Abstract
Background
One of the new current treatment options for Diabetes Mellitus is about increasing glucagon-like peptide-1 (GLP-1) activity. GLP-1 with its incretin effect showed major role in glucose homeostasis. Gastroileostomy can increase GLP-1 secretion by rapid delivery of undigested food to the terminal ileum. We studied the early effects of a gastroileostomy on serum levels of GLP-1, glucose, and insulin in rats.
Methods
Gastroileostomies with side-to-side anastomosis were performed on 15 male New Zealand rats. Blood samples were obtained before and 1 week after the gastroileostomy.
Results
Our results showed that the rats lost a lot of weight from start (330 ± 15 g) to the end (240 ± 25 g) of the experiment (p = 0.048). The data analysis showed that the gastroileostomy surgery elevates the level of GLP-1in plasma significantly (89.1852 vs. 177.440 respectively; p < 0.001) and caused a significant decrease in plasma glucose as well (92.00 and 66.29 mg/dL respectively; p < 0.001). However, the insulin state elevated after the surgery significantly (8.03 vs. 9.89; p < 0.001).
Conclusion
In this study, we showed the effectiveness of gastroileostomy treatment to decrease body weight and plasma glucose with increased GLP-1 in rats. This small rat model suggests the potential of this surgery to treat type 2 diabetes mellitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advances in treatment of type 2 diabetes mellitus (T2DM), optimal treatment control is not entirely achieved specially in obese patients [1]; however, bariatric surgeries are currently used as new therapeutic approaches [1,2,3]. From which, there are studies on using different methods to increase glucagon-like peptide-1 (GLP-1) activity; however, these methods have not been well established [2, 3]. GLP-1 receptor agonists similarly to exenatide and liraglutide have shown acceptable effects [1,2,3,4]. GLP-1, a gastrointestinal peptide with incretin effect, sends postprandial neuroendocrine signal and has a significant role in glucose homeostasis; it is released during a meal and stimulates insulin synthesis and insulin secretion, which does not occur when carbohydrates are administered intravenously [4]. GLP-1 inhibits post-meal glucagon release [5, 6] and affects the appetite center of the brain that causes central satiety. Hence, weight loss remains as one of the predictable effect of elevated GLP-1 [5]. Although GLP-1 can stop the progression of beta cell failure, the glucose-lowering effectiveness dissipates rapidly after cessation of GLP-1 agonist medications [7,8,9]. GLP-1 has a short half-life of about one to 2 min; therefore, continuous infusion of GLP-1 or continuous internal secretion of GLP-1 is necessary to achieve a steady state level in the blood [10]. GLP-1 is currently being evaluated for the therapy of diabetes. Increased secretion of GLP-1 may help to weight loss and be responsible for postprandial hypoglycemia [8]. GLP-1 secretion can be stimulated by undigested foods containing carbohydrates from terminal ileum [1,2,3,4,5,6,7,8,9,10].
Main aims of bariatric/metabolic surgeries include providing functional restriction for foods and malabsorption pathways, which send neuroendocrine signals. Laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass (LRYGB) may change intestinal morphology and the subcellular organization of enterocytes as well as functional proteins behavior in enterocytes. The later results in increased GLP-1 secretion after glucose-rich meals [11,12,13]. Single anastomosis sleeve ileal (SASI) is a new therapeutic approach introduced by Santoro et al. composed of a sleeve gastrectomy with side-to-side gastroileal anastomosis. Recent studies suggest that SASI has two components: the sleeve gastrectomy, the restrictive part, and the gastroileal anastomosis sending neuroendocrine signals. Rapid transit of undigested foods elevates GLP-1 and peptide YY (PYY) secretions from terminal ileum, and the sleeve gastrectomy decreases secretion of ghrelin from gastric fundus. According to modern human diet, less undigested foods are delivered to the distal part of the intestine, resulting in less GLP-1 and PYY secretion. Hence, there is more chance for weight gain and diabetes in people [10,11,12,13,14,15,16,17].
Similarly, gastroileostomy can provide intrinsic increases of GLP-1 secretion in the rapid delivery of undigested foods to the terminal ileum [8]. Although there are multiple types of bariatric surgery, there is a lack of evidence to guide the development of valid and reliable protocols for selection of one technique of bariatric surgery [14, 15]. In recent clinical works reported that SASI was effective for significant weight loss, controlling T2DM, and normalizing lipid profile without any nutrient deficiencies [16, 17]. However, not enough convincing evidence exists on therapeutic effects and complications of SASI.
