Abstract
Background
Management of staple line dehiscence following laparoscopic sleeve gastrectomy (LSG) varies based on local expertise and timing of presentation. We present our experience with an endoscopic suturing platform to treat patients with staple line dehiscence following LSG.
Methods
We included all patients who presented to our institution with a staple line dehiscence following LSG from 2005 through November 2017. All endoscopic suturing procedures were performed by a single interventional endoscopist.
Results
Five patients, ages 25–69 years, received treatment of staple line dehiscence at a median time of 22 days following LSG (range 13–335 days). Four out of the five patients received a stent at some point during their treatment. One patient with a chronic leak required gastrectomy and esophago-jejunostomy as a definitive treatment. The remaining four patients experienced resolution of the leak at a median of 48 days post-operatively (range 21–82 days).
Conclusion
Endoscopic suturing may have a role in the management of leaks following LSG, as a primary treatment or as an adjunct to treatment with a stent. However, given that the technique requires considerable endoscopic expertise and in light of a number of other available therapeutic choices, further studies are required to better define the role of this technology in the algorithm of LSG-related leak management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to its proven efficacy and safety, laparoscopic sleeve gastrectomy (LSG) is now the most popular bariatric surgery in the USA, representing approximately 60% of all bariatric surgeries performed [1]. Staple line dehiscence leading to leakage of gastric content into the abdominal cavity is a relatively rare complication occurring in 0.7% of sleeve gastrectomy patients [2]. Morbidity from this complication is significant, often leading to reoperation, abscess, sepsis, and death if left untreated. Leaks that occur within 48 h of bariatric surgery are thought to be caused by technical failure (e.g., stapler misfire) while those that occur later (e.g., after several days) may be caused by tissue ischemia and increased intragastric pressure from luminal narrowing in the area of the incisura [3]. Although leaks are rare, the grave consequences have spurred the development of several treatment approaches.
Currently, no standard protocol exists for the treatment of gastrointestinal leaks after SG. The most common approach to treat sleeve-associated leaks has been the endoscopic placement of a covered or partially covered expandable stent across the area of the staple line disruption, allowing food contents to bypass the leak and facilitate healing. This method, when successful, allows for early per oral feeding and cessation of the intra-abdominal contamination with gastric contents [3, 4]. Endoscopic stent placement has many limitations. Stents at this location have a tendency to migrate distally, and erosion through the gastrointestinal wall has also been observed [3, 4]. A tight seal between the stent and the esophageal wall above the area of the leak is sometimes difficult to achieve, allowing a persistent leak. When a stent is placed near the gastroesophageal (GE) junction, intense gastroesophageal reflux disease (GERD) symptoms are likely to arise, with patients occasionally developing erosive esophagitis and strictures.
Though no method of leak management is without its flaws, staple line reinforcement through endoscopic suturing is a relatively under-utilized method of addressing this complication. Endoscopic suturing has been used in a diverse array of applications, including closure of perforations and defects in the gastrointestinal wall, stent fixation, closure of mucosal defects during endoscopic submucosal dissection or endoscopic mucosal resection, primary sleeve gastroplasty, endoscopic augmentation of the gastroesophageal junction for the treatment of gastroesophageal reflux disease, and revision of the gastro-jejunal anastomosis and the gastric pouch to promote weight loss after gastric bypass [5,6,7,8]. While the available data are limited, this approach also has promise in the treatment of leaks and fistulas in the context of gastric bypass and sleeve gastrectomy [9, 10]. The Apollo OverStitch system (Endosurgery, Austin, TX) represents a relatively new advance in endoscopic suturing. Advances of the Overstitch system include flexibility (allowing both running and interrupted stitches), curved needle design (affording increased control over the depth of suture placement), visibility of the operative site (improving control and minimizing scope insertions/removals), and a knotless fix-action design, which allows for fast, secure closure while eliminating the need for complex surgical knots.
Here we report on five patients who underwent repair of gastric leaks following LSG via endoscopic suturing using this advanced endoscopic suturing platform.
Materials and Methods
After IRB approval, we performed a retrospective chart review of our bariatric patient registry (n > 5000 patients) to identify patients who developed staple line dehiscence following LSG from 2005 through November 2017 and whose leaks were treated using an endoscopic suturing system. Staple line dehiscence was defined as the documentation in the medical record of a leakage of gastrointestinal content from a staple line following LSG. Each case used the Apollo Overstitch suturing device, currently the only widely available system for endoscopic suturing [5].
