Abstract
Background
Fasting-mimicking diet (FMD) has been recently promoted to achieve similar metabolic changes of fasting. The purpose of our study was to compare the effect of FMD versus continuous energy restriction (CER) on anthropometric measurements, body composition, glucose metabolism, and serum levels of leptin, neuropeptide Y (NPY), and total ghrelin.
Methods
A randomized controlled trial (RCT) was conducted on 60 women with obesity aged 18–55 years. Subjects received either a 5-day FMD (low in energy, sugars, and proteins, but high in unsaturated fats) or a CER (an average daily energy deficit of 500 kcal) for 2 months. Anthropometric and biochemical factors were measured at baseline and the end of the study. Serum levels of leptin, total ghrelin, and NPY were tested with an ELISA kit. Physical activity and dietary intakes were also recorded.
Results
There was no significant difference in weight loss between the two groups: mean weight change for CER was − 2.29 (standard deviation [SD], 1.95) kg compared to − 1.13 (2.27) kg for FMD (p = 0.06). There was more reduction in the basal metabolic rate (BMR) in the CER group (p = 0.045). Favorable effects on fat mass and muscle mass were only seen in the FMD group. Although insulin resistance was reduced in the FMD group compared to the CER group, results were not significant after adjustment. After controlling for potential confounders, there was a significant increase in serum levels of total ghrelin (p = 0.048) and NPY (p = 0.041) following CER; however, results for circulating leptin were not statistically significant (p = 0.48).
Conclusions
There was no significant difference in weight loss following FMD and CER. However, FMD was more effective at reducing insulin resistance and regulating appetite-regulating hormones as well as preserving muscle mass and BMR.
Trial Registration
This trial was registered at the Iranian Clinical Trial Registry (https://www.irct.ir/trial/40881) with the IRCT identification number IRCT20190717044244N1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a growing public health problem that leads to long-term health consequences, including cardiovascular disease, type 2 diabetes mellitus, musculoskeletal disorders, and several types of cancer [1,2,3]. The obesity epidemic has been spreading globally and has reached pandemic status [4]. In 2015, obesity affected 603 million adults and 107 million children worldwide [5]. The treatment modality for obesity is considered to be effective when obesity comorbidities and related costs are reduced [6]. Most guidelines for the treatment of obesity recommend a continuous moderate energy restriction (i.e., 20–30% of energy needs). Energy restriction has been reported to decrease serum concentrations of anorectic gut hormones and increase orexigenic gut hormones [7, 8]. Several issues, however, including poor compliance, weight regain, loss of muscle mass, and increased appetite, limit the potential benefits of such weight loss diets [9,10,11]. In recent years, several variations of intermittent fasting have been used as an alternative strategy for weight management [12]. In these dietary strategies, individuals may not need to restrict energy intake every day, instead using intermittent energy restriction in which energy intake is completely (100%) or partially (≥ 70%) restricted with fasting intervals ranging between 20 and 36 h [13,14,15].
Almost all trials with intermittent fasting resulted in some degree of weight loss, ranging from 2.5 to 9.9%, and fat mass loss [16,17,18]. However, inconsistent findings have been reported for glucose metabolism, insulin sensitivity, and hypothalamic control of appetite [19,20,21]. Water-only fasting for 24–72 h may be difficult to adhere to for the great majority of the population and can lead to adverse effects such as vomiting, nausea, and exacerbation of previous malnutrition, particularly in elderly subjects [22]. Thus, a modified form of fasting, the fasting-mimicking diet (FMD), has been recently developed in which a low intake of energy, protein, and sugar is attained to produce metabolic changes similar to fasting while minimizing the harmful effects of a complete lack of food intake. Mice fed 2 cycles of 4-day FMD, twice a month, and an ad lib diet in the period between FMD cycles experienced an improvement in metabolic changes and body composition [22]. FMD was reported to be safe and feasible when administered for 5 days in 3 monthly cycles in 19 generally healthy adults [22]. In a recent trial with healthy individuals, a 5-day FMD in 3 cycles resulted in moderate weight loss and reduction in total body fat and trunk fat compared to adherence to a normal diet for 3 months [23].
