Abstract
Introduction
Single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) surgery is a modification of the traditional duodenal switch (DS) surgery. SADI-S is relatively a new bariatric surgical procedure and has gone by many names depending on the length of the common channel. In this study, we report our initial experience with this novel technique in the Australian population.
Methods
The medical records of 91 patients who underwent laparoscopic primary SADI-S surgery by one surgeon at a single Australian center from January 2017 through May 2019 were retrospectively studied.
Results
Ninety-one patients were identified for analysis. The mean age and preoperative body mass index (BMI) was 46.2 ± 9 years and 43.2 ± 5.7 kg/m2, respectively. The mean operative time and length of stay were 121.8+/- 25 minutes and 1.4 ± 0.8 days, respectively. At 12 and 24 months, the patients lost an average BMI of 15.2 ± 5.2 kg/m2 and 17.2 ± 5.9 kg/m2, respectively. The short-term and long-term complication rates were 4.3% and 0%, respectively. The mortality rate was 0%. Postoperatively, the obstructive sleep apnea, type 2 diabetes, hyperlipidemia, hypertension, and gastroesophageal reflux disease resolution rates were 94, 94, 75, 68, and 13%, respectively. There was no statistically significant difference between most of the preoperative and postoperative nutritional data.
Conclusions
SADI-S appears to be a safe bariatric surgical procedure with favorable outcomes at 2 years in the Australian population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Single-anastomosis duodenal switch (SADS) is a modification of traditional duodenal switch (DS) surgery [1]. SADS is relatively a new bariatric surgery and has gone by many names, mainly depending on the length of the common channel (cc). It is also known as stomach intestinal pylorus-sparing surgery (SIPS) (cc 300 cm), loop duodenal switch (LDS), and single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) (cc 200–250 cm) [1].
SADI-S was introduced to minimize the problems that are commonly seen with double anastomoses in traditional DS and Roux-en-Y gastric bypass (RYGB) [1]. These problems include but are not limited to dumping syndrome, internal hernias, and ulcers. It involves a loop duodeno-ileostomy rather than traditional Roux-en-Y construction seen in the traditional DS. It is technically simpler than the traditional DS and provides some protection against internal hernias and vitamin deficiencies.
When a 300-cm common channel is used, it has fewer complications like chronic diarrhea, smelly stools, flatulence, and vitamin deficiencies than seen with traditional DS [2, 3]. One of the most significant advantages of this procedure is the preservation of pylorus that provides control of solid emptying, reducing the chances of dumping syndrome and assisting in maintaining a physiologically based rate of gastric emptying [4, 5]. Moreover, it has been reported that the early weight loss outcomes of SADI-S are better than sleeve gastrectomy (SG) but comparable with RYGB and DS [6, 7].
At present, data on the SADI-S are limited with few reports outside of the USA and Spain. However, more and more reports are being published every year [2,3,4,5, 8,9,10,11,12,13,14,15,16]. None have published their experience with this novel technique in the Australian population. In this study, we would like to present our initial two-year experience with SADI-S.
Methods
A formal non-research determination was sought and approved from the Mount Hospital Perth Australia prior to performing our first SADI-S case. Upon demonstration of its safety with the first five cases, formal approval was given to begin doing these cases on a regular basis. No formal IRB is required to begin to do these cases in Australia, so none was sought.
This is a retrospective analysis from one surgeon at a single private institution. Ninety-one patients had primary laparoscopic SADI-S performed from January 2017 through May 2019. During the same time, 651 other primary and revisional metabolic procedures were performed. No revisions were included in the study. Patients signed an informed consent form detailing the laparoscopic SADI-S procedure that included all the potential benefits and risks associated with it. Preoperative data and postoperative outcome data (weight loss, co-morbidity resolution, complications, and mortality) were obtained from a prospectively kept database (Genie Solutions).
