Abstract
Background
The aim of this study was to investigate whether the implementation of enhanced recovery after surgery (ERAS) guidelines according to Thorell and co. in our tertiary referral bariatric center might improve post-operative outcomes.
Methods
ERAS program was introduced in our center since January 1, 2017. Retrospective review of a prospectively collected database identified patients who underwent laparoscopic primary and revisional bariatric surgeries from October 2005 to January 2018. Patients exposed to ERAS program (“ERAS group”) were matched in a 1:1 ratio with patients exposed to conventional care (control group) using a propensity score based on age, gender, preoperative body mass index (BMI), diabetes mellitus, and the type of procedures. The primary outcome was total hospital length of stay (LOS) and the secondary outcomes included the post-operative complications and readmission rates.
Results
During the study period, 464 patients were included, 232 in each group. Implementation of the ERAS protocol was significantly associated with a reduction of LOS (2.47 ± 1.7 vs 5.39 ± 1.9 days, p < 0.00001). One-third of patients was discharged (77/232, 33%) on the first postoperative day (POD) and more than three quarter of patients on POD 2 (182/232, 77%). At the opposite, no patients of the control group were discharged on POD 2. Overall 30-day and 90-day morbidity and readmission rates were the same in both groups. There was no death in each group.
Conclusions
This large case-matched study using a propensity score analysis suggests that implementation of ERAS program significantly reduced length of hospital stay without significant increases on overall morbidity, and readmission rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery has now been established to be safe and effective for long-term weight loss maintenance and control of obesity-related disease as demonstrated by randomized controlled trials [1, 2]. Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are the most widely used surgical procedures, accounting for approximately 75% of all procedures. Enhanced recovery after surgery (ERAS) has deeply changed the approach of perioperative care toward major surgical procedures. Strong evidence of consistent benefits of ERAS has been reported since its initiation by Kehlet et al., especially for colorectal surgery [3,4,5]. The adoption of ERAS pathways has resulted in improved outcome in terms of reduced morbidity, faster recovery, and reduced length of hospital stay in dedicated centers [6, 7]. The literature concerning the implementation of ERAS programs into bariatric surgery is still scarce.
However, two recent systematic reviews with meta-analysis have shown that enhanced recovery programs into bariatric surgery decreased significantly the length of stay with no significant influence on overall morbidity or specific complication rates as compared with conventional care [8, 9]. The limitations to these reviews included the heterogeneity regarding the individual protocols used among the studies (i.e., ERAS, alternative clinical pathways, fast-track programs) and the poor quality of the papers included (only one randomized controlled study [10]). Moreover, 9 studies of the 13 included in the most recent meta-analysis [8] were published before ERAS guidelines were published [11]. In addition, another review which has included five comparative studies applying ERAS protocols [12] was limited by the lack of information of the included studies. To our knowledge, it is useful to establish whether ERAS principles applied to bariatric surgery can improve outcomes.
With our long-term experience in laparoscopic bariatric surgery [13,14,15], our surgery department introduced since January 1, 2017, the ERAS program according to recent guidelines published by Thorell and co. [11]. The current study prospectively investigated both effectiveness and safety of the implementation and validation of ERAS guidelines for bariatric surgery as compared with conventional care, in regard to length of hospital stay, post-operative morbidity, and readmission rates.
Patients and Methods
Study Design
Data were retrospectively collected from a prospectively maintained database of morbidly obese patients undergoing laparoscopic RYGB, LSG, or revision from sleeve gastrectomy to Roux-en-Y gastric bypass (LSG to LRYGB) from October 2005 to January 2018 at our French tertiary referral bariatric center. This study has been approved by the local medical ethics committee; no individual inform consent was necessary as it was a retrospective analysis. The indication for bariatric surgery is assessed using the IFSO criteria [16] and all cases were endorsed in a local interdisciplinary consensus meeting.
Patients were stratified into standard protocol (control group) and ERAS protocol groups. The control group included consecutive bariatric surgeries performed between October 2005 and December 2016 while the ERAS group included consecutive bariatric surgeries performed between January 2017 and January 2018. Only patients with a follow-up longer than 6 months after bariatric surgery were considered for the analysis. This “ERAS group” was matched in a 1:1 ratio with the control group. The matching was made with a propensity score based on age, gender, preoperative body mass index (BMI), diabetes mellitus, and the type of procedures. The investigators (HM and BM) were blinded to the primary end points in both groups during manual matching to reduce bias.