Although earlier reports have shown beneficial effects of FMD on body composition, it has not yet been determined if it is effective on hormonal regulation of energy balance. We hypothesized that a 5-day FMD in 2 monthly cycles and a moderate daily energy restriction may have comparable effects on body composition and appetite-regulating hormones. Therefore, this study aimed to determine changes in body weight, waist circumference, body composition, glucose metabolism, and serum levels of leptin, neuropeptide-Y, and total ghrelin in comparing FMD and CER.
Materials and Methods
Participants
The study was performed from August 24, 2019, and March 5, 2020. Participants were recruited via flyers posted around a university as well as advertisements via SMS. Participants were selected using a questionnaire that contained information on body mass index (BMI), demographic factors, and medical history. Inclusion criteria included metabolically healthy women between 18 and 55 years of age with BMIs ranging from 30 to 35 (ratio of height and weight, expressed as kg/m2). Participants who had hyperandrogenism, polycystic ovary syndrome, heart diseases, or considerable food allergy, or demonstrated features of any kind of metabolic disorder that could affect gluconeogenesis, were not included in the study. We also excluded those who used cigarettes or any other tobacco products or had lost or gained more than 3 kg in weight during the last 3 months. We did not include women who were pregnant or nursing. Participants were requested to prevent pregnancy during the trial. This study was conducted according to the guidelines stipulated in the Declaration of Helsinki. The purpose, procedures, and risks of the study were explained to each participant prior to inclusion, and all participants were enrolled only after obtaining written informed consent. Written informed consent covered publication of the study. This trial was registered at the Iranian Clinical Trial Registry (https://www.irct.ir/trial/40881) with the IRCT identification number IRCT20190717044244N1.
Study Design
Participants were stratified based on age, then randomly assigned using a computer-generated randomization process with a 1:1 allocation ratio to two study groups: 2 months of either a continuous energy restriction (CER) (n = 30) or a FMD (n = 30) diet. To avoid bias, randomization was undertaken by an independent investigator who had no contact with participants before randomization and who was not involved in implementation of dietary interventions. Regarding the nature of the interventions, participants and study personnel were not blinded to allocation. However, study personnel involved in data collection and specimen analysis were blinded to group assignments. To ensure that subjects maintained their usual diet and physical activity levels throughout the intervention, participants were requested to complete a 1-day physical activity record and a 3-day food record for 3 separate nonconsecutive days, including 1 weekend day, before enrollment, and after completion of the trial. Telephone calls occurred every 2 weeks to ensure diet compliance. Household measurements were applied to convert patient-reported portion sizes to grams per day. Quantities of nutrients consumed by study participants were calculated using Nutritionist IV software (First Databank, San Bruno, CA, USA) modified for Iranian foods. Physical activity records were processed manually. The short form of the International Physical Activity Questionnaire (IPAQ) was used to assess the level of physical activity at the beginning and end of the study, reported as metabolic equivalents (METs) in min/day [24].
Group 1 (CER)
Participants in the CER group received a diet plan formulated to produce an average 500 kcal/day energy deficit based on the U.S. National Institutes of Health clinical guidelines on obesity [25], with approximately 30% of energy derived from fat, 50% of energy derived from carbohydrate, and 20% of energy derived from protein based on recommendations from the Institute of Medicine [26]. Energy requirements of each subject were estimated based on resting energy expenditure using the Harris-Benedict equation and physical activity levels.
