Abstract
Background
Sleeve gastrectomy is the most commonly performed bariatric surgery these days but is associated with de novo reflux.
Objective
We aimed to study the influence of hypotonic lower esophageal sphincter (LES) on postoperative gastroesophageal reflux disease (GERD).
Methods
Patients with pre- and postoperative esophageal high-resolution manometry (HRM) and 24-h pH monitoring (pHM) were included retrospectively in our study. Preoperative hypotonic LES was defined by a mean residual pressure of the lower esophageal sphincter < 4 mmHg. Postoperative GERD was defined by a DeMeester’s score > 14.72. We also evaluated postoperative manometric changes at the esophageal-gastric junction.
Results
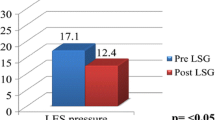
Sixty-nine patients (54 females and 15 males) had pre- and postoperative HRM and pHM. The mean age was 45.9 ± 9.8 years. The mean body mass index (BMI) was 47.5 ± 7.5 kg/m2. Hypotonic LES concerned 21 patients (30.4%) before sleeve gastrectomy. The mean time between the two esophageal monitorings was 32.1 ± 24.1 months. The sensitivity, specificity, positive predictive value, and negative predictive value of hypotonic LES to predict GERD were 31, 70, 52, and 48% respectively. The LES minimal residual pressure was not statistically decreased after sleeve gastrectomy (p = 0.24). Only the wave speed, esophageal length, and LES length were significantly reduced after SG (p = 0.029, 3.8 × 10−7 and 0.00032).
Conclusion
Hypotonic LES has a poor predictive value on postoperative GERD. The LES’s length is significantly reduced after SG and this could be a factor explaining de novo reflux.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction/Purpose
Hypotonic lower esophageal sphincter (LES), hiatal hernia, and increased intra-abdominal pressure are causes of gastroesophageal reflux syndrome (GERD) [1]. Moreover, prevalence of GERD is increased in obese patients with greater consequences [2]. The International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) reported that sleeve gastrectomy (SG) accounts for 43.6% of all primary bariatric procedures globally performed between 2013 and 2017 [3]. Recent literature reports high incidence of Barrett’s esophagus (BE) going up to 18.8% at 5 years in patients who underwent SG [4,5,6]. Furthermore, there is a lack of correlation between GERD symptoms and esophageal lesions [7].
For all these reasons, we must identify clear risk factors to develop GERD and, in the long term, BE in patients who will undergo SG. Unfortunately, even pH monitoring (pHM) is not efficient enough to determine appropriate candidates for this procedure because of the important proportion of de novo reflux [8]. As hypotonic LES plays a role in the appearance of GERD, we aimed to evaluate the impact of a preoperative hypotonic LES on post-SG GERD. We also studied the influence of hypotonic LES on DeMeester’s score and postoperative esophagitis. Manometric changes at the esophageal-gastric junction after SG were investigated too.
Materials and Methods
Patients
All patients who underwent SG in the Department of Digestive and Endocrine Surgery at the University Hospital of Nantes from January 2011 to December 2018 were included. During the study period, the vast majority (more than 95%) of surgical bariatric procedures were SG. It was a doctor’s choice as our population had a mean BMI (body mass index) > 45 kg/m2 to reduce postoperative complications. The eligibility criteria for SG were age > 18 years, indication for a bariatric surgery procedure according to the French health authorities (HAS) guidelines: obesity with a BMI > 35 kg/m2 with secondary comorbidities (type 2 diabetes (TD2) and high blood pressure (HBP), obstructive sleep apnea syndrome (OSA), impact on locomotor system), or BMI > 40 kg/m2 [9]. All patients underwent an extensive preoperative multidisciplinary evaluation according to the National French guidelines that are similar to the recommendations of the National Institute of Health. Patients with esophageal monitoring before and after SG were included retrospectively in our study (details are shown in Fig. 1).