Technique
A needle driver controlled by a cable running alongside the dual lumen scope sits locked into the orifice of the secondary channel at the end of the scope. The needle (with attached suture) is passed back and forth from the needle driver to a metallic receiving catheter, which is located within the primary channel of the scope. This movement allows the suture to follow the needle across tissues and to then be reloaded for a grasp of tissue on the contralateral lip of a defect. To lock a stitch the needle is released and a pledget is advanced over the suture. Suture traction closes the defect. The released needle serves as an anchor on one site of the defect. The pledget locks onto the suture as the opposing anchor. The stitch can thus be tied off either after a single stitch or as a running stitch.
We generally perform sleeve leak closure following three main steps. First, we identify the leak site. We find that this is best accomplished using a diagnostic endoscope. The typical site is located along the apex of the suture line close to the gastroesophageal junction. Next, we cauterize the edges of the fistulae, usually by argon plasma coagulation. The goal here is that secondary inflammation will lead to better adherence between the contralateral walls of the defect. The burn also serves to help identify the location when the dual lumen scope is subsequently passed to the site with the suturing attachment affixed to its end. The use of the less dexterous larger diameter dual lumen scope with the attached suturing device reduces visualization of the fistula within the narrow lumen. This reduction is one of the primary impediments to good endoscopic suture closure. Because of this limitation, we recommend using single stitches over running stitches. As the suture runs through the defect, the result is a narrowing of both the defect and the GI lumen, further reducing visualization and sometimes even impairing the ability of the needle driver to adequately pull back from the needle. These issues can prevent placement of a second stitch. If necessary, clips can be placed for additional support of the suture line closure. Visualization is again best accomplished using the narrower diameter diagnostic gastroscope.
Results
The chart review yielded 5 patients who met inclusion criteria (Table 1). Each case is described in detail below.
Case 1
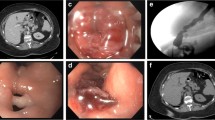
A 69-year-old female with an extensive surgical history, including multiple abdominal wall hernia repairs with mesh, underwent LSG using a staple line buttressing material and extensive adhesiolysis and was discharged on post-operative day (POD) #2 after a negative upper gastrointestinal (UGI) study. She was re-admitted on POD#4 with chest pain. A computed tomography (CT) scan with contrast showed multiple collections of intra-abdominal air fluid, suggesting a staple line leak. She was taken for a laparoscopic-converted-to-open exploration and oversewing of the staple line. An intraoperative leak test was negative. Three drains were placed at the conclusion of the operation. Postoperatively, she was managed with total parenteral nutrition (TPN) and antibiotics. An additional abdominal drain was placed by Interventional Radiology to address the collection of fluid. Due to a persistent leak, she was transferred on POD #19 to our institution for further management. Re-imaging showed a persistent proximal leak. On POD #22, she underwent endoscopic suturing of the dehisced staple line (Fig. 1a, b). Following this intraluminal suturing, it was noted that the GE junction was mildly narrow, necessitating placement of a covered stent over this area. The patient had significant dysphagia, nausea, and chest discomfort until the stent was removed on POD #40. She remained on TPN for nutritional support during this time. A small and contained leak with no communication to the surgical drains persisted until POD #48. The patient’s symptoms resolved completely, and all drains were removed.
Case 2
A 54-year-old female underwent LSG using a staple line buttressing material and was discharged on POD #3. On POD #17, she presented with abdominal pain and food intolerance. Abdominal CT revealed a contained gastric leak and she was initially treated by making her NPO and starting TPN. Due to persistence of the leak, she underwent placement of a covered stent on POD #34. She never became septic or required drainage of the fluid collection. A large sympathetic left pleural effusion required percutaneous drainage. The stent was removed 22 days after placement due to distal migration which caused gastric obstruction and a large inflammatory pseudopolyp (Fig. 2a). She continued to be symptomatic with a small fistula and, 2.5 months after the initial surgery, she underwent endoscopic closure of the gastric fistula using the aforementioned suturing platform (Fig. 2b). A small leak (visualized by CT) persisted for 1 week and subsequently resolved concurrently with her symptoms.
Case 3
A 41-year-old male underwent an uneventful LSG using staple line buttressing material and was discharged on POD #2. He presented on POD #15 with high-grade fevers and chills. A CT scan was positive for a contained leak. He underwent a diagnostic laparoscopy and placement of drains. Although no fluid collection was found, the dense adhesions between his omental fat and the buttressing material were not disrupted. On POD #18, he underwent endoscopic suturing of a small staple line disruption. Post-procedure upper gastrointestinal series (UGI) showed complete resolution of the leak. He was kept on TPN and antibiotics for 10 days and was discharged home without any drains and with complete resolution of the leak.