Bariatric ERAS Intervention Protocol
Prior to the start of ERAS protocol in our department until January 2017, all members of the multidisciplinary team including surgeon, anesthesiologists, nurses, and management staff have been to the Catharina Hospital in Eindhoven, in order to train, update, and set up the project [17, 18].
Our ERAS protocol has been developed with the Thorell et al.’s guidelines [11] and summarized in Table 1. All patients were treated according to this protocol and the surgery was performed only if they fulfilled the mandatory conditions. The protocol was approved by all members of the bariatric team [19].
Preoperative Management
The minimal preoperative follow-up was 6 months during which patients were received several times in preoperative appointments with the surgeon, the anesthetist, the dietician, the endocrinologist, the physiotherapist, and the psychologist [11]. Each steps of our CP were explained during all those consultations. During the first consultation with the surgeon, the biometrics values were collected. All along the preoperative follow-up, the surgery prehabilitation of the patients included the practice of exercise to improve their physiological function without exceeding their maximal resistance, the cessation or reduction (maximum 2 cigarettes per day) of smoking at least 4 weeks before surgery, and the complete cessation of alcohol for 2 years in case of history of alcohol abuse [11]. Obstructive sleep apnea (OSA) was systemically screened and a continuous positive airway pressure therapy was introduced if the diagnostic was made to decrease the risk of postoperative apnea [20]. Two weeks before the surgery, a preoperative low-calorie diet (1000–1200 kcal/day) was initiated to reduce the liver volume [11]. In patients with type 2 diabetes, this diet was associated with a modification of their treatment by their endocrinologist to prevent hypoglycemia. Nutritional issues were systematically explained in a dedicated group meeting led by a dietician. Two days before surgery, the patient was called by a dedicated nurse (bariatric coordination nurse) to do a preoperative checklist. They were reminded to recommendations of fasting for 6 h prior to the anesthetic’s induction and all recent events were recorded. The patients were admitted in a bariatric-dedicated ward on the morning of their surgery except the high-risk patients (insulin-dependent diabetes patients) who were admitted the night before surgery. The surgeon and a dedicated nurse checked if the patient complied with all the preoperative conditions: negative βhCG levels for women the week before the surgery and weighing of the patients. A weight gain at the admission led to an adjournment of the surgery.
Surgical Technique
All procedures were standardized to be administered in the same way by all the surgeons of our center. The surgery was performed by laparoscopy, in beach chair position with flexed hip and a local analgesia by ropivacaine infiltration was performed. No drain, urinary catheter, or nasogastric tube were left [11]. The LRYGB operative technique included systematic bisection of the omentum, creation of a 30-ml vertical gastric pouch, formation of an antecolic and antegastric Roux-en-Y limb, a totally manual gastrojejunostomy with absorbable suture and a linear-stapled jejuno-jejunostomy with sutured closure of the defect. The Petersen’s and intermesenteric defect were closed with a nonabsorbable suture. All the staple lines were reinforced by absorbable suture. The gastrojejunostomy was tested with 60 ml of methylene blue given with distal bowel occlusion.
The LSG was performed by multiple firings of a linear stapler sizing over a 36F calibrating tube. We start the stapling 6 cm from the pylorus to the cardiac notch without section of the left paracardial lymph nodes. The staple line was reinforced by absorbable suture. The LSG was tested with 150 ml of methylene blue.
The revision from LSG to RYGB was a single-stage procedure of which surgical technique was previously reported [14].
Perioperative Management
Two hours before the induction, a preoperative carbohydrate conditioning was made by the ingestion of a carbohydrate drink (e.g., apple juice) [11]. All patients wear compression stockings during the surgery except for the patients with BMI > 50 kg/m2 or history of deep venous thrombosis/pulmonary embolism (DVT/PE) who wore pneumatic stockings. The transportation of patients was made in their bed but they make the transfer from their bed onto the operation table alone to prevent malposition [21]. Their monitoring was followed by a pulse oximeter, EKG, blood pressure cuff, and a Bispectral Index [11, 22]. Before surgery, the patients received intravenous analgesics and antibiotics. To prevent postoperative nausea and vomiting (PONV), they received intravenous glucocorticoids. All anesthetists were dedicated to this kind of surgery and so aware of the specific difficulties in the management of obese patient [11]. Our ERAS anesthesia protocol was standardized as describe in Fig. 1 and based on the recommendations of the guidelines [11]. The dose of drugs was indexed on the ideal weight. After the surgery, patients were monitored in the recovery room for 2 h after the surgery.