Group 2 (FMD)
Participants in the FMD group received instructions on how to consume the FMD for 5 continuous days and how to return to their usual diet after completion until the next cycle that would commence about 25 days later. Subjects were asked not to alter their usual physical activity during the trial. FMD is a diet based on plants comprising limited food options such as vegetable soups, drinks, and snacks, with a supplement tablet to cover micronutrients and essential fatty acid needs. The diet is designed to achieve fasting-like effects on serum concentrations of glucose and ketone bodies while providing both macronutrients and micronutrients to minimize the burden of fasting and adverse effects [22]. Individuals in this diet plan were required to consume 4600 kJ (1099 kcal) comprising 11% protein, 46% fat, and 43% carbohydrate on the first day, and 3000 kJ (717 kcal) comprising 9% protein, 44% fat, and 47% carbohydrate during days 2 to 5. All FMD meals were provided using half-ready soups. All subjects were also given a multivitamin-mineral supplement and an omega-3 capsule that provided 100% of daily value to take over the 5 days of the FMD cycle. Subjects were allowed to freely drink water and other calorie-free beverages. Nutritional information of the FMD is provided in Supplementary Figure 1. After completion of the first cycle, another package containing soups and supplements was delivered to participants for the second cycle. Participant compliance was monitored by asking them to record all the ingested foods during the 5 days of FMD and to return their empty soup and supplement packages after the completion of each cycle.
Assessment of Anthropometric Measurements and Body Composition
Anthropometric measurements were taken by a trained dietitian. Body composition, weight, height, and waist circumference were measured at study baseline and at study completion 2 months after the intervention began. To measure height and waist circumference, a non-stretch tape measure (Seca) was used to the nearest 0.1 cm. Waist circumference was measured in the slimmest area with no pressure on the body surface at the end of a normal exhalation. For height measurement, participants were asked to stand without shoes with both the shoulder blades, buttocks, and heels touching the wall and their heads situated in the Frankfurt position. Body mass index (kg/m2) was calculated as weight in kilograms divided by height in meters squared. Body weight, fat mass, muscle mass, and basal metabolic rate (BMR) were measured with participants wearing minimal clothing and no shoes standing on the weighing platform without bending their knees on the morning following a night fasting using the TANITA BC-418 body analyzer that measured to the nearest 0.1 kg [27]. All testing was conducted in a quiet, mildly lit, heated room. Participants were required to avoid caffeine intake for at least 10 h and to abstain from engaging in strenuous activity for 24 h prior to the test.
Assessment of Biochemical Measurements
Fasting venous blood was obtained from each participant at the beginning and the end of the study to measure serum levels of fasting plasma glucose (FPG), insulin, leptin, total ghrelin, and neuropeptide-Y (NPY). FPG levels were measured on the day of blood sampling. To separate serum, blood samples were centrifuged immediately (Hettich D-78532, Tuttlingen, Germany) at 1465g for 10 min and then the serum was frozen at − 70 °C until further measurements. Commercially available kits were used to measure FPG (Pars Azmun, Tehran, Iran). Intra- and inter-assay CVs for FPG were 1.5% and 2.7%, respectively. ELISA kits (Cobas Integra 800 Autoanalyzer, Roche Diagnostics, Germany) were used to assay serum insulin levels. Intra- and inter-assay CVs for serum insulin were 1.9% and 2.6%, respectively. Homeostatic model assessment for insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) were calculated based on suggested formulas [28]. Serum levels of leptin, total ghrelin, and NPY were quantified using ELISA kits (EASTBIOPHARM, China, under license by the USA) with intra- and inter-assay CVs of < 10% and < 12%, respectively. QUICKI was calculated from FPG and insulin levels according to Katz et al. [29] using the formula QUICKI = 1/(log [insulin in mU/l] + log [FPG in mg/dl]). HOMA-IR was calculated from FPG and serum according to Matthews et al. [30] using the formula HOMA-IR = insulin in mU/l × FPG in mg/dl/405. 1/HOMA-IR was also calculated.