Bariatric Procedure
All SG procedures were performed by laparoscopic approach. Gastrolysis was debuted 7 cm away from the pylorus for antral preservation. No extensive esophageal-gastric junction dissection was performed. Gastric resection was calibrated on a 42-Fr tube. Continuous oversuture was done in stapling line with a non-absorbable suture. In case of hiatal hernia, concomitant repair was not performed during SG.
24-h pH Monitoring
GERD was studied using ambulatory 24-h pH measurements after withdrawal of proton pump inhibitors for at least 2 weeks. The DeMeester score (DMS) is a composite score of acid exposure during a prolonged ambulatory pH monitoring. The results were expressed using five standard components: percentage of total time of pH < 4, percentage of upright time of pH < 4, percentage of supine time of pH < 4, number of reflux episodes > 5 min, longest reflux episode (min) [10]. Using these parameters, the DMS has been calculated. In our study, GERD was defined by a DMS > 14.72 irrespective of the presence of GERD clinical symptoms or/and an association of clinical symptoms.
High-Resolution Manometry
High-resolution manometry (HRM) was performed with a 4.2-mm outer diameter solid-state assembly with 36 circumferential pressure sensors spaced at 1-cm interval (approximately 3 intragastric sensors). The ManoView software was utilized to perform high-resolution manometry analysis. Manometry was done by 12 successive 5-ml water swallows. Preoperative hypotonic LES was defined by a mean residual pressure of the lower esophageal sphincter < 4 mmHg.
Esogastroduodenal Endoscopy
Biopsies were made with a local anesthesia to confirm the diagnosis of BE. In case of non-tolerance, the exam could be performed under general anesthesia. Erosive esophagitis was defined during esogastroduodenal endoscopy by the Los Angeles classification [11] and BE by the Prague classification [12], and also with biopsies to search for gastric/intestinal metaplasia or even dysplasia. Presence of Helicobacter pylori was also searched.
Statistical Analysis
Quantitative results are presented in mean and standard deviation. Student’s t test or Welch’s test was used to compare variations between two groups. Univariate comparisons for quantitative variable analyses were performed using Pearson’s chi-squared tests or Fisher’s tests for independent groups. To compare quantitative variables with qualitative ones, the paired Student’s t test was used. Multivariate analysis was performed using a logistic regression model. For all tests, a two-sided p < 0.05 was considered statistically significant. All statistical analyses were performed with the use of the R software®. The database was built using the Microsoft Excel® (Microsoft, Redmond, WA, USA).
Results
Sixty-nine patients (54 females and 15 males) had pre- and postoperative HRM and pH monitoring. The mean age was 45.8 ± 9.8 years. The mean BMI was 47.6 ± 7.1 kg/m2. Among the 12 patients with esophagitis, 6 were grade A and 6 were grade B. One patient had preoperative endoscopic BE (C0M0 without dysplasia). Baseline characteristics are described in Table 1.
Mean % total body weight loss at 1 year was 21.3%. Two patients (2.9%) had 90-day postoperative complications: one scar disunion (Dindo-Clavien 1) and one intra-abdominal abscess treated by antibiotics (Dindo-Clavien 2).
Causes of the second esophageal monitoring are described in Fig. 1. The mean time between the two esophageal monitorings was 32.1 ± 24.1 months.
The sensitivity, specificity, positive predictive value, and negative predictive value of preoperative hypotonic LES to determine postoperative GERD were 31, 70, 52, and 48% respectively. Contingency table is in Table 2. Postoperatively, there were no correlation between a hypotonic LES and GERD on pHmetry (Cohen’s k = − 0.005). There were no statistically significant difference between patients with and without hypotonic LES in regard to the evolution of GERD status (p = 0.72), DeMeester’s score (p = 0.38), or its components (Table 3).