Case 4
A 45-year-old female underwent removal of an adjustable gastric band, conversion to LSG using a staple line buttressing material and was discharged on POD #4. She presented on POD #9 with a fever, chest pain, and epigastric pain. A CT scan of the abdomen and pelvis was positive for suture dehiscence and a contained leak. She was taken to the operating room for a laparoscopic washout and placement of surgical drains. On POD #13, the patient underwent endoscopic suturing with the aforementioned technique. At the conclusion of the procedure, the fistula appeared to be closed except for a questionable area along the proximal aspect of the closure that could not be well visualized. A persistent leak was confirmed through a UGI study and the patient underwent repeat endoscopic suturing the following day. A follow-up UGI study showed persistent extravasation of contrast into a collection in the left upper quadrant that did not communicate with any of the three surgical drains. A repeat endoscopy on POD #22 showed that the endoscopic sutures remained in place; however, 3 very small entrances still opened into the cavity. These sites were not amenable to endoscopic suturing, and therefore, a covered stent was placed. The stent had to be upsized on POD #26 due to persistence of the leak. On POD #48, she underwent esophageal stent removal. In addition, contrast was injected endoscopically with proximal and distal balloons obstructing the lumen, a maneuver that did not result in any visible contrast extraluminally. Despite radiographic persistence of a small contained area of contrast pooling adjacent to the proximal staple line, the patient continued to show clinical improvement, and with gradual advancement in diet, her drains were removed on POD #76.
Case 5
A 25-year-old male was transferred to our practice with a chronic gastrocutaneous fistula secondary to LSG staple line disruption. The patient had previously undergone a number of failed surgical and endoscopic procedures to control the gastric leak. He arrived at our hospital on POD #326 with an esophageal stent in place. The patient underwent percutaneous drainage of abdominal abscesses which, at an outside hospital, had grown pseudomonas and methicillin-resistant Staphylococcus aureus. On POD #328, he underwent an upper endoscopy which revealed diffuse inflammation. In order to attempt endoscopic suturing, the esophageal stent was removed. However, after stent removal, the gastric tissue was inflamed and edematous, and endoscopic suturing was not feasible. One week later, he underwent a two-layer closure of the gastric leak via endoscopic suturing. The endoscopic procedure led to temporary closure of the fistula as evidenced by a negative UGI study. The patient exhibited dietary noncompliance with continued tobacco use, which may have contributed to leak recurrence. He required definitive treatment with gastric resection, Roux-en-Y esophago-jejunostomy, and placement of a feeding jejunostomy tube on POD # 457.
Discussion
Physicians have used a variety of methods to manage leaks following SG in order to minimize the need for major revisional surgery and to prevent potentially fatal sepsis [11]. The most common of these include endoscopic stent placement, endoscopic placement of over-the-scope clip, endoscopic pigtail catheter placement, and endoscopic placement of a vacuum-assisted closure (VAC) system. An endoscopically placed pigtail catheter allows for internal drainage of the peri-gastric abscess cavity; however, optimal positioning and maintenance of the drain may be challenging given the mobile and dynamic nature of the anatomy and the time needed for complete healing of the leak (118 days) [12]. Endoscopic use of VAC technology involves application of negative pressure on the fistula cavity via a sponge attached to a nasogastric tube, which gently draws fluid out of the gastric leak area, stimulating the edges to come back together over time [13]. This method is labor-intensive, requiring use of a nasogastric tube for several days and necessitating multiple endoscopies to change the VAC sponge. Endoscopic placement of an over-the-scope clip has been used successfully to close gastric leaks [14]. We have recently acquired this technology at our institution, and we are exploring its role in the management of LSG leaks, especially as it may be applied to endoscopic suturing. Finally, endoscopic septotomy has been applied to resolve well-established contained disruptions of the gastric sleeve staple line. With this technique, the septum separating the lumen containing the disrupted staple line and the lumen of the sleeved stomach is endoscopically divided so the leak flow is directed away from the abscess cavity. Although no consensus exists on the timing of such intervention, some authors recommend its use after the first month when a stable septum has developed [15].
The present case series supports the usefulness and effectiveness of endoscopic suturing as either a primary treatment or as an adjunct to other therapeutic interventions for gastric leaks after LSG. The advantages of this approach include the potential for early leak resolution and resumption of peroral diet. Further, this technique avoids the complications associated with stent placement, including stent migration and erosion. It also avoids the prolonged use of nasogastric tubes associated with the endoscopic VAC technique. Although the endoscopically placed stitches may not be durable, as they tend to cut through the tissue over time, they may provide adequate closure of the disrupted staple line long enough to allow complete healing. Our experience with endosuturing has been limited to the treatment of early leaks, with the exception of case #5, in which we attempted endosuturing prior to embarking on a major revisional surgery. Based on this limited series, it appears that this approach is more successful in patients with early leaks.
