Abstract
Introduction
Sub-optimal weight loss following Roux-en-Y gastric bypass (RYGB) represents an important clinical challenge in a significant number of patients. Early identification of such patients would be advantageous, as it could aid in the selective implementation of targeted adjunct interventions during the first post-operative year.
Methods
Clinical audit data from 1137 patients undergoing RYGB between 2013 and 2016 at the Instituto Sallet in Brazil were prospectively registered in an online database (BOLD) and analyzed.
Results
Forty-eight percent of patients achieving less than 5% total weight loss after the first post-operative month achieved a 20% total weight loss at 1 year (n = 626; OR = 0.6 CI = 95%). Eighty-three percent of patients losing between 5 and 10% at 1 month and 95% of patients losing greater than 10% at 1 month had lost at least 20% of total body weight after the first post-operative year. Forty-four percent of patients achieving less than 10% total weight loss after the third post-operative month achieved 20% total weight loss at 1 year (n = 494; OR = 0.3 CI = 95%).
Conclusion
Total bodyweight reduction after RYGB of < 5% at 1 month and < 10% at 3 months is associated with suboptimal weight loss at 1 year. These results reinforce findings from other studies reporting that patients tend to follow a common weight loss trajectory. Identifying the patients with weight trajectory requiring adjunct therapies early on is crucial so appropriate adjustments can be made to post-operative care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients undergoing Roux-en-Y gastric bypass (RYGB) surgery typically achieve maximal weight loss after 12 to 18 months. Total body weight loss at this point is generally in the region of 20–30%. Large-scale clinical series demonstrate that on average, weight loss of up to 20% is sustained over a 20-year follow-up [1]. However, poor weight loss of less than 20% at 1 year represents an important clinical challenge in a number of patients [2, 3]. Early identification of such patients would be advantageous as it could support the selective implementation of targeted adjunct intervention during the first post-operative year. This is a period of time at which most patients remain closely engaged with clinical services and in a position to avail of tailored follow-up to optimize weight loss.
Pre-operative predictors of weight loss outcomes are weak [4]. However, similar to what has been observed following lifestyle and medication-based weight loss interventions, the degree of weight loss in the first few months after surgery may be a strong predictor of longer-term outcomes. As exemplified in the Look AHEAD study, the majority of patients that lost more than 10% bodyweight during the first year of an intensive lifestyle intervention were able to maintain these results for 8 years [5]. Similarly, in the SCALE study of 3 mg/day liraglutide as a weight loss intervention, there was a correlation between early and long-term weight loss [6]. Wise et al. demonstrated that after bariatric surgery, patients follow a characteristic post-operative weight loss trajectory and early results are a useful predictor [7, 8]. In the present study, we sought to identify the prognostic value of using predefined weight loss threshold values at 1 and 3 months after surgery as a means of predicting weight loss at 12-month follow-up.
Methods
Clinical audit data from 1137 patients undergoing RYGB between 2013 and 2016 at the Instituto Sallet in Brazil were prospectively registered in an online database (BOLD) and analyzed. Weight and height were measured before and after surgery to calculate BMI. Eligibility criteria for surgery included a BMI greater than 40 kg/m2, or greater than 35 kg/m2 plus comorbidities in patients who had unsuccessfully tried to lose weight using non-surgical methods in the past.
Patients that had completed longitudinal follow-up with data available at 1 month (25 to 35 days),1 year (330 to 390 days), 3 months (85 to 95 days) or all 3 time-points were included in the analysis. The patients were separated into three cohorts according to the availability of complete data at each specific time point; in cohort 1, 626 patients had follow-up data available at baseline, 1 month, and 1 year after surgery. In cohort 2, 494 patients had available data at baseline, 3 months, and 1 year after surgery. In cohort 3, 338 patients had available data at baseline, 1 month, 3 months, and 1 year after surgery. To identify patients at risk of suboptimal weight loss, we evaluated patients’ 1- and 3-month results in relation to weight loss at 1 year. We considered less than 20% total weight loss (TWL) at 1-year follow-up as suboptimal as it represented the 14th centile of weight loss in our cohort. According to Van de Laar et al., %TWL-based percentile charts constitute a neutral benchmark for defining sufficient postoperative weight loss over time [9]. The software SPSS (IBM®) was used to perform a multivariate logistic regression and to plot true positive rates against false positive rates to generate a ROC curve (Table 1).
Results
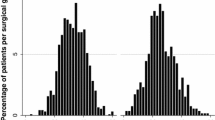
One-year follow-up data after RYGB was available for 1137 patients. Mean percentage weight loss at 1 year was 30.1 ± 10%. The 626 patients that had follow-up data available at 1 month and 1 year after surgery (cohort 1) lost an average of 10.1 ± 3% of bodyweight 1 month after surgery. The 494 patients that had follow-up data available at 3 months and 1 year after surgery (cohort 2) lost on average 18.7 ± 5% of bodyweight at 3 months. The 338 patients that had follow-up data available at 1 month, 3 months, and 1 year after surgery (cohort 3) lost on average 10 ± 4% bodyweight at 1 month and 18 ± 5% of bodyweight at 3 months. From cohort 1, patients that presented with less than 5% total weight loss at 1 month had a 48% chance of achieving a 20% total weight loss at 1 year (OR = 0.6; CI = 95%). However, patients that lost between 5 and 10% bodyweight in 1 month had an 83% chance of achieving 20% total weight loss in 1 year. Ninety-five percent of patients that presented with total weight loss greater than 10–15% at 1 month went on to lose more than 20% bodyweight in 1 year (Fig. 1).
