Abstract
Background
Gastric leak is the most common and dreaded post-operative infectious complication (PIC) after laparoscopic sleeve gastrectomy (LSG). Accurate identification of patients at risk postoperatively is of cardinal importance.
Objective
The aim of this study is to assess the diagnostic performance of C-reactive protein (CRP) in predicting PICs and the most optimal time to measure it.
Methods
CRP results were collected in patients undergoing LSG between 2011 and 2015. CRP was systematically measured on post-operative days (POD) 1, 3, and 5.
Results
Of 1326 patients, 42 (3.2%) developed a PIC at a median of 5 days after surgery. The incidence of leakage was 1.9%. The best area under the curve was observed on POD5 (0.87; 95% CI 0.77–0.96). At this time point, a cut-off of 115 mg/L yielded a sensitivity of 66.7% (95% CI 46.5–86.8%), a specificity of 95.1% (95% CI 93.9–96.3%), a positive and negative predictive values of 19.4% (95% CI 10.3–28.6%) and 99.4% (95% CI 99.0–100%), respectively, and a positive and negative likelihood ratios (LRs) of 13.62 and 0.35, respectively. The combination of sequential assessments of CRP on POD3 and 5 provided a sensitivity of 84.4% (95% CI 71.8–97.0%), a specificity of 91.1% (95% CI 89.5–92.8%), a positive and negative predictive values of 20.9% (95% CI 14.0–27.9%) and 99.5% (95% CI 99.1–99.9%), respectively, and a positive and a negative LRs of 9.58 and 0.17, respectively.
Conclusions
CRP may be useful to identify patients at risk of PICs after LSG and, therefore, to prompt early investigation. However, CRP does not help rule out PICs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laparoscopic sleeve gastrectomy (LSG) has become a first-line treatment in bariatric surgery. It is considered as a simple and easy procedure, yet it involves some risk of post-operative infectious complications (PICs). Gastric leaks are the most common and the most feared PICs after LSG [1]. They are the second most common cause of death after bariatric surgery [2] and occur in around 2% of patients [3].
A significant proportion of leaks occur well after surgery. In a systematic review of more than 4000 patients published in 2012, 79% of leaks occurred more than 10 days after surgery and after hospital discharge [4]. In particular, the increasing trend towards the implementation of fast-track protocols in bariatric surgery [5] runs the risk of discharging patients before PICs could arise, leading to increased morbidity and hospital readmission. Hence, there is an acute need for reliable tools in order to identify patients at high risk of PICs.
Arguably, clinical surveillance and computed tomography (CT) scans remain the mainstay for leak detection. Still, a widely available fast and cheap biological marker that could be used alongside careful clinical assessment is desirable. The correlation of the acute phase protein CRP (C-reactive protein) with PICs after major abdominal surgery is well-known [6]. Nevertheless, laboratory examinations are generally regarded as non-contributory in diagnosing PICs after bariatric surgery, and published evidence remains scarce [7]. Two studies on the diagnostic performance of CRP to detect PICs after LSG have recently been published [8, 9]; however, they were underpowered to produce precise estimates.
Thus, in this study, we aimed to evaluate the usefulness of CRP measurement after LSG in predicting PICs. We addressed this question by using measurements of CRP levels on post-operative days (POD) 1, 3, and 5 to determine the performance of a single measurement of CRP based on assessment on any of the aforementioned days. A secondary objective was to determine the performance of CRP based on a combination of two sequential measurements. The diagnostic performance of the two sequential determinations of CRP can be thought as a way to take into account its variation along the post-operative period.
Methods
Study Design
This is a retrospective study, with a prospectively maintained database of consecutive patients undergoing LSG at a single university hospital in France (Bichat–Claude-Bernard University Hospital), between January 2011 and July 2015. Patients with at least one CRP measurement between POD1 and 5 were included in the final analysis. Surgical procedure and pre- and post-operative management are described in Appendix 1, which can be found in the supplemental data in the online version of this article.
The following data were recorded in the prospectively maintained database: patient’s demographics and anthropometrics, the presence and the day of a PIC registered within a 30-day period after surgery, the presence and the day of other complications, and whether the patient has been readmitted to hospital within 30 days from surgery. All these data were anonymized and then linked to two other databases using hospital identification number. The two databases, namely, a laboratory database (for CRP data) and an administrative database related to the French Medical Information System (for length of hospital stay (LOS) and simultaneous ring ablation or cholecystectomy procedure data), were prospectively maintained and provided by Bichat Hospital.