Co-morbidities included were obstructive sleep apnea (OSA), type 2 diabetes (T2D), hyperlipidemia (HLD), hypertension (HTN), and gastroesophageal reflux disease (GERD). The resolutions of co-morbidities were defined according to the American Society for Metabolic & Bariatric Surgery (ASMBS) and the American Diabetes Association (ADA) guidelines [17, 18]. Complete remission of OSA, T2D, HLD, HTN, and GERD was defined as follows: discontinuation of continuous positive airway pressure, normal measures of glucose metabolism in the absence of antidiabetic medications, normal lipid panel without antilipidemic medications, being normotensive and off antihypertensive medication, and absence of symptoms and discontinuation of GERD medication, respectively. The A1c and HLD normal values ranged from 48 to 53 mmol/mol and LDL 3.36–4.11 mmol/L, total cholesterol 0.8–9.2 mmol/L, and triglycerides 1.69–2.25 mmol/L. High-risk patients were defined based on the preoperative body mass index (BMI) (BMI ≥ 55 kg/m2), age (≥ 65 years), and American Society of Anesthesiologists (ASA) score (≥ IV) [19].
Patients were asked to follow-up with the surgeon at 2 weeks, 3 months, 6 months, 9 months, 12 months, 18 months, and then every year after the surgery. In addition, all our patients were seen by a registered dietitian who offered behavioral modification suggestions and vitamins and mineral supplements. The dietitian’s appointments were scheduled at different time intervals than the surgeon appointments so that there was broader coverage of patient care. Unlike the USA and Europe, Australia does not have the companies selling vitamin and mineral supplements that cater to a specific bariatric procedure. It has been difficult for the US and European companies to supply its products to Australia due to strict Australian Therapeutic Goods Administration compliance requirements. Hence, this practice prescribes compounds and dispenses its own vitamin and mineral supplements based on the ASMBS Guidelines Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient (supplementary material). [20].
Statistical Methods
Descriptive statistics were used to calculate the mean and standard deviation of the preoperative characteristics such as age, weight, BMI, ideal body weight (IBW), and excess body weight (EBW). Postoperative weight loss data were analyzed using non-linear regressions. Fisher’s exact test was used to analyze the nutritional data. All statistical analyses were done using R: A language and environment for statistical computing [21].
Surgical Technique
Laparoscopic SADI-S surgery was performed with a five-port technique (Fig. 1). Initial access was through a 5-mm Applied Medical Kii Fios first entry port at Palmer’s point using a 0°scope. Pneumoperitoneum was established to a pressure of 15 mmHg with CO2 gas. A 5-mm 45° laparoscopic camera was used. Next, a 15-mm port was introduced through the umbilicus. Two 5-mm ports were then introduced two finger breadths beneath the right and left subcostal margins. The last 5-mm port was placed in the right lumbar quadrant in the mid-clavicular line at a variable point based on the patient’s body habitus (Fig. 1).
A Snowden Pencer Liver retraction system was used with the retractor introduced through the right subcostal port. The surgeon (standing on the right side of the patient) operated through the right midclavicular line lumbar and the umbilical port. The assistant surgeon (standing on the left side) assisted through the left subcostal port.
The first step was to locate the ileocecal valve. The small bowel was counted 300-cm proximal to the ileocecal valve. The two bowel graspers used to walk the bowel had markings at 10 cm with a steri-strip that ensured accurate measurement of the small bowel. The antimesenteric border of the bowel at this point was attached loosely to the omentum just below the pyloric valve to mark the site for anastomosis. This suture was later cut prior to the duodeno-ileostomy being performed.