Postoperative Management
All patients were admitted in the dedicated ward and there was no admission in intensive care unit (ICU) even for high-risk patients. The systematic post-operative PONV protocol contained four times daily 1 g paracetamol, four times daily 20 mg droperidol, two injections of parecoxib, tramadol 50 mg if necessary, and ondansetron 4 mg if necessary [11]. Four hours after the surgery, patients were mobilized to reduce both the risk of DVT/PE and the pain [23, 24] and encouraged to drink. We maintained a low intravenous volume of fluids at the evening of the surgery in order to promote oral intake [11, 25]. Patients with CPAP followed their treatment in the postoperative period to decrease complications rates [11, 26].
At postoperative day 1, the intravenous route was removed and all the patients who underwent bariatric surgery ate together in a dedicated room. The diabetic patients were screened by an endocrinologist to adjust the dosage of their medication. If the patients met all discharge criteria (Table 2), they would be discharged in the afternoon of the POD 1. Those criteria were based on some studies which study risk factors for long LOS or readmission after discharge [25, 27,28,29,30]. The written prescriptions were given by the surgeon and contained analgesics, one-time daily proton pump inhibitors for 3 months, vitamin and minerals supplements, an adjusted dose to BMI of enoxaparin (i.e., 8000 IU for BMI > 40 kg/m2 and 10,000 IU for BMI > 50 kg/m2) during 15 days, and 5 sessions of physiotherapy to promote the mobilization after discharge. All information about diet, exercises, and alarm symptoms were given by a dedicated dietician and nurse. Two days after surgery, the patient was called by the bariatric nurse to do a postoperative checklist (regarding pain, nausea, vomiting, and mobility). The first follow-up visit with the surgeon is scheduled 1 month after the surgery.
Data Collection
The relevant information for each patient were prospectively collected. Patient characteristics (gender, age), biometrics values (i.e., weight, height, body mass index BMI, percentage of excess body weight), comorbidities (diabetes, hypertension, OSAS, dyslipidemia…), the ASA physical status classification system (ASA), surgical past history, medications, and habitus were retrieved. For each patient, the surgeon’s experience and the operative time were collected. A surgeon was considered as a junior if he performed less than 50 cases of the same procedures [31]. Because some associated intervention during surgery (i.e., adhesiolysis, cholecystectomy, gastric band ablation, reparation of intestinal wound, parietal reparation) may adversely influence postoperative recovery or LOS, their frequency was also recorded for analysis.
The postoperative data recorded included early and late postoperative complications, length of hospital stay (LOS), the rate of readmission in emergency room after discharge, and the rate of rehospitalization and reintervention. Early complications were defined as those that occurred until postoperative day 30 (POD 30) or at any time during the primary hospital stay. Late complications were defined as those that occurred within 90 days postoperative (POD 90). We considered “surgery-related morbidity” to be any complication resulting from the surgical procedure, such as anastomotic leakage, peritonitis, intraperitoneal bleeding, anastomotic bleeding, or any other event directly caused by the surgery. All the complications were stratified according to the Dindo-Clavien scale [32]. A Dindo-Clavien classification of three points or higher was considered as a severe complication. Readmission rate was defined as unplanned hospitalization after discharge from bariatric care unit within 90-day postoperative period.
Outcomes
Primary outcome of this study was total hospitalization length of stay. Secondary outcomes were the rate of overall and severe complications, rate of readmission in emergency room for medical consultation, rehospitalization rate, and reoperation rate within both 30 and 90 days, respectively.
Statistical Analysis
Statistical analysis was performed with R studio R3.2.1. Data were presented as medians with 95% confidence interval or mean with standard deviation (SD) as appropriate.
A propensity score matching (PSM) analysis was calculated to reduce selection biases. Considering the knowing criteria of ERAS failing, patients matched in order to get two identical population in the preoperative data to estimate which factors influence the feasibility of ERAS protocol in bariatric surgery. Patients were matched in 1:1 analysis with the closest estimated PS within 0.2 of the PSM standard deviation. For PSM, we chose variables which were known to affect our main outcome. As a result, PS was performed using logistic regression including the following criteria: age, gender, preoperative body mass index (BMI), diabetes mellitus, and the type of procedures. After the matching process, the demographic data of both groups were compared in order to demonstrate adequate matching. Finally, both groups were analyzed regarding the different variables in this study.