In univariate analysis, no preoperative variable on esophageal monitoring was a significant risk factor to develop postoperative GERD (Table 4). Patients were more likely to have a DMS > 14.72 after sleeve (from 36 to 52%) without statistical significance (p = 0.09). There is a trend after SG to develop more esophageal lesions such as esophagitis (from 17.4 to 27.5%) and BE (from 1.4 to 7.2%) without statistical significance (Table 5). In multivariate analysis, only preoperative esophagitis was a significant risk factor to develop postoperative esophagitis (OR = 19.7, p = 0.003). There is a trend to a lower LES minimal residual pressure after SG (p = 0.24). Only the wave speed, esophageal length, and LES length were significantly reduced after SG.
Discussion
We hereby present the largest series of patients with pre- and post-SG complete esophageal morphologic assessment. Prevalence of de novo GERD and, therefore, esophageal lesions after SG, is of concern for every bariatric surgeon.
There is a disparity of opinion regarding the reflux after SG. A survey of the Fifth International Consensus Conference for the current status of SG revealed that only 23.3% of expert bariatric surgeons felt that GERD was an absolute contraindication to SG, whereas a higher proportion (52.6%) of general surgeons considered this an absolute contraindication [13]. The problem is the use of symptomatology alone as a screening tool for GERD.
Our results showed that hypotonic LES before SG does not increase significantly DeMeester’s score. Mostly, the diagnostic value of preoperative hypotonic LES to determine postoperative GERD is poor. LES’s length is significantly reduced after SG and this could be one of the factors explaining de novo reflux.
The physiopathology of post-SG GERD is still unclear, but the modification of the esophageal-gastric junction seems determinant with a reduction of LES pressure after surgery [14]. In our series, there was a trend toward a postoperative reduction of LES pressure without statistical significance. Antral preservation was done in our series and it appears to reduce “de novo” GERD [15] even if it seems to impair weight loss in the literature [16]. Braghetto et al. showed a significant decrease in LES pressure without antral preservation [14]. Likewise, a 42-Fr bougie was used to limit complications such as staple line leak [13]. A recent meta-analysis showed that the bougie size [17] does not influence the incidence of de novo GERD. Technical aspect of SG seems crucial and needs to be investigated later on. Valezi et al. excluded patients with GERD (clearly a bias in obese population) but showed clearly intragastric pressure rise and LES damage after SG [8]. It seems that a raised intragastric pressure constitutes SG signature but is not correlated with esophagitis or GERD [18].
RYGB is a well-known treatment of GERD in obese population. Literature based on the BOLD database of the American Society for Metabolic and Bariatric Surgery clearly showed it on more than 20,000 patients [19]. Dupree et al. considered that GERD is a relative contraindication to SG [20]. Recently, the SM-BOSS study attested that 60.4% of patients with RYGB were cured of GERD symptoms (only 25% in the SG group) and that 31.6% of patients in the SG group had de novo GERD (only 10.7% in RYGB group) [21].
An aspect often laid aside is that the DeMeester’s score does not evaluate the sensibility to acid gastric fluid of the esophageal mucosa while it is directly linked to the appearance of esophagitis and BE.
The strength of our study is the precise method for evaluating LES and GERD before and after SG in more than fifty patients. We acknowledge some limitations. Firstly, the patients included had a postoperative esophageal monitoring most of the time for weight regain. It represented a significant bias as higher BMI is a known factor of de novo GERD, and only 13.4% of our patients had a postoperative esophageal monitoring. Furthermore, second monitoring was done at different periods of time for each patient and maybe too precociously (mean delay of 32 months after SG), to show important changes in esophageal status. We need more studies to better apprehend the good candidate for SG, especially prospective ones with esophageal monitoring before and after SG according to a precise timing to eliminate biases present in our study. For the time being, esophagitis diagnosis by esogastroduodenal fibroscopy seems to be the simplest and best way to select patients and to avoid an increased risk of BE.
Conclusion
Hypotonic LES has a poor predictive value on postoperative GERD. The LES’s length is significantly reduced after SG and this could be a factor explaining de novo reflux.
References
Liu L, Li S, Zhu K, et al. Relationship between esophageal motility and severity of gastroesophageal reflux disease according to the Los Angeles classification. Medicine. 2019;98(19):e15543.