Figure 2 shows that patients from cohort 2 who had less than 10% weight loss at 3 months had a 44% chance of achieving 20% weight loss at 1 year (OR = 0.3; CI = 95%). Patients who achieved 10–15% weight loss at 3 months had a 76% chance of achieving 20% weight loss at 1 year, while those achieving more than 15% weight loss at 3 months had a 99% chance of achieving 20% weight loss at 1 year (Fig. 2).
Figures 3 and 4 show the true positive rate plotted as a function of the false positive rate for different cut-off points, generating a receiver operating characteristic (ROC) curve. Each point on the ROC curve represents a sensitivity/specificity pair corresponding to a specific decision threshold. The point at which initial weight loss best predicted 1-year outcome was at the 14% weight loss at the 3-month mark corresponded to a sensitivity of 92% and a specificity of 74%.
Compared with the data at 1 month, sensitivity and specificity at 3 months were better, as seen by the greater area under the curve (AUC) at 3 vs. 1 month on the respective ROCs. The AUC measures how well the different cut-off points (1 month and 3 months after surgery) can distinguish between two parameters (weight loss of > 20% and weight loss of < 20%). In this case, the AUC value was 0.75 when 1 month after surgery data was used and 0.88 when 3 months after surgery data was used.
Patients with less than 5% weight loss at 1 month lost on average 17.2 ± 3% weight at 1 year, although the range of weight loss was 0–43%. Patients with less than 10% weight loss at 3 months lost on average 17 ± 9% weight at 1 year while the range of weight loss was 4–33%. The sensitivity of < 10% weight loss at 3 months predicting < 20% weight loss at 1 year was 98%, while the specificity was 65%. This means that the positive predictive value was 95% while the negative predictive value was 42%.
In Fig. 5, we used the cohort of patients that had data available at 1 month, 3 months, and 1 year to validate our findings (cohort 3; n = 338). The patients who lost more than 5% body weight in the first month and more than 10% at 3 months went on to lose an average of 33.2 ± 9% at 1 year. The patients who lost more than 10% body weight in the first month and more than 15% at 3 months went on to lose an average of 35 ± 9% at 1 year. The patients that lost less than 10% body weight at 1 month and less than 15% at 3 months went on to lose on average 23 ± 9% at 1 year. Patients who lost less than 5% at 1 month and less than 10% at 3 months went on to lose 14 ± 5% of body weight at 1 year.
Discussion
One-month and three-month bodyweight loss predicted weight loss at 1 year. Using logistic regression to quantify the strength of the association between these two predictors and RYGB outcome, we determined that weight loss less than 5% bodyweight at 1 month and less than 10% bodyweight at 3 months was negatively correlated with the achievement of 20% bodyweight loss at 1 year (OR = 0.6 and 0.3, respectively). Weight loss of 10–15% at 3 months appears to place patients at intermediate risk, while more than 15% weight loss at 3 months predicts that most patients will achieve more than 20% weight loss at 1 year.
The ROC curve demonstrated that 3-month weight loss was a more sensitive and specific cut-off point for predicting outcome 1 year after surgery. The true positive rate was higher for the 3-month cut-off point compared with 1 month. The furthest point from the line of equality on the 3-month mark showcased how this cut-off point can be a more sensitive and specific predictor of outcome than the 1-month cut-off point. This was confirmed by the AUC for the ROC which was larger at the 3-month mark compared with the 1-month mark.
These findings are aligned with results published by Mor et al. in 2012. They reported that patients in the first and fourth quartiles for weight loss 1 month after surgery continued to be in the same weight loss quartile 1 year after RYGB surgery [2, 10, 11]. In 2017, McNickel et al. reported similar findings in patients undergoing sleeve gastrectomy. They demonstrated that pre-operative performance is not a good predictor for 1-year outcomes in LSG and better correlation was observed in %EWL at post-surgery visits [12].
Using initial weight loss as a guideline, we can identify patients who are more likely to be poor responders to RYGB. This may allow us to consider better and more tailored post-operative approaches, although we still need to determine which early interventions may impact on long-term weight loss. Options include changes in eating behavior, psychological interventions, diet, exercise, or pharmacotherapy.
In 2019, Wharton et al. demonstrated that patients that presented with insufficient weight loss or weight regain after undergoing RYGB were able to lose a significant amount of weight while taking liraglutide 3.0 mg [13]. Comparably, Istfan et al. reported that phentermine and topiramate, used individually or in combination, are associated with weight loss in patients that present with weight regain after RYGB [14].