Comparison of variables used for the matching process between the two groups was undertaken using chi-square or Fisher’s exact tests as indicated (*), for categorical data. Continuous variables were analyzed by two-sided Student’s t test.
Results
Between January 1, 2017 and January 1, 2018, 232 consecutive patients undergoing bariatric surgery were exposed to ERAS program (“ERAS group”). Our propensity score matched those patients with 232 patients who underwent bariatric with conventional care (“control group”). Characteristics of all patients are summarized in Table 3. No significant differences were observed between both groups except for a significant higher prevalence of OSAS, hypercholesterolemia, and use of antiplatelet and anticoagulant among patients exposed to the ERAS group. No-morbidity was significantly more observed in patients of the control group as compared with the ERAS group (70/232 vs 51/232, p = 0.03).
Postoperative outcomes are summarized in Table 4. Both groups were comparable according to bariatric surgical procedures of which 4.5% of cases were revisional. There was no statistically significant difference in the number of additional concomitant procedures performed in both groups. All procedures were performed laparoscopically, except for 3 patients who required conversion into laparotomy (0.65%). Mean operative time was similar between both groups (130.1 min in ERAS group vs 127.4 min in control group, p = 0.49).
Implementation of the ERAS protocol decreased significantly the mean LOS as compared with conventional care (2.47 ± 1.7 5.39 ± 1.9, p < 0.00001). Implementation of the ERAS protocol has led to discharge one-third of patients (77/232, 33.2%) on the POD 1 and more than three quarter of patient on POD 2 (182 (77 on POD 1 and 105 on POD 2)/232, 78.4%). At the opposite, no patients of the control group were discharged on POD 2. Main reasons for delayed discharge in the ERAS group (50/232 > POD 2) included minor postoperative complications (n = 10, 20%), major complications (n = 2, 4%), inadequate control of pain at the beginning of our experience (n = 16, 32%), patient’s social factors (n = 7, 14%; e.g., patient reluctant to be discharged home because of living alone or > 2-h drive from hospital), postoperative nausea and poor oral intake (n = 13, 26%), and difficulty to control diabetes mellitus (n = 2, 4%). Among the 33 patients who came for readmission in emergency room, 29 (87%) were admitted in the hospital. In the ERAS group, 5/15 patients were discharged on POD 1, 3/15 were discharged on POD 2, and 7 were discharged after POD 2 (4 at POD 4, 2 at POD 10, and 1 at POD 16). One hundred percent of patients who came for readmission in emergency room were admitted in the hospital in the ERAS group. Eighty-seven percent of patients (13/15) in the ERAS group came for readmission in emergency room between POD 13 and POD 20.
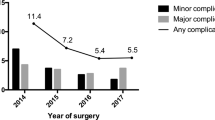
Number and type of complications within 30 and 90 days respectively are shown in Table 5. In total, 65 of 464 (14%) patients (32 patients in ERAS group and 33 patients in control group, respectively) developed a postoperative complication within 30 days from the original operation. Fifty-four of 464 (11.6%) patients (29 (12.5%) in ERAS group and 25 (10.8%) patients in control group, respectively) developed a minor complication (Dindo-Clavien grade I–II) and 11 of 464 (2.4%) (3 (1.3%) patients in ERAS group and 8 (3.5%) patients in control group, respectively) a severe complication (Dindo-Clavien grade III–IV). There was no statistical difference in 30-day major or minor complications rates according to Dindo-Clavien classification, between the two groups (13.8% vs 14.2%, ns; Table 5). Six patients (two in ERAS group and four in control group, respectively), needed a reoperation during the first hospitalization within index operation due to bleeding on trocar wound (n = 3), stenosis of the gastrojejunostomy of a LRYGB (n = 2), and a wound of the pulmonary artery by a central catheter (n = 1).
Twenty-nine patients (15 in the ERAS group and 14 in the control group, respectively) were readmitted within 30 days after discharge. Half of these patients in the ERAS group were discharged during the first 2 days postoperatively. Three patients (1 in the ERAS group and 2 in the control group) experienced severe post-operative complications: 2 of them required a reoperation due to a stenosis of the gastrojejunostomy of a LRYGB and a bleeding on trocar wound in the second case. In the patient with bleeding on the trocar wound, an endoscopic prosthesis was necessary for a gastrojejunal anastomotic leakage. In the ERAS group, 30 patients had antiplatelet agents and 7 had anticoagulation. In the control group, 14 patients had antiplatelet agents and 1 had anticoagulation. At POD 30, in the ERAS group, 8 patients had bleeding complications (2 patients only had antiplatelet agents and none had anticoagulation) and in the control group, 8 patients had bleeding complications (1 patients only had antiplatelet agents and none had anticoagulation). There was no significant difference between the two groups for the rate of readmission in emergency room for medical consultation, hospitalization, and reintervention rates (Table 5).