Eusebi LH, Fuccio L, Bazzoli F. The role of obesity in gastroesophageal reflux disease and Barrett’s esophagus. Dig Dis. 2012;30(2):154–7.
Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;
Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis. 2017;13(4):568–74.
Sebastianelli L, Benois M, Vanbiervliet G, et al. Systematic endoscopy 5 years after sleeve gastrectomy results in a high rate of Barrett’s esophagus: results of a multicenter study. Obes Surg. 2019;29(5):1462–9.
Felsenreich DM, Langer FB, Prager G. Weight loss and resolution of comorbidities after sleeve gastrectomy: a review of long-term results. Scand J Surg. 2019;108(1):3–9.
Soricelli E, Casella G, Baglio G, et al. Lack of correlation between gastroesophageal reflux disease symptoms and esophageal lesions after sleeve gastrectomy. Surg Obes Relat Dis. 2018;14(6):751–6.
Thereaux J, Barsamian C, Bretault M, et al. pH monitoring of gastro-oesophageal reflux before and after laparoscopic sleeve gastrectomy. Br J Surg. 2016;103(4):399–406.
Haute Autorité de Santé - Obésité: prise en charge chirurgicale chez l’adulte. [cité 18 juill 2017]. Disponible sur: https://www.has-sante.fr/portail/jcms/c_765529/fr/obesite-prise-en-charge-chirurgicale-chez-l-adulte
Johnson LF, Demeester TR. Twenty-four-hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62(4):325–32.
Kasyap AK, Sah SK, Chaudhary S. Clinical spectrum and risk factors associated with asymptomatic erosive esophagitis as determined by Los Angeles classification: a cross-sectional study. PLoS One. 2018;13(2):e0192739.
Alvarez Herrero L, Curvers WL, van Vilsteren FG, et al. Wong Kee Song LM, et al. Validation of the Prague C&M classification of Barrett’s esophagus in clinical practice. Endoscopy. 2013;45(11):876–82.
Gagner M, Hutchinson C, Rosenthal R. Fifth International Consensus Conference: current status of sleeve gastrectomy. Surg Obes Relat Dis. 2016;12(4):750–6.
Braghetto I, Lanzarini E, Korn O, et al. Manometric changes of the lower esophageal sphincter after sleeve gastrectomy in obese patients. Obes Surg. 2010;20(3):357–62.
Del Genio G, Tolone S, Limongelli P, et al. Sleeve gastrectomy and development of “de novo” gastroesophageal reflux. Obes Surg. 2014;24(1):71–7.
McGlone ER, Gupta AK, Reddy M, et al. Antral resection versus antral preservation during laparoscopic sleeve gastrectomy for severe obesity: systematic review and meta-analysis. Surg Obes Relat Dis. 2018;14(6):857–64.
Wang Y, Yi XY, Gong LL, et al. The effectiveness and safety of laparoscopic sleeve gastrectomy with different sizes of bougie calibration: a systematic review and meta-analysis. Int J Surg. 2018;49:32–8.
Mion F, Tolone S, Garros A, et al. High-resolution impedance manometry after sleeve gastrectomy: increased intragastric pressure and reflux are frequent events. Obes Surg. 2016;26(10):2449–56.
Pallati PK, Shaligram A, Shostrom VK, et al. Improvement in gastroesophageal reflux disease symptoms after various bariatric procedures: review of the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis. 2014;10(3):502–7.
DuPree CE, Blair K, Steele SR, et al. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: a national analysis. JAMA Surg. 2014;149(4):328–34.
Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. Jama. 2018;319(3):255–65.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Statement
The study was reviewed by the local institutional review board according to the Helsinki Declaration.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Greilsamer, T., de Montrichard, M., Bruley des Varannes, S. et al. Hypotonic Low Esophageal Sphincter Is Not Predictive of Gastroesophageal Reflux Disease After Sleeve Gastrectomy. OBES SURG 30, 1468–1472 (2020). https://doi.org/10.1007/s11695-019-04335-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04335-z