This study has several limitations. The learning curve associated with endoscopic suturing is steep; our case series was performed by an experienced endoscopist using a commercially available endosuturing platform. The treatment in many cases included a hybrid approach with endoscopic suturing and stent placement. Even if endoscopic suturing did not provide complete closure of the gastric wall defect, decreasing its size may have contributed to the eventual healing with stenting. In this series, variable adherence to dietary recommendations proved to be a challenge. One patient in our study required gastrectomy and esophago-jejunostomy as a definitive treatment of a chronic gastric leak, following initial resolution with endosuturing. The patient’s dietary non-compliance and smoking may have contributed to the recurrence of his leak. Four patients achieved successful leak management with no major adverse events and no signs of recurrence at a median follow-up of 361 days (range 190–1253 days). Finally, our ability to generalize is hampered by our small sample size.
The initial approach to treatment of staple line dehiscence following LSG depends on the timing of the presentation, the clinical picture of the patient and the clinician’s technical expertise. Stent placement remains the most widely used treatment approach. Stable patients with localized fluid collection adjacent to a dehisced proximal staple line may be managed by internal drainage such as endoscopic pigtail, VAC placement, or septotomy. External drainage can be achieved with laparoscopy or via percutaneous drains placed by interventional radiology. Based on our experience, we have adopted an initial approach using endosuturing or over-the-scope clipping, depending on the anatomy, the size of the defect, and the expertise of the interventional endoscopist. We reserve the placement of stents for patients who have persistent leaks following these initial procedures.
Conclusion
Endoscopic suturing may have a role in the management of leaks following LSG, as a primary treatment or as an adjunct to treatment with a stent. Given the endoscopic expertise required to perform the procedures and the number of other therapeutic choices that are available, further studies are required to better define the role of this technology in the algorithm of LSG-related leak management.
References
American Society of Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers available at https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed June 7, 2019.
Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–20. discussion 420–2
Walsh C, Karmali S. Endoscopic management of bariatric complications: a review and update. World J Gastrointest Endosc. 2015;7(5):518–23.
Rached A, Basile M, El Masri H. Gastric leaks post sleeve gastrectomy: A review of its preention and management. World J Gastroenterol. 2014;20(38):13904–10.
Stravropoulos S, Modayil R, Friedel D. Current applications of endoscopic suturing. World J Gastrointest Endosc. 2015;7(8):777–89.
Han J, Chin M, Fortinsky K, et al. Endoscopic augmentation of gastroesophageal junction using a full-thickness endoscopic suturing device. Endoscopy International Open. 2018;06:E1120–5.
Jirapinyo P, Slattery J, Ryan MB, et al. Evaluation of an endoscopic suturing device for transoral outlet reduction in patients with weight regain following Roux-en-Y gastric bypass. Endoscopy. 2013;45:532–6.
Thompson CC, Chand B, Chen YK, et al. Endoscopic suturing for transoral outlet reduction increases weight loss after roux-en-Y gastric bypass surgery. Gastroenterology. 2013;145:129–37.
Modayil R, Friedel D, Marotta-Kollarus M, et al. Endoscopic suturing registry: a single center’s two-year experience. Am J Gastroenterol. 2014;109:AB1968.
Beck C and Mikami D. Endoluminal surgery with OverStitch device for treatment of complications post bariatric surgery: single institution experience. Poster abstract Available at https://www.sages.org/meetings/annual-meeting/abstracts-archive/endoluminal-surgery-with-overstitch-device-for-treatment-of-complications-post-bariatric-surgery-single-institution-experience/. Accessed June 10, 2019.
Singh R, Nussbaum J, Kumta N. Endoscopic management of perforations, leaks and fistulas. Transl Gastroenterol Hepatol. 2018;3:85.
Giuliani A, Romano L, Marchese M, et al. Gastric leak after laparoscopic sleeve gastrectomy: management with endoscopic double pigtail drainage. A systematic review. Surg Obes Relat Dis. 2019;5(8):1414–9.
Smallwood NR, Fleshman JW, Leeds SG, et al. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc. 2016;30(6):2473–80.
Shoar S, Poliakin L, Khorgami Z, et al. Efficacy and safety of the over-the-scope clip (OTSC) system in the management of leak and fistula after laparoscopic sleeve gastrectomy: a systematic review. Obes Surg. 2017;27(9):2410–8.
Angrisani L, Hasani A, Santonicola A, et al. Endoscopic septotomy for the treatment of sleeve gastrectomy fistula: timing and indications. Obes Surg. 2018;28(3):846–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
DT received honorariums from Medtronic, served as a consultant for ConMed, and received educational support from Intuitive. None of the other authors have anything to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lamb, L.C., Lawlor, MK., Tishler, D.S. et al. Use of an Endoscopic Suturing Platform for the Management of Staple Line Dehiscence After Laparoscopic Sleeve Gastrectomy. OBES SURG 30, 895–900 (2020). https://doi.org/10.1007/s11695-019-04344-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04344-y