Statistical Analysis
The sample size was estimated taking into consideration type 1 (α) and type 2 (β) errors of 0.05 and 0.10 (power = 90%), respectively, and body weight as a key variable; the difference of body weight was 3 [22]. Therefore, the sample size was estimated to be 25 participants in each group using the suggested formula for parallel clinical trials [31]. To account for probable dropouts during the study, 30 participants were recruited for each group. To determine if the distribution of variables was normal, a Kolmogorov-Smirnov test was performed, and the histograms of each variable were generated to visually assess the distribution. The intention-to-treat (ITT) approach was used for data analysis. ITT analysis includes every participant who is randomized according to randomized treatment assignment and ignores noncompliance, protocol deviations, withdrawal, and anything that happens after randomization [32]. Within-group comparisons were performed using a paired-samples t test. ANOVA of mixed-model repeated measures was applied to examine effects of FMD and CER on primary study outcomes. The Chi-square test was used for categorical variables and 1-factor ANOVA for continuous variables. Concerning the assumption that missing values are randomly missing, we did not impute these values because the mixed-model analysis without ad hoc imputation is the same power as analysis using mixed methods (http://www.rti.org/rtipress). To avoid potential bias, all analyses were adjusted for baseline levels of corresponding variables and dietary intake of energy and macronutrients using ANCOVA in order not to affect the magnitude of the change in dependent variables. p values of less than 0.05 were considered significant. All statistical analyses were done using SPSS 17.
Results
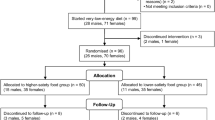
The process of assessing and selecting participants is presented in Fig. 1. The age range of participants was 21 to 48 years old. A total of 10 participants dropped out during the study, of whom 60% (n = 6) were from the FMD group and 40% (n = 4) were from the CER group. The main reasons for dropout from FMD were abdominal discomforts, gastrointestinal reflux, pregnancy, problems adhering to the diet, and traveling. The four participants in the CER group did not complete the intervention due to personal issues and adherence problems. The baseline characteristics of participants did not differ significantly between the two groups (Table 1). However, there were statistically significant differences in participants’ age (FMD: 34.03 ± 1.29 years, CER: 31.17 ± 1.35 years; p = 0.04) and marital status (FMD: 76% married, CER: 50% married; p = 0.04).
The dietary intake of the study population during the intervention is shown in Table 2. There were no significant differences in mean dietary intake of energy, total protein, total fat, cholesterol, saturated fatty acids (SFAs), polyunsaturated fatty acids (PUFAs), monounsaturated fatty acids (MUFA), or vitamins A and E between the two groups. However, differences in dietary intake of carbohydrate, fiber, and vitamin C were approaching significance between the two groups.
A comparison of anthropometric measurements, body composition, and glucose metabolism as well as serum levels of leptin, neuropeptide-Y, and total ghrelin between the FMD and CER groups is shown in Table 3. Looking at mean (SD), body weight was significantly reduced from 82.43 (10.29) kg to 81.29 (10.19) kg in the FMD group (p = 0.023), as well as from 79.27 (10.41) kg to 76.98 (9.23) kg in the CER group (p = 0.069). Both FMD and CER were associated with significant within-group reductions in body weight, BMI, and waist circumference (p < 0.05), but the difference was not statistically significant between the two groups (p > 0.05). A comparison of baseline versus post-trial measurements showed a significant reduction in fat mass for the FMD group (mean difference (SD) − 0.63 (2.99) kg, p < 0.001), while a significant loss of muscle mass was observed for the CER group (mean difference (SD) − 0.78 (1.25) kg, p = 0.04). Between-group comparisons were significant for fat mass (p = 0.042) but not for muscle mass (p = 0.89). BMR was significantly reduced in both FMD and CER groups (p < 0.05), but the comparison of change values between the study groups was not statistically significant (p = 0.56).
Compared to the FMD group, a significant reduction in serum levels of FPG was observed in subjects in the CER group (p < 0.001). Between-group comparisons revealed a significant reduction in serum insulin (p = 0.03) and HOMA-IR (p = 0.048), as well as a significant increase in QUICKI (p = 0.044) in the FMD group compared to the CER group. However, within-group changes in serum levels of insulin, HOMA-IR, and QUICKI were not statistically significant in either the FMD or CER group. A comparison of change values between and within the study groups revealed no significant alterations in serum levels of leptin and NPY. Considering the change values of serum total ghrelin, a statistically significant difference was found between FMD and CER groups (p = 0.01).
When the analyses were controlled for baseline levels of corresponding variables and dietary intake of macronutrients, more reduction was observed in muscle mass (p = 0.048) and BMR (p = 0.045) following CER compared to those who received FMD (Table 4). However, change values for fat mass were not statistically significant between the two groups. There was also a significant increase in serum levels of total ghrelin (p = 0.048) and NPY (p = 0.041) among participants in the CER group when compared to the FMD group.