The greater omentum of the stomach was resected similar to the technique used in sleeve gastrectomy (SG), with a LigaSure device (Medtronic, Mansfield, MA, USA). The dissection was then carried medially to the first and second of the duodenum [22]. Retrogastric and retroduodenal adhesions were taken down with the LigaSure device (Medtronic, Mansfield, MA, USA). The limit of this dissection was determined by the gastroduodenal artery embedded in the pancreas. This way, we had at least 3 to 4 cm of duodenum dissected beyond the pylorus. It is our preference not to take down the right gastric artery routinely, as in almost all cases, there was enough space created by this dissection method to introduce the stapler safely without any blunt dissection. Once we were sure that the duodenal transection would be possible safely, we then moved on to do the SG portion first. This was done using a 36 french bougie for sizing. The gastric resection began 4 to 6 cm away from the pylorus hugging loose on the bougie and ended 1 to 2 cm off the angle of His. The first two stapler firings were with 45-mm black Endo GIA™ reinforced Tri-Staples™ (Medtronic, Mansfield, MA, USA). The next firings were with 60-mm Black Endo GIA™ reinforced Tri-Staples™ (Medtronic, Mansfield, MA USA).
The duodenum was then transected at least 2-3 cm beyond the pylorus with a 60-mm purple Endo GIA™ reinforced Tri-Staples™ (Medtronic, Mansfield, MA USA).
We then created an end-to-side (duodenum-to-ileum) anastomosis. The antimesenteric border of the bowel at the marked point was attached to the end of the proximal duodenum staple line using a continuous 2 ‘0’ polysorb suture using an Endo Stitch Device™ (Medtronic, Mansfield, MA, USA). The loop was set up so that the efferent limb was descending on the patient’s right, and the afferent limb was ascending coming up from the left. A duodenotomy and enterotomy was made that was approximately 2 cm. We initially used diathermy to create the enterotomy; however, after we had one patient who bled through the duodenal cut edge requiring a transfusion, we subsequently started using the LigaSure device (Medtronic, Mansfield, MA, USA) to make these cuts which resulted in a bloodless field for suturing. The enterotomy was closed with a running posterior layer and a running anterior layer using 2 0 polysorb sutures with the Endo Stitch™ Device (Medtronic, Mansfield, MA, USA) (Fig. 2).
The patients were discharged when oral intake was adequate; the pain was well controlled and ambulating well. All patients attended a follow-up visit with the operating surgeon 2 weeks post-op. The patients were all instructed about postoperative vitamin supplementation (supplementary material).
Results
Ninety-one patients were identified for the study. The mean age of the study population was 46.2 ± 9 years (Table 1). The mean preoperative BMI and weight were 43.2 ± 5.7 kg/m2 and 123.4 ± 20 kg, respectively. The mean IBW and EBW were 61.4 ± 10.2 kg and 61.4 ± 16.2 kg. The study had 32.9% of male and 67% of the female population. The preoperative OSA, T2D, HLD, HTN, and GERD were seen in 23%, 38.4%, 29.6%, 29.6%, and 9.8% of patients, respectively. Apart from T2D patients, the study also had one insulin resistance (1%), one prediabetes (1%), and three T1D (3.2%) patients. In this study, preoperatively, eight patients were on insulin, and 32 patients on oral hypoglycemic agents. The study had 5.4% and 7.6% high-risk patients and tobacco users, respectively.
The mean operative time (skin-to-skin) was 121.8 ± 25 min (range 75–182) (Table 2). The mean length of stay was 1.4 ± 0.8 days (range 1–7). The intraoperative complication rate was 0%.
Sixty-two patients and 25 patients were out 12 and 24 months, respectively. Of the eligible patients, data were available for 66.1% of patients and 68% of patients at 12 and 24 months, respectively.
Short- and Long-Term Complication
Of all the patients, four patients (4.3%) experienced short-term complications (Clavien-Dindo Grade II) (Table 3). Of the four complications, two patients had bleeding requiring blood transfusions. One from the enterotomy edge and the other from the right upper quadrant port incision site developing an abdominal wall hematoma. Both of these patients were asymptomatic despite an HB drop to ten. However, as they were going to be on a modified dietary regimen for the next 4 weeks, it was deemed appropriate to give them an IV iron infusion and one unit of blood to prevent them from becoming tired and weak in the postoperative period. One patient was diagnosed with euglycemic diabetic ketoacidosis (EDKA) on postoperative day three, requiring readmission to ICU for a prolonged period. One patient experienced alveolar hypoventilation postoperative day three, which was related to obesity hypoventilation syndrome due to inadequate continuous positive airway pressure (CPAP) usage pre- and post-surgery and not the procedure itself. This patient was admitted to the ICU and was initially placed on bilevel positive airway pressure (BiPAP) and then on CPAP.