This study was reported in accordance with the guidelines from the Standards for Reporting of Diagnostic Accuracy statement [10].
Index Test
CRP testing was performed as part of routine measurements on POD1, 3, and 5. All patients had CRP measurements independently of clinical suspicion of infection. The quantitative determination of CRP was carried out using an automated analytical system (Siemens Dimension Vista® 1500) with a reference range <3 mg/L and a measurement range of 2.90–190 mg/L.
Reference Standard
The outcome of interest was the occurrence of a PIC within 30 days after surgery, defined as the presence of any septic complication including adverse events associated with surgery and medical conditions that are not specific to bariatric surgery (Appendix 2). Patients presenting with fever, abdominal pain, tachycardia, elevated levels of inflammatory markers, or abnormal TOGD tests underwent double-contrast CT scan with oral and intravenous ingestion.
Data Analysis
The diagnostic accuracy of CRP as a marker of PICs was assessed for each POD by receiver operating characteristic (ROC) analysis. Patients with a PIC occurring before the POD of interest were excluded from the analysis in order to compute the diagnostic performance estimates with respect to the day of CRP measurement. A number of true−/false positives, true−/false negatives, sensitivity (Se), specificity (Sp), area under the curve (AUC), positive−/negative predictive values (PPV/NPV), positive−/negative likelihood ratio (LR) with 95% confidence intervals (CI), and optimal cut-off values were calculated for each POD. The optimal cut-off point was determined by maximizing the sum of sensitivity and specificity (Youden’s index) [11]. As multilevel LRs of a test with multiple cut-off points are more powerful and useful than one single cut-off point [12], multilevel LRs were calculated across 11 quantiles cut-off points of CRP values [13]. The probability of a PIC after CRP testing (post-test probability) was obtained using LRs and formulas based on Bayesian theorem.
We subsequently assessed the diagnostic performance of two strategies of CRP testing combining measurements of CRP on the POD that provided the best diagnostic accuracy, and either one of the other measurements. Respective cut-off values determined for separate CRP measurements were used to compute diagnostic performance estimates of CRP testing combinations. For each CRP testing combination, a negative test was defined by CRP below the cut-off at both POD measurements, whereas a positive test was defined by CRP above the cut-off at either of the two measurements. Sensitivity analysis, including diagnostic accuracy of CRP in predicting only gastric leak, was undertaken as described in Appendix 3. All statistical analyses were performed using R version 3.3.0 and SAS (version 9.3, SAS institute Inc., Cary North Carolina). P values are two sided, and values <0.05 were considered statistically significant.
Results
During the study period, 1482 patients underwent LSG at Bichat–Claude-Bernard University Hospital. One hundred fifty-six patients did not have their CRP checked on the exact PODs considered in this study. Thus, 1326 (89%) patients were available for the present analysis (Fig. 1). The majority of patients were female (82.6%). The median age was 39 years (IQR 30–48) at the time of LSG, and median BMI was 41.9 kg/m2 (IQR 38.7–46.3) (Table 1).
Of the 1326 patients, 42 (3.2%) developed a PIC within 30 days of surgery. Median time to diagnosis of PICs was 5 days (IQR 4–8; range 1–18) after surgery. The incidence of PICs after POD1, 3, and 5 was 2.9% (39/1326), 2.7% (36/1326), and 1.6% (21/1326), respectively. Median post-operative LOS was 7 days (IQR 7–8) and was significantly longer in patients with PICs (P < 0.0001) (Table 1). Among the 42 cases of PICs, 9 (9/42, 21%) occurred after hospital discharge. The rate of each type of complication is summarized in Table 2. There were no in-hospital or outpatient deaths within 30 days of operation in this consecutive series.