Discussion
There was no statistically significant difference between groups in terms of weight loss. Although a greater loss of body weight, BMI, and waist circumference was observed in the group following CER compared to the group following the 5-day FMD, the amount was not clinically relevant. While the CER group exhibited a higher reduction in BMR, favorable effects on fat mass and muscle mass were only seen in the FMD group. Insulin resistance was reduced in participants who followed FMD compared to those in the CER group. When analyses were controlled for potential confounders (age, marital status, baseline levels of variable, energy intake, dietary fat, dietary carbohydrate, dietary fiber, and vitamin C), significant increases in serum levels of total ghrelin and NPY were seen in the CER group; however, results for serum leptin were not statistically significant.
Finding an effective therapy for weight loss, dietary approaches with chronic energy restriction can lead to adverse effects that make it difficult to be adopted by the great majority of the population [22]. In recent years, a periodic diet very low in energy and protein has been developed that produces metabolic effects similar to traditional fasting while minimizing the negative impacts of fasting. Therefore, we decided to compare the effect of FMD versus CER on anthropometric values, body composition, and appetite-regulatory hormones in metabolically healthy women with obesity.
In this randomized trial, we found that FMD did not produce superior weight loss or decreased waist circumference compared to CER. Although a significant loss of fat mass was seen in the FMD group, the results were not significant after adjusting the analysis for confounding factors. These results are similar in part to previous animal and human studies. In mice, 4 days of FMD induced a gradual weight loss following a temporary weight fluctuation immediately after the FMD, a reduction trend for total adipose tissue, and an unchanged lean body mass when compared to a normal diet [22]. A 7-day FMD administered in diabetic mice showed a fluctuation in body weight [33]. Three cycles of a 5-day FMD for 3 months reduced body weight by 3% and showed a reducing trend for trunk fat relative to a normal diet; however, lean body mass remained similar in both groups [22]. In the only crossover RCT performed in generally healthy individuals, compared to a normal diet group, body weight, BMI, waist circumference, and total body fat were significantly reduced and lean body mass was increased in those who completed three FMD cycles [23]. These previous studies compared FMD to a control group in which participants were instructed to maintain their regular eating habits, whereas in the current study FMD was compared against a comparison group who received CER. Moreover, to our knowledge, the effects of FMD on circulating concentrations of hormones known to be involved in body weight regulation have not been previously identified.
Insulin resistance is regarded as an underlying mechanism in resistance to weight reduction [34]. Therefore, increasing insulin sensitivity and glycemic control might be effective approaches in the management of obesity. More reduction of FPG in the CER group may have contributed to the greater weight loss that occurred in this group; however, FMD reduced serum insulin concentrations and insulin resistance significantly compared to CER. Notably, these changes became non-significant when the analysis was adjusted for potential confounders. Similar effects of FMD on glycemic control have been previously reported. A 10-fold decline in serum insulin concentrations was observed in mice after 4 days of FMD, but levels returned to baseline after re-feeding [22]. Moreover, FMD reduced glucose tolerance and insulin resistance as well as restored beta-cell function in diabetic mice [33]. In an RCT on generally healthy individuals following 3 cycles of FMD, serum levels of insulin and FPG were reduced only in subjects with elevated risk factors [23].
It was surprising that the reduction of leptin during the intervention was not statistically significant, since many trials with dietary weight loss report a decrease over time with different types of fasting interventions [35,36,37]. This is, however, expected in the absence of a significant reduction in fat mass since the exclusive source of leptin expression and secretion is the adipocytes of white adipose tissue; and therefore, the circulating levels of leptin are proportional to fat mass [38]. Although not significant, leptin increased following FMD, but decreased after CER. Decreased levels of plasma leptin after weight loss may represent a strong tendency for weight regain [39]. Moreover, we found that muscle mass and BMR, a major component of total daily energy expenditure, were less decreased after FMD compared with CER. Thus, with the use of the FMD approach, diet-induced thermogenesis appeared to be maintained better than CER. Future studies with objective measurements of diet-induced thermogenesis are indicated to confirm this finding.