Gastrointestinal surgery as a bariatric/metabolic surgery might be an option for patients with T2DM without adequate clinical control [18, 19]. The underlying mechanisms of bariatric surgery to achieve T2DM remission are unclear and might involve changes in gut hormones such as GLP-1 [20].
To our knowledge, this pre-clinical study is the first experimental assessment of the anti-glycemic effect of gastroileostomy in rat as a promising model to control T2DM. In this experiment, the gastroileostomy is SASI without sleeve procedure; therefore, our study was conducted to compare and evaluate efficacy of gastroileostomy on plasma levels of insulin, glucose, and GLP-1.
Materials and Methods
Animals
Fifteen healthy male New Zealand rats with the similar weight (the means and standard deviation (mean ± SD) 330 ± 15 g body weight), and no history of surgery or other medical interventions were included from Central Laboratory of our University. The animals were maintained under controlled conditions in a pathogen-free environment under constant ambient temperature and humidity with free access to food and water at all times, excluding a mandatory overnight fast before the surgery (at least 8 h). This research commenced after fulfilling all ethical statements while receiving its approval from department of ethics in medical research. All procedures were conducted in agreement with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Interventions
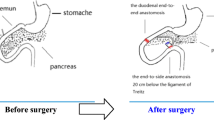
Gastroileostomy was performed on 15 male rats after an overnight fast. Surgery for all rats was carried out under the same standard conditions by one surgeon. Anesthesia was induced and maintained with 0.1 mg/kg ketamine, (Ketamine Hydrochloride Rotexmedica, Germany) (Fig. 1), while each anesthetized rat was laid in the supine position on a surgical table (Fig. 2), and the abdominal skin was shaved using hair removal cream (Veet ®, French) and disinfected with 10% betadine. Under sterile conditions, a 4.5-cm midline incision with a No. 10 scalpel was performed on the abdomen. A piece of peritoneal surface was removed from the abdominal wall with surgical scissors. Gastroileostomy surgery was performed on all animals by anastomosing 1 cm of antrum to terminal ileum (side-to-side). We measured the whole small intestine, located the ileum, and create an upward loop from its distal one third, about 10 cm from ileocecal part. A side-to-side 10-mm anastomosis was made to antral part of the stomach. To complete anastomosing, a single layer running technique with absorbable poly-p-dioxanone suture (4/0, Monoplus®) was applied. We tested the opening of the anastomosing with an index finger (Fig. 3). Finally, the incised area of the skin and fascia were repaired with silk sutures (4/0 Taft, Yazd, Iran), and the rats were left at a suitable temperature (23–25 °C) to regain consciousness. All animals were kept on the same diet after surgery. The study took 7 days after surgery. Silk sutures were removed on day 5 of treatment, under general anesthesia.
Blood samples were obtained from the vein in medial cantus at the base line and 1 week after gastroileostomy (Fig. 4).
Outcome Assessment
The effectiveness was determined by any reduction in body weight and plasma glucose level.
Plasma insulin was measured using the LIAISONR Analyzer. GLP-1 was measured using the human GLP-1ELISA kit (Biotech Co., Germany).
All side effects of the intervention, including adverse effects related to systemic anesthesia, local infection, anastomosing leakages, and death, were assessed by the same surgeon using a questionnaire.
Statistical Analysis
Mean ± SD values were determined for plasma GLP-1, glucose, and insulin level as the base line and 1 week after the surgery. For statistical analysis and to compare two mean ± SD values, paired Student’s t test of IBM SPSS version 21.0 (Chicago, USA) was used. p value of < 0.05 was considered significant.
Results
The experiments were performed with 15 male New Zealand rats. One rat died after the operation due to not regaining consciousness.
One week after the gastroileostomy, the body weight of rats on standard diet decreased significantly compared to baseline (330 ± 15 vs. 240 ± 25 g before and after the surgery respectively; p = 0.048).
We detected a similar reduction of plasma glucose level during the first week after the gastroileostomy has been recorded. Blood glucose decreased significantly from 92.00 ± 14.21 mg/dL before the surgery to 66.29 ± 16.06 mg/dL after that (p < 0.001) (Table 1).
Data shows that gastroileostomy surgery elevates the level of GLP-1in plasma significantly (89.1852 ± 77.261618 vs. 177.440 ± 40.939613; p < 0.001) with simultaneous increase in insulin secretion (8.03 ± 0.01 vs. 9.89 ± 0.36; p < 0.001) (Table 1).