Six patients (2 in the ERAS group and 4 in the control group, respectively) were readmitted between the POD 30 and POD 90. Only two of them, in the control group, experienced severe postoperative complications. One patient required emergency operation for incisional hernia and the second was successfully treated by endoscopic dilatation for a gastrojejunal stenosis. Finally, at day 90, the two groups were comparable according to overall morbidity rate (15.5% vs 16.8%, p = 0.71), reintervention rate (1.3% vs 2.6%, p = 0.31), and readmission rate (7.3% vs 7.8%, p = 0.86) respectively. No 90-day mortality was observed in each group. All patients were followed-up at 6 months after surgery (Table 6).
Discussion
This case-matched study suggested that implementation of ERAS protocol according to Thorell’s guidelines into bariatric surgery is a safe and feasible program which improve outcomes. In our experience, it reduced significantly the length of stay by around 3 days without any statistically significance on the overall morbidity rate, specific complication rate, and readmission rate, respectively.
ERAS protocols have changed the approach of perioperative care toward many colorectal surgical procedures with strong evidence of consistent benefits [33]. However, data from studies evaluating the impact of ERAS pathways according to Thorell et al.’ guidelines in patients undergoing bariatric surgery remain sparse. To our knowledge, three meta-analysis [8, 9, 12] were available in the literature and suggested that the use of ERAS protocols in bariatric surgery shortened LOS compared to conventional care without significantly increased complications.
In our study, length of hospital stay was 2.47 days which was congruent with results found by other ERAS team [34]. LOS was chosen as the primary outcome in our study because it is a measure of efficacy while the rate of both complications and readmissions, chosen as secondary outcomes are measures of safety. According to recent analysis, most of patients who underwent LGYBP required at least 2 days in the hospital for adequate recovery [29]. Several comorbidities such as diabetes mellitus, chronic obstructive pulmonary disease, renal insufficiency, and the experience of the surgeon were predictive of prolonged hospitalization. These findings would not be appropriate in theory for fast-tracking [29, 35]. With our long-term experience in laparoscopic bariatric surgery, our team consider at the opposite that the higher the risks are, the more effort should be given to enhance recovery: in our study, there were the same number of patients who underwent surgery by a junior or a senior surgeon, One quarter of patient’s exposed to the ERAS group was classified as ASA score = 3, the prevalence of OSA, hypercholesterolemia, and the use of antiplatelet and anticoagulant were significantly higher among patients exposed to the ERAS group as compared with patients in the standard group with the same results on postoperative morbidity.
After implementation of the program according to guidelines [11], we discharged one-third of patients within 24 h from the index operation and more than three quarter (78.4%) of patients within 48 h from the index operation. At the opposite, no patient without ERAS program was discharged before the POD 2. This discrepancy may be explained by establishment of clearly defined discharge criteria, which may decrease the possibility of patients to stay longer than required. In the early stage of implementation of our ERAS protocol, reasons for delayed discharge beyond the POD 2 included inadequate control of pain, poor postoperative nausea control, and poor oral intake in more than half of the patients. Furthermore, if we exclude the 100 first patients in the ERAS group—according to the learning curve for an ERAS protocol [36, 37], the LOS was 1.96 days and 87% of patients were able to discharge within 48 h from the index operation (data not shown).
Our overall 30-day hospital readmission rate was 6.5% (n = 15) which is lower that the rate reported in the literature (0.9 to 8.3%) [38]. Six (40%) of these patients were admitted to the emergency department because of reported inadequate pain control and were discharged the following day with stronger analgesia. To minimize any risk, specific instructions were given to all patients on discharge, with a 24/7 direct contact telephone number and the recommendation to return to our hospital if any problem was experienced at home. Our low hospital readmission rate can be explained because we had facility to review the patient in emergency room and discharge.