There was also a significant increase in circulating total ghrelin and NPY in the CER group compared to the FMD group. It has been demonstrated that ghrelin stimulates food intake in both laboratory animals [40] and humans [41]. In addition to increasing food intake, exogenous ghrelin decreases both the metabolic rate [42] and the catabolism of fat [43]. Several lines of evidence propose that the appetite-stimulating effect of ghrelin is mediated through neuropeptide-Y (NPY) neurons in the arcuate nucleus [44,45,46]. NPY has been implicated in the promotion of responses to eating as daily food intake was increased in NPY-injected rats [47] and the secretion of NPY increased during food deprivation [48]. The ablation of NPY led to a greater weight loss during fasting and slower weight recovery during refeeding [49]. Moreover, serum levels of NPY increased both during and 2 weeks after Ramadan fasting in pregnant women [50]. Our finding suggests that ghrelin and NPY may play a part in increased satiety and maintenance of weight loss during FMD.
To our knowledge, the current study is the first RCT comparing the effect of FMD and CER on serum levels of appetite-regulating hormones and anthropometric measurements. We examined diets among women with obesity in free-living situations since this group seems to beneficially respond to energy restriction. However, some limitations should be considered when interpreting our findings. First, we considered premenopausal women only due to the potential impacts of menopausal status on metabolic factors. The benefits of FMD in women with obesity cannot be extrapolated to men due to lower levels of adiposity, low expression of the ob gene, and dissimilar secretion of sex hormones [51,52,53]. Other forms of fasting have shown greater acceptability among men than women [54, 55]. In addition, self-reported estimations of physical activity may have low agreement with objectively measured levels. However, the validity of the IPAQ has been confirmed in previous studies [56, 57], and the difference between self-reported and accelerometer-measured physical activity has been reported to increase activity and intensity levels [58]. Future studies may benefit from including accelerometer-based measures of physical activity as a complementary method. Despite the relatively high validity reported for a 3-day food diary, there may be some underreporting in women with obesity [59, 60]. To overcome underreporting, dietary intake was also evaluated by 24-h dietary recalls biweekly through telephone calls. Although the measurement condition was tightly controlled, using estimated BMR is another limitation of the study as estimated rather than measured. It is also noted that the length of the menstrual cycle may affect the results of this trial. Some studies have shown that estradiol may play a role in the secretion of insulin, leptin, ghrelin, and NPY [61,62,63,64], although there are contradictory findings from other studies [65, 66].
In summary, 2 cycles of FMD did not produce superior weight loss compared with a CER approach. Our findings showed an increase in total ghrelin and NPY levels after CER compared to FMD. Although FMD was not easier to follow than CER, particularly over the long term, FMD may be more feasible than previously studied fasting regimens. FMD regimens may be proposed in clinical practice for some individuals who are interested in energy reduction for less than a week, rather than every day. Future research could assess quantitative 24-h leptin data to fully reveal the association of FMD with leptin concentration dynamics. Long-term follow-up of trial participants may be indicated to find the comparing effect of these methods on the maintenance of weight loss. In addition, psychological studies may be useful to elucidate behavioral factors affecting compliance with regimens and subjective appetite (e.g., hunger and fullness).
Data Availability
Because of ethical restrictions on sharing a de-identified dataset (data contain potentially identifying or sensitive patient information), data from this study are available upon request. These restrictions were imposed by the Ethics Committee and principal investigator.
References
Jiang L, Rong J, Wang Y, et al. The relationship between body mass index and hip osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2011;78(2):150–5.
Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer--viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8.
Singh GM, Danaei G, Farzadfar F, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8(7):e65174.
Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–67.
Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
Colagiuri S, Lee CM, Colagiuri R, et al. The cost of overweight and obesity in Australia. Med J Aust. 2010;192(5):260–4.
Deighton K, Batterham RL, Stensel DJ. Appetite and gut peptide responses to exercise and calorie restriction. The effect of modest energy deficits. Appetite. 2014;81:52–9.