None of the patients experienced a long-term complication. The mortality rate was 0%.
Weight Loss Analysis
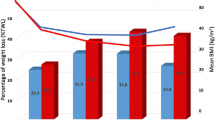
The mean change in BMI at 12, 18, and 24 months was 15.2 ± 5.2 kg/m2, 17.8 ± 5.5 kg/m2, and 17.2 ± 5.9 kg/m2, respectively (Table 4). The mean % total weight loss (%TWL) at 12, 18, and 24 months was 34.6 ± 9.2%, 39.8 ± 8.9%, and 38.8 ± 9.9%, respectively. The mean %excess body weight loss (%EWL) at 12, 18, and 24 months was 69.2 ± 16.4%, 78.1 ± 13.2%, and 77 ± 15.7%, respectively.
Obesity-Related Co-morbidity
Postoperatively, the resolution rate for OSA, T2D, HLD, HTN, and GERD was 94.4%, 94.2%, 75%, 68%, and 12.5%, respectively (Table 5).
Nutritional Status
We compared the nutritional outcomes (number of patients with abnormal values) between baseline and 12 months. At 1 year, the labs were available for 35 patients (60.3%). There was no statistically significant difference between most of the preoperative and postoperative nutritional data except vitamins D and B12 and calcium (Table 6).
Discussion
The results of the study confirm that the early outcomes of SADI-S in Australia have similar results to the rest of the world. The procedure was very effective in treating obesity and co-morbidities like OSA and T2D.
In this study, the mean EWL was 77 ± 15.7%. The reported mean EWL was 85% at 2 years (72–100%), with the outlier of 100% having a 200-cm common channel [15, 16]. The reported complication rate following SADI-S was between 1.6 and 13% [2, 23]. In the present study, the short- and long-term complication rates were 4.3% and 0%, respectively. The most commonly reported complication following SADI-S was diarrhea [24]. Interestingly, none of our patients experienced diarrhea following surgery. Very few reported bowel movements up to 2.5 times a day. In most of these patients, the first bowel movement was in the morning, and then immediately 20–40 min later and very rarely later in the day. Most of the patients reported no change in their bowel habits after surgery apart from the change in the consistency of stools to soft, fluffy, or liquid in nature. We do not know what may attribute to this positive outcome apart from the fact that the measure of 300 -cm of bowel may have been accurate due to our measurement technique described above. Moreover, of the four short-term complications, two complications were rare and have never been reported following the SADI-S procedure [2,3,4,5, 8,9,10,11,12,13,14,15,16]. One patient that was on sodium-glucose cotransporter 2 (SGLT2) inhibitors experienced DKA. There have been few reports in the literature that have reported the association of SGLT2 inhibitors and DKA [25, 26]. This patient required ICU admission. The second rare short-term complication that occurred was alveolar hypoventilation. The patient had a history of OSA, which was diagnosed preoperatively by the treating surgeon and was on CPAP for at least 3 weeks prior to surgery. However, she was drowsy through recovery post-op. We initially thought it might be due to the PCA and other antiemetics like droperidol and stopped them, but on day two, the patient still was very drowsy and hypertensive, leading to the diagnosis and ICU admission. We felt that this might have been due to suboptimal and inadequate CPAP therapy, both in the pre- and postoperative period in the ward. Amongst all the co-morbidities that were studied, the resolution rates were highest for OSA and T2D. In the case of T2D patients, the complete resolution was seen in 94.2% of diabetic patients at the end of 2 years. The reported overall T2D resolution following SADI-S was 60–80% [15, 16, 24]. Interestingly, apart from T2D, our study population also had one insulin resistance, one prediabetes, and three T1D patients. Postoperatively, the serum insulin and blood sugar levels returned to normal in patients with insulin resistance and prediabetes. Improvement was noted in all three T1D patients with a 40–60% reduction in the dosage of the antidiabetic medications.