Furthermore, in a review published by Belligoli et al. in 2020, weight regain after bariatric surgery was more influenced by post-operative behavioral factors than by pre-operative predictors [15]. Since most of these behavioral factors are modifiable, intensive behavioral interventions may help to prevent poor postoperative outcomes.
Applying all these interventions in an indiscriminate way can be costly and time-consuming, limiting access for patients who may need them most. Identifying the correct patients who need intervention early should increase access to the right treatment options, improve the health economic case, and may enhance patients’ chances of long-term success.
Limitations of our study include the follow-up period of only 1 year and that we could not investigate weight regain, which usually occurs after the 1-year mark. We also did not have complete data available for all patients at 1, 3, and 12 months of follow-up which is reflective of usual clinical practice. Patients knowing that weight loss may be predicted by early follow-up may increase attendance to routine clinic visits.
Conclusion
The percentage of patients having a sub-optimal weight loss after bariatric surgery remains reasonably constant, but as more surgery is done, the absolute number of patients with poor outcomes will increase. This may result in more revisional surgery. Considering the increased risks and costs of revisional surgery, early recognition and treatment of patients at risk of being poor responders may be much safer and more cost-effective.
Patients should be evaluated at 1 and 3 months to help predict weight loss at 1 year. Three-month weight loss was a more sensitive and specific predictor of outcome when compared with 1 month. Bodyweight reduction after RYGB of less than 5% at 1 month and 10% at 3 months suggests suboptimal weight loss at 1 year. These patients may now be considered for prospective studies to test whether additional interventions at an early post-operative stage will improve long-term weight loss.
References
Courcoulas A, King W, Belle S, et al. Seven-year weight trajectories and health outcomes in the longitudinal assessment of bariatric surgery (LABS) study. JAMA Surg. 2018;153(5):427–34.
Mor A, Sharp L, Portenier D, et al. Weight loss at first postoperative visit predicts long-term outcome of Roux-en-Y gastric bypass using Duke weight loss surgery chart. Surg Obes Relat Dis. 2012;8(5):556–60. Available from:. https://doi.org/10.1016/j.soard.2012.06.014.
Shukla A, He D, Saunders K, et al. Current concepts in management of weight regain following bariatric surgery. Expert Rev Endocrinol Metab. 2018;13(2):67–76.
Livhits M, Mercado C, Yermilov I, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012;22:70–89.
Look AHEAD Research Group, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–75. https://doi.org/10.1001/archinternmed.2010.334.
Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes. 2013;37:1443–51.
Wise E, Felton J, Kligman M. Exponential decay modeling can define parameters of weight loss trajectory after laparoscopic Roux-en-Y gastric bypass. Am J Surg. 2018;216:120–3.
Welch G, Wesolowski C, Piepul B, et al. Physical activity predicts weight loss following gastric bypass surgery: finding from a support group survey. Obes Surg. 2008;18:517–24.
Van de Laar AWJM, Acherman YIZ. Weight loss percentile charts of large representative series: a benchmark defining sufficient weight loss challenging current criteria for success of bariatric surgery. Obes Surg. 2014, 24:727–34. Available from:. https://doi.org/10.1007/s11695-013-1130-9.
Hindle A, de la Piedad Garcia X, Brennan L. Early postoperative psychosocial and weight predictors of later outcome in bariatric surgery: a systematic literature review. Obes Rev. 2017;18:317–34.
Manning S, Pucci A, Carter N, et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Endosc. 2015;29:1484–91.
McNickle AG, Bonomo SR. Predictability of first-year weight loss in laparoscopic sleeve gastrectomy. Surg Endosc. 2017;31:4145–9. Available from:. https://doi.org/10.1007/s00464-017-5467-3.
Wharton S, Kuk JL, Luszczynski M, et al. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post-bariatric surgery. Clin Obes. 2019;9:e12323. Available from:. https://doi.org/10.1111/cob.12323.
Istfan NW, Anderson WA, Hess DT, et al. The mitigating effect of phentermine and topiramate on weight regain after Roux-en-Y gastric bypass surgery [Internet]. Obesity. 2020:1023–30. Available from:. https://doi.org/10.1002/oby.22786.
Belligoli A, Bettini S, Segato G, et al. Predicting responses to bariatric and metabolic surgery. Curr Obes Rep. 2020; Available from:. https://doi.org/10.1007/s13679-020-00390-1.
Funding
No funding was allocated for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
CWlR is supported by a Science Foundation Ireland grant (ref 11/YI/B2480) and Health Research Board grant (USIRL-2016-2). The other authors declare no competing interests.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
For this type of study, formal consent is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silveira, F.C., Docherty, N., Sallet, P.C. et al. Early Post-operative Weight Change After Roux-en-Y Gastric Bypass Predicts Weight Loss at 12-Month Follow-up. OBES SURG 30, 5020–5025 (2020). https://doi.org/10.1007/s11695-020-04942-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04942-1