Lean MEJ, Malkova D. Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence? Int J Obes. 2016;40(4):622–32.
Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes. 2015;39(8):1188–96.
Dansinger ML, Gleason JA, Griffith JL, et al. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53.
Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12(7):487–91.
Harvie M, Howell A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects-a narrative review of human and animal evidence. Behav Sci (Basel). 2017;7(1)
Ash S, Reeves MM, Yeo S, et al. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with type II diabetes: a randomised trial. Int J Obes Relat Metab Disord. 2003;27(7):797–802.
Catenacci VA, Pan Z, Ostendorf D, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity. 2016;24(9):1874–83.
Zuo L, He F, Tinsley GM, et al. Comparison of high-protein, intermittent fasting low-calorie diet and heart healthy diet for vascular health of the obese. Front Physiol. 2016;7:350.
Byrne NM, Sainsbury A, King NA, et al. Intermittent energy restriction improves weight loss efficiency in obese men: the MATADOR study. Int J Obes. 2018;42(2):129–38.
Heilbronn LK, Smith SR, Martin CK, et al. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81(1):69–73.
Seimon RV, Roekenes JA, Zibellini J, et al. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol Cell Endocrinol. 2015;418:153–72.
Harvie M, Wright C, Pegington M, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110(8):1534–47.
Trepanowski JF, Kroeger CM, Barnosky A, et al. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: secondary analysis of a randomized controlled trial. Clin Nutr. 2018;37(6 Pt A):1871–8.
Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes. 2011;35(5):714–27.
Brandhorst S, Choi IY, Wei M, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22(1):86–99.
Wei M, Brandhorst S, Shelehchi M, et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med. 2017;9(377)
Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–81.
National Heart L, Blood Institute %J Washington D, USA: United States Department of Health, Services H. NIH Publication No. 98–4083. 1998.
Lupton JR, Brooks J, Butte N, et al. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. 2002;5:589–768.
Roubenoff R. Applications of bioelectrical impedance analysis for body composition to epidemiologic studies. Am J Clin Nutr. 1996;64(3 Suppl):459s–62s.
Song Y, Manson JE, Tinker L, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women’s Health Initiative Observational Study. Diabetes Care. 2007;30(7):1747–52.
Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Suresh K, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012;5(1):7–13.
Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109–12.
Wei S, Han R, Zhao J, et al. Intermittent administration of a fasting-mimicking diet intervenes in diabetes progression, restores β cells and reconstructs gut microbiota in mice. Nutr Metab. 2018;15(1):80.
Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. 2012;19(2):81.
Kassab SE, Maklady,FAJSCUMJ. Changes in serum leptin concentrations during Ramadan fasting in lean and obese individuals. 2000;3(1):83–91.
Dubuc GR, Phinney SD, Stern JS, et al. Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women. Metabolism. 1998;47(4):429–34.
Lamošová D, Máčajová M, Zeman MJAVB. Effects of short-term fasting on selected physiological functions in adult male and female Japanese quail. 2004;73(1):9–16.
Murphy K, Bloom S. Gut hormones in the control of appetite. Exp Phsysiol. 2004;89(5):507–16.
Havel PJ, Kasim-Karakas S, Mueller W, et al. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996;81(12):4406–13.
Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. 2001;50(11):2540–7.
Wren A, Seal L, Cohen M, Brynes A, Frost G, Murphy K, et al. Ghrelin enhances appetite and increases food intake in humans. 2001.
Asakawa A, Inui A, Kaga O, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. 2001;120(2):337–45.
Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–13.
Cummings DE, Shannon MH. Roles for ghrelin in the regulation of appetite and body weight. Arch Surg. 2003;138(4):389–96.
Clark JT, Kalra PS, Crowley WR, et al. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115(1):427–9.
Morley J, Levine A, Gosnell B, et al. Effect of neuropeptide Y on ingestive behaviors in the rat. Am J Phys Regul Integr Comp Phys. 1987;252(3):R599–609.
Stanley BG, Kyrkouli SE, Lampert S, et al. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7(6):1189–92.