Protein malnutrition following traditional DS is a major concern [27]. In a study by Sanchez-Pernaut et al., a common channel of 200–250 cm was performed [11]. At 1 year, 34% and 13.7% of the patients had abnormal levels of protein and albumin, respectively. At 3 years, 29% and 12% of the patients had abnormal levels of protein and albumin, respectively. The SADI-S with a common channel of 300 -cm was introduced to eliminate this problem. With a common channel of 300 -cm, at 1 year, Enochs et al. reported protein and albumin deficiency in 7.6% and 3.1% of the patients, respectively [28]. In a study by Mitzman et al., the mean albumin levels were normal at 1 year. Only two patients had hypoalbuminemia [4]. Surve et al. reported abnormal albumin and protein in 10% of the patients [2]. Moon et al. reported 8% abnormal protein levels and 13% abnormal albumin levels in their group [29]. Our data were consistent with these studies. In this study, 6.6% and 6.2% of the patients had an abnormal protein and albumin levels, respectively.
The retrospective nature and small sample size of the study were a few of the limitations. In addition, the study lacked a few important data points, like preoperative vitamin E and B1, zinc, and copper levels. Also, the study did not report the nutritional outcome at 2 years. Additional long-term follow-ups and a larger study population would be required to further evaluate the outcomes of the SADI-S surgery. We intend to collect data diligently and report the 5- and 10-year outcomes.
Conclusions
SADI-S appears to be a safe bariatric surgical procedure with favorable outcomes at 2 years in the Australian population. Nutritional outcomes at 1 year appear promising, but long-term follow-up is needed.
References
Surve A, Cottam D, Sanchez-Pernaute A, et al. The incidence of complications associated with loop duodeno-ileostomy after single-anastomosis duodenal switch procedures among 1328 patients: a multicenter experience. Surg Obes Relat Dis. 2018;
Surve A, Zaveri H, Cottam D, et al. A retrospective comparison of biliopancreatic diversion with duodenal switch with single anastomosis duodenal switch (SIPS-stomach intestinal pylorus sparing surgery) at a single institution with two year follow-up. Surg Obes Relat Dis. 2017;13(3):415–22.
Cottam A, Cottam D, Portenier D, et al. A matched cohort analysis of stomach intestinal pylorus saving (SIPS) surgery versus biliopancreatic diversion with duodenal switch with two-year follow-up. Obes Surg. 2017;27(2):454–61.
Mitzman B, Cottam D, Goriparthi R, et al. Stomach intestinal pylorus sparing (SIPS) surgery for morbid obesity: retrospective analyses of our preliminary experience. Obes Surg. 2016;26(9):2098–104.
Sánchez-Pernaute A, Rubio MA, Pérez-Aguirre E, et al. Single-anastomosis duodenoileal bypass with sleeve gastrectomy: metabolic improvement and weight loss in first 100 patients. Surg Obes Relat Dis. 2013;9:731–5.
Cottam A, Cottam D, Roslin M, et al. A matched cohort analysis of sleeve gastrectomy with and without 300 cm loop duodenal switch with 18-month follow-up. Obes Surg. 2016;26(10):2363–9.
Cottam A, Cottam D, Medlin W, et al. A matched cohort analysis of single anastomosis loop duodenal switch versus Roux-en-Y gastric bypass with 18 month follow up. Surg Endosc. 2016;30(9):3958–64.
Surve A, Zaveri H, Cottam D, et al. Mid-term outcomes of gastric bypass weight loss failure to duodenal switch. Surg Obes Relat Dis. 2016;12(9):1663–70.
Neichoy BT, Schniederjan B, Cottam DR, et al. Stomach intestinal pylorus-sparing surgery for morbid obesity. JSLS. 2018;22(1)
Surve A, Zaveri H, Cottam D. A step-by-step surgical technique video with two reported cases of common channel lengthening in patients with previous stomach intestinal pylorus sparing surgery to treat chronic diarrhea. Surg Obes Relat Dis. 2017;13(4):706–9.