Discussion
A significant reduction of body weight and plasma glucose level was achieved in the first week after the gastroileostomy surgery, and simultaneously, the insulin level and GLP-1 increased significantly. Weight loss improves blood glucose and insulin level, which are independent from increased secretion of GLP-1. Our rats lost one third of their initial weight after 1 week and had higher level of GLP-1 and insulin, which suggests the independent effect of gastroileostomy on neuroendocrine signals of the intestine leading to increased secretion of GLP-1, decreased plasma glucose, and therefore ends with weight reduction. Our results are first experimental surgery suggesting the early effects of gastroileostomy on body weight and glucose homeostasis in rat model.
The gastroileostomy resembles the SASI surgery without performing the restrictive component, sleeve gastrectomy. According to a newly published clinical research with limited patients, SASI decreases fasting blood sugar (FBS), glycosylated hemoglobin A (HbA1c), and lipid profile until 2 years after the surgery continuously and progressively without nutrient deficiency. C-peptide did not change significantly in this study suggesting that SASI improves T2DM by decreasing insulin resistance but not its secretion level in blood [16, 17]. More compelling evidences are required to show the clinical applicability of this procedure.
Consistent with the previous results [21, 22], we observed that experimental gastroileostomy in rats model changed glucose absorption restriction and increased GLP-1 secretion [23, 24]. We verified that gastroileostomy surgery increases glucose-stimulated GLP-1 secretion in the rats by measuring GLP-1 in the blood before and after the surgery in the same nutritional conditions. GLP-1 increases glucose-dependent insulin secretion, exhibits trophic effects on β cells of the pancreas, inhibits glucagon secretion from α cells, inhibits glucose production in the liver, increases glucose uptake in the heart, inhibits gastric emptying, decreases postprandial gastrointestinal motility, and reduces appetite [25, 26].
Bariatric procedures send glucose-rich meals to the terminal ileum and lead activation of L-cells by these glucose, which may explain the glucose-induced GLP-1 secretion after gastroileostomy surgery [27, 28]. The increased glucose-induced release of GLP-1 from L-cells observed in animals after bariatric surgery, including Ileal Interposition surgery, can be due to an increased stimulation of L cells because of contact with higher intraluminal glucose concentrations after surgery [22]. In gastroileostomies, the duodenum and the jejunum are removed to increase the amount of glucose reaching the ileum after a glucose-rich meal is increased [11, 22, 27].
Shortening of the food retention time in the GI tract after gastroileostomy is also thought to increase the glucose concentration in the ileum after glucose-rich meals. Gastroileostomy forces more than half of the meal to pass from the anastomosis and the remaining from pyloric sphincter.
It is unclear which one of the two major types of bariatric surgery achieves the greatest and the most durable remission of T2DM and induced weight loss [29].
There are limited randomized clinical trials in this topic [15, 30]. Each technique has some advantages and disadvantage. LRYGB is fully reversible; however, the irreversible LSG is a faster and simpler procedure with potentially less dumping [29,30,31].
Technical difficulties are involved in performing LRYGB in severely obese patients; therefore, these patients may have limited success from LRYGB attributed to pouch dilation and loss of restriction at the gastrojejunal anastomosis over time [29,30,31].
Therefore, a simple technique with fast efficacy and minimal complications is desirable.
We chose gastroileostomy over other glycemic controls by bariatric surgery and anti-metabolic procedures, since it causes a rapid and continuous elevation in the level of GLP-1.
This procedure may change intestinal morphology and an increased number of GLP-1-secreting L cells per cross section, as shown in previous studies [22, 23].
Gastroileostomy leads to increased GLP-1 secretion after glucose-rich meals and can be considered as a promising option for control of hyperglycemia in type 2 diabetes mellitus.
The limitations of our study include the relatively small sample size and the lack of control rats in our study design. Therefore, side effects related to general anesthesia and midline laparotomy were not studied. This study revealed the early outcomes of gastroileostomy. The authors recommend further clinical studies evaluating the efficacy and safety of this surgery to find the most efficient and safe surgical technique that can be used in a human model. Like other researches in this field, long-term results of this surgery should be evaluated too.
Conclusion
Based on the results of this study, gastroileostomy is an effective way of improving metabolism of glucose in rats and will help to decrease body weight and to treat type 2 diabetes mellitus.
References
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53.
Dupre J, Behme MT, McDonald TJ. Exendin-4 normalized postcibal glycemic excursions in type 1 diabetes. J Clin Endocrinol Metab. 2004;89(7):3469–73.
Shyangdan DS, Royle P, Clar C, et al. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;10:CD006423.
Lee YS, Jun HS. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism. 2014;63(1):9–19.
Koliaki C, Doupis J. Incretin-based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2011;2(2):101–21.
Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36(8):741–4.
Xu G, Stoffers DA, Habener JF, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270–6.
Vilsboll T, Krarup T, Deacon CF, et al. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50(3):609–13.
Stoffers DA, Desai BM, DeLeon DD, et al. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes. 2003;52(3):734–40.
Parkes DJC, Smith P, et al. Pharmacokinetic actions of exendin-4 in the rat: comparison with glucagon-like peptide-1. Drug Dev Res. 2001;53:260–7.
Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol. 2009;297(5):G950–7.
Johannessen H, Kodama Y, Zhao CM, et al. Eating behavior and glucagon-like peptide-1-producing cells in interposed ileum and pancreatic islets in rats subjected to ileal interposition associated with sleeve gastrectomy. Obes Surg. 2013;23(1):39–49.
De Robertis E, Zito Marinosci G, Romano GM, et al. The use of sugammadex for bariatric surgery: analysis of recovery time from neuromuscular blockade and possible economic impact. Clinicoecon Outcomes Res. 2016;8:317–22.
Murphy R, Evennett NJ, Clarke MG, et al. Sleeve gastrectomy versus Roux-en-Y gastric bypass for type 2 diabetes and morbid obesity: double-blind randomised clinical trial protocol. BMJ Open. 2016;6(7):e011416.
Keidar A, Hershkop KJ, Marko L, et al. Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia. 2013;56(9):1914–8.
Mahdy T, Schou C. Efficacy of single anastomosis sleeve ileal (SASI) bypass for type-2 diabetic morbid obese patients: gastric bipartition, a novel metabolic surgery procedure: a retrospective cohort study. Int J Surg. 2016;34:28–34.
Salama TMS, Sabry K, El Ghamrini Y. Single anastomosis sleeve ileal bypass: new step in the evolution of bariatric surgeries. J Investig Surg. 2017;30(5):291–6.
Campos J, Ramos A, Szego T, et al. The role of metabolic surgery for patients with obesity grade I and clinically uncontrolled type 2 diabetes. Arq Bras Cir Dig. 2016;29:102–6.
Rizvi AA. The evolving role of bariatric surgery in patients with type 1 diabetes and obesity. Integr Obes Diabetes. 2016;2(2):195–9.
Vetter ML, Cardillo S, Rickels MR, et al. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009;150(2):94–103.
Ashrafian H, le Roux CW. Metabolic surgery and gut hormones—a review of bariatric entero-humoral modulation. Physiol Behav. 2009;97(5):620–31.
Jurowich CF, Otto C, Rikkala PR, et al. Ileal interposition in rats with experimental type 2 like diabetes improves glycemic control independently of glucose absorption. J Diabetes Res. 2015;2015:490365.
Nausheen S, Shah IH, Pezeshki A, et al. Effects of sleeve gastrectomy and ileal transposition, alone and in combination, on food intake, body weight, gut hormones, and glucose metabolism in rats. Am J Physiol Endocrinol Metab. 2013;305(4):E507–18.
Zhang H, Li J, Li Z, et al. Increased GLP-1 response after gavage-administration of glucose in UCP2-deficient mice. Horm Metab Res. 2012;44(2):86–90.
Imeryuz N, Yegen BC, Bozkurt A, et al. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Phys. 1997;273(4 Pt 1):G920–7.
Zhao T, Parikh P, Bhashyam S, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317(3):1106–13.
Taqi E, Wallace LE, de Heuvel E, et al. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg. 2010;45(5):987–95.
Patriti A, Aisa MC, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced proglucagon gene expression and L-cell number. Surgery. 2007;142(1):74–85.
Li J, Lai D, Wu D. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes Surg. 2016;26(2):429–42.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Mahawar KK, Parikh C, Carr WR, et al. Primary banded Roux-en-Y gastric bypass: a systematic review. Obes Surg. 2014;24(10):1771–92.
Acknowledgments
The authors acknowledge Mr. Amirsalar Moazen Safaei and Mr. Aria Azarshahi for their help in proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Resource
No fund received for this research.
Ethics Statements
This research commenced after fulfilling all of the ethical statements and receiving its approval from department of ethics in medical research. All procedures were conducted in agreement with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Rights and permissions
About this article
Cite this article
Keleidari, B., Mohammadi Mofrad, R., Shahabi Shahmiri, S. et al. The Impacts of Gastroileostomy Rat Model on Glucagon-like Peptide-1: a Promising Model to Control Type 2 Diabetes Mellitus. OBES SURG 28, 3246–3252 (2018). https://doi.org/10.1007/s11695-018-3312-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3312-y