Kalra SP, Dube MG, Sahu A, et al. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci U S A. 1991;88(23):10931–5.
Patel HR, Qi Y, Hawkins EJ, et al. Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. 2006;55(11):3091–8.
Khoshdel A, Kheiri S, Nasiri J, Heidarian EJMjotIRoI. The effect of Ramadan fasting on serum leptin, neuropeptide Y and insulin in pregnant women. 2014;28:92.
Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 2009;97(2):199–204.
Nicklas BJ, Katzel LI, Ryan AS, Dennis KE, Goldberg AP. Gender differences in the response of plasma leptin concentrations to weight loss in obese older individuals. 1997;5(1):62–68.
Fuente-Martín E, Argente-Arizón P, Ros P, et al. Sex differences in adipose tissue: it is not only a question of quantity and distribution. Adipocyte. 2013;2(3):128–34.
Williams KV, Mullen ML, Kelley DE, Wing RRJDc. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. 1998;21(1):2–8.
Truby H, Baic S, DeLooy A, Fox KR, Livingstone MBE, Logan CM, et al. Randomised controlled trial of four commercial weight loss programmes in the UK: initial findings from the BBC “diet trials”. 2006;332(7553):1309–14.
Cleland C, Ferguson S, Ellis G, et al. Validity of the International Physical Activity Questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med Res Methodol. 2018;18(1):176.
Motl RW, Sasaki JE, Cederberg KL, et al. Validity of sitting time scores from the International Physical Activity Questionnaire-Short Form in multiple sclerosis. Rehabil Psychol. 2019;64(4):463–8.
Dyrstad SM, Hansen BH, Holme IM, et al. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc. 2014;46(1):99–106.
Yang YJ, Kim MK, Hwang SH, et al. Relative validities of 3-day food records and the food frequency questionnaire. Nutr Res Pract. 2010;4(2):142–8.
Jeon KJ, Lee O, Kim H-K, et al. Comparison of the dietary intake and clinical characteristics of obese and normal weight adults. Nutr Res Pract. 2011;5(4):329–36.
Kalra S, Kalra P. NPY: a novel on/off switch for control of appetite and reproduction. Neuropeptide Y and related peptides: Springer; 2004. p. 221–49.
Sirotkin AV, Rafay J, Kotwica J, et al. Role of ghrelin in regulating rabbit ovarian function and the response to LH and IGF-I. Domest Anim Endocrinol. 2009;36(3):162–72.
Sirotkin AV, Rafay J, Kotwica J. Leptin controls rabbit ovarian function in vivo and in vitro: possible interrelationships with ghrelin. Theriogenology. 2009;72(6):765–72.
Pulido JME, Salazar MA. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30(1):19–22.
Dafopoulos K, Chalvatzas N, Kosmas G, et al. The effect of estrogens on plasma ghrelin concentrations in women. J Endocrinol Investig. 2010;33(2):109–12.
Yki-Järvinen H. Insulin sensitivity during the menstrual cycle. J Clin Endocrinol Metab. 1984;59(2):350–3.
Funding
The study was approved and financially supported by Ahvaz Jundishapur University of Medical Sciences (project number NRC-9806).
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows—AZ and SAH: conceived and designed the experiments; MS and AZ: wrote the paper; MS and AM: performed the experiments; KAA: analyzed the data; AZ had primary responsibility for the final content.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
All procedures were reviewed and approved by the ethics committee of Ahvaz Jundishapur University of Medical Sciences and was registered on the Iranian Registry of Clinical Trials website; IRCT registration no. IRCT20190717044244N1, accessed at https://www.irct.ir/trial/40881.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 15.7 kb)
Rights and permissions
About this article
Cite this article
Sadeghian, M., Hosseini, S.A., Zare Javid, A. et al. Effect of Fasting-Mimicking Diet or Continuous Energy Restriction on Weight Loss, Body Composition, and Appetite-Regulating Hormones Among Metabolically Healthy Women with Obesity: a Randomized Controlled, Parallel Trial. OBES SURG 31, 2030–2039 (2021). https://doi.org/10.1007/s11695-020-05202-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-05202-y