Our overall morbidity rate within 30 days was 13.8% in patients exposed to ERAS program. Although this value was high, it is in accordance with the one reported in the literature (0.6 to 18.3%) [9, 12, 38]. However, this rate was three times higher than Deneuvy et al.’s have recently reported. This result must be interpreted with caution because in their study, 56% of patients were included in the ERAS while all consecutive patients were exposed to ERAS program in our study. No differences in overall and specific complications were noted between both groups [10] that are consistent with other bariatric ERAS studies that yielded similar results [39]. Additionally, no influence of the type of surgery was noted, including revisional bariatric procedures (data not shown). In our experience, the majority of post-bariatric surgery complications developed within the in-hospital. Only 3 patients (0.65%) have required a reoperation within 90 days after discharge. Furthermore, no 90-day mortality was observed in each group. Therefore, it is reasonable to assume that its introduction is safe, regardless the type of operation.
However, implementation of ERAS protocol in our experience did not decrease significantly overall morbidity and specific complications rates as compared with the standard protocol. These results might be explained by the fact that elective laparoscopic bariatric surgery led to postoperative complication rate which is lower than in other fields of surgery. Further reduction in these may be difficult to achieve and probably may require a highly powered study to show a significant decrease.
The present study has several potential limitations: the study design was retrospective but relevant information for each patient was prospectively collected in our tertiary center of obesity management. Comparisons with historical controls may introduce significant risk of bias but the use of a propensity score allow to control and reduce the effect of confounding factors. Furthermore, inclusion of consecutive patients exposed to ERAS program without any exclusion reduces the risk of selection bias. This study did not focus on our follow-up rate. Although our team has followed a training program, we considered that continuous auditing remains the main key to achieving a high compliance rate.
In conclusion, this case-matched study with a propensity score highlights that implementation of ERAS protocol according to Thorell’s guidelines into bariatric surgery is feasible and safe. This multidisciplinary strategy reduced significantly the length of stay by around 3 days without any statistical increase on overall morbidity, specific complications, and readmission rates. In the future, assessment of quality of life and cost-effectiveness analysis will be key elements in evaluating the success of ERAS in the bariatric settings. Moreover, ERABS needs prospective randomized controlled study to be completely used in surgical bariatric management of patients.
References
Mingrone G et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet (London, England). 2015;386(9997):964–73.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376(7):641–51.
Spanjersberg WR, Reurings J, Keus F, et al. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. 2011;2:CD007635.
Gustafsson UO et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg. 2018.
Beamish AJ, Chan DSY, Blake PA, et al. Systematic review and meta-analysis of enhanced recovery programmes in gastric cancer surgery. Int J Surg. 2015;19:46–54.
Basse L, Raskov HH, Hjort Jakobsen D, et al. Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. Br J Surg. 2002;89(4):446–53.
Basse L, Hjort Jakobsen D, Billesbølle P, et al. A clinical pathway to accelerate recovery after colonic resection. Ann Surg. 2000;232(1):51–7.
Ahmed OS, Rogers AC, Bolger JC, et al. Meta-analysis of enhanced recovery protocols in bariatric surgery. J Gastrointest Surg. 2018;22(6):964–72.
Małczak P, Pisarska M, Piotr M, et al. Enhanced recovery after bariatric surgery: systematic review and meta-analysis. Obes Surg. 2017;27(1):226–35.
Lemanu DP, Singh PP, Berridge K, et al. Randomized clinical trial of enhanced recovery versus standard care after laparoscopic sleeve gastrectomy. Br J Surg. 2013;100(4):482–9.
Thorell A, MacCormick AD, Awad S, et al. Guidelines for perioperative care in bariatric surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg. 2016;40(9):2065–83.
Singh PM, Panwar R, Borle A, et al. Efficiency and safety effects of applying ERAS protocols to bariatric surgery: a systematic review with meta-analysis and trial sequential analysis of evidence. Obes Surg. 2017;27(2):489–501.
Contival N, Menahem B, Gautier T, et al. Guiding the non-bariatric surgeon through complications of bariatric surgery. J Visc Surg. 2018;155(1):27–40.
Gautier T, Sarcher T, Contival N, et al. Indications and mid-term results of conversion from sleeve Gastrectomy to Roux-en-Y gastric bypass. Obes Surg. 2013;23(2):212–5.
Vallois A, et al. Revisional Roux-en-Y gastric bypass: a safe surgical opportunity? Results of a case-matched study. Obes Surg. 2018.
Fried M et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24(1):42–55.