Post-operative Time Course of CRP Levels
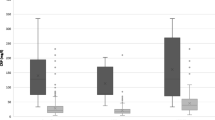
The median levels of CRP were significantly higher at each post-operative measurement in complicated patients relative to uncomplicated patients: 43.2 (IQR 20.5–88.5), 136 (IQR 62–185), and 170.5 mg/L (IQR 89.1–247) on POD1, 3, and 5, respectively, versus 18.3 (IQR 12–29.4), 47 (IQR 30–72.9), and 44.6 mg/L (IQR 28–67.1) on the same respective days (P < 0.0001). Greater levels of CRP were observed in patients with leaks or abscess complications. CRP increased on POD3 in all patients. In patients with PICs, CRP further increased on POD5, while it decreased in uncomplicated patients (Table 3, Appendix 4). Furthermore, CRP levels at each post-operative measurement were significantly higher in patients with simultaneous procedure (P < 0.001) (Appendix 5).
Diagnostic Performance of CRP Levels
The diagnostic performance of separate and combined CRP testing is depicted in Table 4. The greatest diagnostic accuracy as measured by the area under the ROC curve was observed on POD5 (AUC = 0.87) (Fig. 2). The highest cut-off value was observed on POD3, which was the day of the highest mean CRP level. The highest sensitivity and specificity were reached on POD5, using a cut-off of 115 mg/L (Se = 66.7%; Sp = 95.1%). The vast majority of patients with no PIC were correctly predicted, with an increasing NPV value per POD (97.9, 98.6, and 99.4% on POD1, 3, and 5, respectively). However, the PPV remained low (14.5, 22.0, and 19.4% on POD1, 3, and 5, respectively). Among the two combination strategies, CRP testing on POD3 and 5 provided the highest sensitivity and specificity (Se = 84.4%; Sp = 91.1%). The positive LRs on POD1, 3, and 5 were respectively 4.04, 9.59, and 13.62. On the same respective days, the negative LRs were 0.51, 0.47, and 0.35. The multilevel LRs for CRP levels in identifying PICs are shown in Table 5. Sensitivity analysis results are presented in Appendixes 6 and 7.
Discussion
CRP is often routinely determined as part of the post-operative assessment of patients undergoing surgery. Our study is, to the best of our knowledge, the largest study by far addressing the diagnostic value of CRP levels for the detection of PICs following LSG. It sheds some light regarding the optimum use of CRP measurement and its role in identifying patients who are at higher risk postoperatively. With several systematic reviews and meta-analysis on the diagnostic performance of CRP in abdominal surgical patients published just within the past year [6, 14, 15], there exists a substantial body of evidence on the role of CRP measurement in facilitating a safe early discharge in these patients. However, limited data are available regarding the added value of measuring CRP in the specific field of bariatric surgery in general and in LSG in particular.
In uncomplicated patients, it is well understood from previous research that there is a peak at 48–72 h post-operatively, which decreases thereafter [16]. On the other hand, in patients with post-operative complications, CRP level may remain high or even increase further [17]. Our study offers further confirmation of the post-operative peak of CRP observed at 72 h. In addition, we found that CRP levels were generally higher in patients who underwent simultaneous procedure. This is supported by the notion that the degree of CRP increase after surgery is correlated with the magnitude of operative injury and operative procedure [18]. Interestingly enough, we found that CRP was significantly higher in complicated patients even on POD1. Given the median time to diagnosis of 5 days, this increase suggests that CRP is produced ahead of clinical manifestation, which is in accordance with a previous study [19].
The best discriminative ability was reached on POD5 in this study, as it was the case in a meta-analysis and systematic review of 2215 patients who had major abdominal surgery [6]. This is plausible, as by day 5, that the inflammatory reaction after surgery would have resolved, whereas the inflammation induced by infection would have increased [20]. The same meta-analysis obtained a NPV ranging from 82% on POD1 to 92% on POD5. In our study, the NPV was notably higher. This may be due to a much lower incidence of PICs in the setting of LSG, as compared to other surgeries included in the meta-analysis (mainly colorectal surgery). Yet, it would be presumptuous to claim that such a high NPV is transferrable to other settings. One must keep in mind that, on the contrary of sensitivity and specificity, predictive values not only depend on the discriminative value of the test but also strongly vary with disease prevalence [21]. The leak rate observed in our study is quite low (1.9%) and in the lower range of what has been reported in the literature (0–7%) [4], consistent with the fact that our hospital is a high-volume center for LSG. Nonetheless, since the incidence of complications post-sleeve gastrectomy is generally relatively low, a high NPV is rather likely to be maintained across various settings. Finally, our results indicate that CRP may be useful in ruling in a diagnosis on POD5, owing to the high specificity and positive likelihood ratio (>10) on that day [13].
