129:235–40.
Ahiskalioglu A, Tulgar S, Celik M, et al. Lumbar erector spinae plane block as a main anesthetic method for hip surgery in high risk elderly patients: initial experience with a magnetic resonance imaging. Eurasian J Med. 2020;52:16–20.
Altiparmak B, Korkmaz Toker M, Uysal AI, et al. Ultrasound-guided erector spinae plane block versus oblique subcostal transversus abdominis plane block for postoperative analgesia of adult patients undergoing laparoscopic cholecystectomy: randomized, controlled trial. J Clin Anesth. 2019;57:31–6.
Chen N, Qiao Q, Chen R, et al. The effect of ultrasound-guided intercostal nerve block, single-injection erector spinae plane block and multiple-injection paravertebral block on postoperative analgesia in thoracoscopic surgery: a randomized, double-blinded, clinical trial. J Clin Anesth. 2020;59:106–11.
Krishna SN, Chauhan S, Bhoi D, et al. Bilateral erector spinae plane block for acute post-surgical pain in adult cardiac surgical patients: a randomized controlled trial. J Cardiothorac Vasc Anesth. 2019;33:368–75.
Tulgar S, Kapakli MS, Senturk O, et al. Evaluation of ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: a prospective, randomized, controlled clinical trial. J Clin Anesth. 2018;49:101–6.
Tulgar S, Kose HC, Selvi O, et al. Comparison of ultrasound-guided lumbar erector spinae plane block and transmuscular quadratus lumborum block for postoperative analgesia in hip and proximal femur surgery: a prospective randomized feasibility study. Anesth Essays Res. 2018;12:825–31.
Adhikary SD, Liu WM, Fuller E, et al. The effect of erector spinae plane block on respiratory and analgesic outcomes in multiple rib fractures: a retrospective cohort study. Anaesthesia. 2019;74:585–93.
Alici HA, Ahiskalioglu A, Aydin ME, et al. High volume single injection lumbar erector spinae plane block provides effective analgesia for lower extremity herpes zoster. J Clin Anesth. 2019;54:136–7.
Chung K, Kim ED. Continuous erector spinae plane block at the lower lumbar level in a lower extremity complex regional pain syndrome patient. J Clin Anesth. 2018;48:30–1.
Tekin E, Ahiskalioglu A, Aydin ME, et al. High-thoracic ultrasound-guided erector spinae plane block for acute herpes zoster pain management in emergency department. Am J Emerg Med. 2019;37(375):e1–3.
Ueshima H, Otake H. Continuous erector spinae plane block for pain management of an extensive burn. Am J Emerg Med. 2018;36(2130):e1–2.
Forero M, Adhikary SD, Lopez H, et al. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621–7.
Subendran J, Siddiqui N, Victor JC, et al. NSAID use and anastomotic leaks following elective colorectal surgery: a matched case-control study. J Gastrointest Surg. 2014;18:1391–7.
Hilvering B, Draaisma WA, van der Bilt JD, et al. Randomized clinical trial of combined preincisional infiltration and intraperitoneal instillation of levobupivacaine for postoperative pain after laparoscopic cholecystectomy. Br J Surg. 2011;98:784–9.
Barazanchi AWH, MacFater WS, Rahiri JL, et al. Evidence-based management of pain after laparoscopic cholecystectomy: a PROSPECT review update. Br J Anaesth. 2018;121:787–803.
Gurusamy KS, Nagendran M, Guerrini GP, et al. Intraperitoneal local anaesthetic instillation versus no intraperitoneal local anaesthetic instillation for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;3:CD007337.
Brogi E, Kazan R, Cyr S, et al. Transversus abdominal plane block for postoperative analgesia: a systematic review and meta-analysis of randomized-controlled trials. Can J Anaesth. 2016;63:1184–96.
Albrecht E, Kirkham KR, Endersby RV, et al. Ultrasound-guided transversus abdominis plane (TAP) block for laparoscopic gastric-bypass surgery: a prospective randomized controlled double-blinded trial. Obes Surg. 2013;23:1309–14.
Lopez MB, Cadorniga AG, Gonzalez JM, et al. Erector spinae block. a narrative review. Cent Eur J Clin Res. 2018;1:28–39.
Ueshima H. Pneumothorax after the erector spinae plane block. J Clin Anesth. 2018;48:12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Perioperative pain management is mostly challenging in bariatric surgery.
• ESPB reduces intraoperative opioid dose.
• ESPB provides effective postoperative pain control.
• ESPB is a simple and effective technique that can be used in bariatric surgery.
Rights and permissions
About this article
Cite this article
Zengin, S.U., Ergun, M.O. & Gunal, O. Effect of Ultrasound-Guided Erector Spinae Plane Block on Postoperative Pain and Intraoperative Opioid Consumption in Bariatric Surgery. OBES SURG 31, 5176–5182 (2021). https://doi.org/10.1007/s11695-021-05681-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05681-7