Sánchez-Pernaute A, Rubio MÁ, Cabrerizo L, et al. Single-anastomosis duodenoileal bypass with sleeve gastrectomy (SADI-S) for obese diabetic patients. Surg Obes Relat Dis. 2015;11(5):1092–8.
Surve A, Zaveri H, Cottam D. Retrograde filling of the afferent limb as a cause of chronic nausea after single anastomosis loop duodenal switch. Surg Obes Relat Dis. 2016;12(4):e39–42.
Moon RC, Kirkpatrick V, Gaskins L, et al. Safety and effectiveness of single- versus double-anastomosis duodenal switch at a single institution. Surg Obes Relat Dis. 2019;15(2):245–52.
Surve A, Zaveri H, Cottam D, et al. Laparoscopic stomach intestinal pylorus-sparing surgery as a revisional option after failed adjustable gastric banding: a report of 27 cases with 36-month follow-up. Surg Obes Relat Dis. 2018;
Topart P, Becouarn G. The single anastomosis duodenal switch modifications: a review of the current literature on outcomes. Surg Obes Relat Dis. 2017;13(8):1306–12.
Martini F, Paolino L, Marzano E, et al. Single-anastomosis pylorus-preserving bariatric procedures: review of the literature. Obes Surg. 2016;26(10):2503–15.
Brethauer SA, Kim J, El Chaar M, et al. ASMBS clinical issues committee. Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. 2015;25:587–606.
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S13.
Doyle DJ, Garmon EH. American Society of Anesthesiologists Classification (ASA class). Treasure Island: StatPearls Publishing LLC; 2017.
Aills L, Blankenship J, Buffington C, et al. ASMBS allied health nutritional guidelines for the surgical weight loss patient. Surg Obes Relat Dis. 2008;4:S73–S108.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for statistical computing; 2013. URL http://www.R-project.org/
Surve A, Zaveri H, Cottam D. A safer and simpler technique of duodenal dissection and transection of the duodenal bulb for duodenal switch. Surg Obes Relat Dis. 2016;12(4):923–4.
Nelson L, Moon RC, Teixeira AF, et al. Safety and effectiveness of single anastomosis duodenal switch procedure: preliminary results from a single institution. Arq Bras Cir Dig. 2016;29(Suppl 1):80–4.
Shoar S, Poliakin L, Rubenstein R, et al. A single anastomosis duodeno-ileal switch (SADIS): a systematic review of efficacy and safety. Obes Surg. 2018;28(1):104–13.
Modi A, Agrawal A, Morgan F. Euglycemic diabetic ketoacidosis. Curr Diabetes Rev. 2017;13:315–21.
Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7:135–8.
Lupoli R, Lembo E, Saldalamacchia G, et al. Bariatric surgery and long-term nutritional issues. World J Diabetes. 2017;8(11):464–74.
Enochs P. The laparoscopic stomach, intestinal and pylorus sparing (SIPS) procedure: a single center analysis of our first 100 patients. Surg Obes Relat Dis. 2015;11(6):S165–6.
Nelson L, Moon RC, Teixeira AF, et al. Safety and effectiveness of single anastomosis duodenal switch procedure: preliminary result from a single institution. Arq Bras Cir Dig. 2016;29Suppl 1(Suppl 1):80–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of Human and Animal Rights
I certify that the manuscript did not involve the use of animal or human subjects.
Since this is a retrospective study, formal consent is not required for this type of study.
Conflict of Interest
Author 3, the corresponding author, reports personal fees and other from Medtronic and GI Windows, outside the submitted work.
All other authors have no conflicts of interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Surve, A., Rao, R., Cottam, D. et al. Early Outcomes of Primary SADI-S: an Australian Experience. OBES SURG 30, 1429–1436 (2020). https://doi.org/10.1007/s11695-019-04312-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04312-6