The multilevel LRs for several intervals of serum CRP showed the cut-off points above which PIC is likely (63, 132, and 125 mg/L on POD1, 3, and 5, respectively). However, no cut-off point below which we can strongly exclude the possibility of a PIC could be identified. For example, the post-test probability of a PIC in an average patient with a high CRP level (>132 mg/L) on POD3 was 30% versus the pre-test probability of approximately 3%, but only small changes from pre-test to post-test probability were observed for lower CRP ranges. From a practical clinical point of view, the aforementioned cut-off values identify patients with a high probability of a PIC, thereby prompting further diagnostic procedures in a timely manner.
Taken together, these results could serve as a basis for a rational approach to monitoring CRP after bariatric surgery, in order to identify patients most likely to develop gastric leaks and other septic complications. Notably, it is important to remember that gastric leaks are particularly difficult to diagnose. Patients with gastric leaks have a poor prognosis, which is inexorably worsened by delays in diagnosis and treatment [22]. Patients with increased CRP levels (>120 mg/L on POD3 and/or >115 mg/L on POD5) would require additional investigation. As such, CRP would be even more useful for detecting septic complications in fast-tack patients (usually discharged between POD1 and POD3). Ambulatory testing of CRP would be performed, and patients would be specifically instructed to return to hospital if they had elevated CRP levels. Hence, for those centers that already implement fast-track protocols, sequential ambulatory CRP determinations would be appropriate. On the other hand, for the remaining centers—including ours—the findings of this study should encourage and facilitate adoption of fast-track protocols combined with ambulatory testing of CRP.
When performing sensitivity analysis using early PICs as an outcome, similar results were obtained regarding the day with the best diagnostic accuracy and the best testing combination. However, sensitivity was generally higher for detection of early PICs, as compared to PICs in overall. Conversely, specificity was lower as one would expect, since sensitivity and specificity usually vary in opposite directions. Given that approximately 20% of PICs appeared after POD7, it is not surprising that the ability of CRP testing up to 5 days post-operatively is more sensitive for early PICs. Hence, further studies are warranted to investigate the role of CRP measurement from the second week onwards following surgery, in order to detect late complications that would have been otherwise missed by early CRP testing.
The present study did not reiterate any of the results of the two previous studies that assessed the diagnostic performance after LSG [8, 9]. This may be due, we believe, to the difference in the definition of study outcomes reported in those studies (which did not include septic complications other than leaks, intra-abdominal abscess, and surgical site infection). In addition, the study setting was different in the study of Munoz et al. (ERAS Program). Furthermore, although information regarding statistical certitude (i.e. confidence intervals) is missing in these reports, the precision of diagnostic performance estimates may be questionable because of the small sample sizes of these studies, thus making it difficult to provide incontestably precise estimates.
There are limitations to this study. First and foremost, the study was undertaken in a center reporting a median LOS of 7 days, thereby limiting the generalizability of the study results to hospitals where fast-track programs are implemented. Second, the study is based on a retrospective design. However, the retrospective design did not lead to selective measurement of CRP in patients clinically suspected of PICs. Indeed, CRP is routinely checked on POD1, 3, and 5 in our center, as part of post-operative follow-up. Third, as the vast majority of previous studies assessing the diagnostic value of CRP after abdominal surgery, only patients with clinical suspicion or elevated CRP measurements underwent reference tests. Hence, we cannot exclude the possibility that the findings of this study are biased through selective disease verification (work-up bias). Indeed, it has been shown that if positive results are more likely to receive the gold standard, the sensitivity is being overestimated and the specificity is being underestimated. However, when the probability of selecting patients only depends on the observed data (the test being evaluated), the estimates of PPV and NPV are unbiased [23]. A further limitation is that the outcome assessors were not blinded to CRP levels; therefore, knowledge of the CRP value might have influenced the interpretation of the reference test. A final limitation is that clinical data such as tachycardia and fever were not available, which did not allow us to investigate the diagnostic performance of CRP combined with clinical symptoms. Nevertheless, as mentioned above, CRP seemingly begins to rise before the appearance of clinical symptoms, which suggests that it is a better candidate marker for predicting PICs.