Mannaerts GHH, van Mil SR, Stepaniak PS, et al. Results of implementing an enhanced recovery after bariatric surgery (ERABS) protocol. Obes Surg. 2016;26(2):303–12.
Proczko M, Kaska L, Twardowski P, et al. Implementing enhanced recovery after bariatric surgery protocol: a retrospective study. J Anesth. 2016;30(1):170–3.
Stepaniak PS, Heij C, Buise MP, et al. Bariatric surgery with operating room teams that stayed fixed during the day: a multicenter study analyzing the effects on patient outcomes, teamwork and safety climate, and procedure duration. Anesth Analg. 2012;115(6):1384–92.
Giles T, Lasserson T, Smith B, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. In: Giles T, editor. Cochrane Database of Systematic Reviews, no. 3. Chichester: John Wiley & Sons, Ltd; 2006. p. CD001106.
Ogunnaike BO, Jones SB, Jones DB, et al. Anesthetic considerations for bariatric surgery. Anesth Analg. 2002;95(6):1793–805.
Lemmens HJM, Brodsky JB. General anesthesia, bariatric surgery, and the BIS monitor. Obes Surg. 2005;15(1):63.
Dobesh PP, Wittkowsky AK, Stacy Z, et al. Key articles and guidelines for the prevention of venous thromboembolism. Pharmacotherapy. 2009;29(4):410–58.
Schug SA, Raymann A. Postoperative pain management of the obese patient. Best Pract Res Clin Anaesthesiol. 2011;25(1):73–81.
Major P, Wysocki M, Torbicz G, et al. Risk factors for prolonged length of hospital stay and readmissions after laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2018;28(2):323–32.
Liao P, Yegneswaran B, Vairavanathan S, et al. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth. 2009;56(11):819–28.
Gagnière J, Slim K. Don’t let obese patients be discharged with tachycardia after sleeve gastrectomy. Obes Surg. 2012;22(9):1519–20.
Pike TW, White AD, Snook NJ, et al. Simplified fast-track laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2015;25(3):413–7.
Carter J, Elliott S, Kaplan J, et al. Predictors of hospital stay following laparoscopic gastric bypass: analysis of 9,593 patients from the National Surgical Quality Improvement Program. Surg Obes Relat Dis. 2015;11(2):288–94.
Lane JC, Wright S, Burch J, et al. Early prediction of adverse events in enhanced recovery based upon the host systemic inflammatory response. Color Dis. 2013;15(2):224–30.
Geubbels N, de Brauw LM, Acherman YIZ, et al. The preceding surgeon factor in bariatric surgery: a positive influence on the learning curve of subsequent surgeons. Obes Surg. 2015;25(8):1417–24.
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Greco M, Capretti G, Beretta L, et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38(6):1531–41.
Deneuvy A, Slim K, Sodji M, et al. Implementation of enhanced recovery programs for bariatric surgery. Results from the francophone large-scale database. Surg Obes Relat Dis. 2018;14(1):99–105.
Khorgami Z, Petrosky JA, Andalib A, et al. Fast track bariatric surgery: safety of discharge on the first postoperative day after bariatric surgery. Surg Obes Relat Dis. 2017;13(2):273–80.
Bamgbade OA, Adeogun BO, Abbas K. Fast-track laparoscopic gastric bypass surgery: outcomes and lessons from a bariatric surgery service in the United Kingdom. Obes Surg. 2012;22(3):398–402.
Søvik TT, Aasheim ET, Kristinsson J, et al. Establishing laparoscopic Roux-en-Y gastric bypass: perioperative outcome and characteristics of the learning curve. Obes Surg. 2009;19(2):158–65.
Hahl T, Peromaa-Haavisto P, Tarkiainen P, et al. Outcome of laparoscopic gastric bypass (LRYGB) with a program for enhanced recovery after surgery (ERAS). Obes Surg. 2016;26(3):505–11.
Lam J, et al. An ERAS protocol for bariatric surgery: is it safe to discharge on post-operative day 1? Surg Endosc. 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study has been approved by the local medical ethics committee; no individual inform consent was necessary as it was a retrospective analysis.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meunier, H., Le Roux, Y., Fiant, AL. et al. Does the Implementation of Enhanced Recovery After Surgery (ERAS) Guidelines Improve Outcomes of Bariatric Surgery? A Propensity Score Analysis in 464 Patients. OBES SURG 29, 2843–2853 (2019). https://doi.org/10.1007/s11695-019-03943-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-03943-z