Conclusion
To conclude, the present results suggest that sequential testing of CRP on POD3 and 5 may be useful to identify patients at risk of a PIC occurring within 8 days of LSG and, therefore, to prompt early investigation. However, CRP is not helpful to reliably rule out PICs after LSG. For those centers that already implement fast-track protocols, sequential ambulatory CRP determinations would be appropriate. On the other hand, for the remaining centers, the findings of this study should encourage and facilitate adoption of fast-track protocols combined with ambulatory testing of CRP. In both cases, other subsequent measurements relevant for screening late complications should be performed. The proposed thresholds require validation in blinded clinical trials in a fast-track setting.
References
Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240–5.
Iossa A, Abdelgawad M, Watkins BM, Silecchia G. Leaks after laparoscopic sleeve gastrectomy: overview of pathogenesis and risk factors. Langenbecks Arch. Surg. 2016;1–10.
Parikh M, Issa R, McCrillis A, et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257:231–7.
Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26:1509–15.
Elliott JA, Patel VM, Kirresh A, et al. Fast-track laparoscopic bariatric surgery: a systematic review. Updat Surg. 2013;65:85–94.
Gans SL, Atema JJ, van Dieren S, et al. Diagnostic value of C-reactive protein to rule out infectious complications after major abdominal surgery: a systematic review and meta-analysis. Int J Color Dis. 2015;30:861–73.
Abou Rached A, Basile M, El Masri H. Gastric leaks post sleeve gastrectomy: review of its prevention and management. World J Gastroenterol. 2014;20:13904–10.
Albanopoulos K, Alevizos L, Natoudi M, et al. C-reactive protein, white blood cells, and neutrophils as early predictors of postoperative complications in patients undergoing laparoscopic sleeve gastrectomy. Surg Endosc. 2013;27:864–71.
Muñoz JL, Ruiz-Tovar J, Miranda E, et al. C-reactive protein and Procalcitonin as early markers of septic complications after laparoscopic sleeve gastrectomy in morbidly obese patients within an enhanced recovery after surgery program. J Am Coll Surg. 2016;222:831–7.
Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527.
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5.
Sackett DL. Evidence-based medicine: how to practice and teach EBM. 2nd ed: Aufl. Edinburgh, Churchill Livingstone; 2001.
Grimes DA, Schulz KF. Refining clinical diagnosis with likelihood ratios. Lancet Lond Engl. 2005;365:1500–5.
Straatman J, Harmsen AMK, Cuesta MA, Berkhof J, Jansma EP, van der Peet DL. Predictive value of C-reactive protein for major complications after major abdominal surgery: a systematic review and pooled-analysis. PLoS ONE [Internet]. 2015 [cited 2016 May 10];10. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4503561/.
Adamina M, Steffen T, Tarantino I, et al. Meta-analysis of the predictive value of C-reactive protein for infectious complications in abdominal surgery. Br J Surg. 2015;102:590–8.
MacKay GJ, Molloy RG, O’Dwyer PJ. C-reactive protein as a predictor of postoperative infective complications following elective colorectal resection. Colorectal dis. Off J Assoc Coloproctology G B Irel. 2011;13:583–7.
Straatman J, van der Peet DL. C-reactive protein after major abdominal surgery: biochemical and clinical aspects. ResearchGate [Internet]. 2015 [cited 2016 Sep 21];2. Available from: https://www.researchgate.net/publication/279212476_C-reactive_protein_after_major_abdominal_surgery_biochemical_and_clinical_aspects.
Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery. 2015;157:362–80.
Welsch T, Müller SA, Ulrich A, Kischlat A, Hinz U, Kienle P, et al. C-reactive protein as early predictor for infectious postoperative complications in rectal surgery. Int J Colorectal Dis. 2007;22:1499–507.
van der Meer W, Pickkers P, Scott CS, van der Hoeven JG, Gunnewiek JK. Hematological indices, inflammatory markers and neutrophil CD64 expression: comparative trends during experimental human endotoxemia. J Endotoxin Res. 2007;13:94–100.
Brenner H, Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med. 1997;16:981–91.
Montravers P, Augustin P, Zappella N, Dufour G, Arapis K, Chosidow D, et al. Diagnosis and management of the postoperative surgical and medical complications of bariatric surgery. Anaesth Crit Care Pain Med. 2015;34:45–52
Zhou XH. Effect of verification bias on positive and negative predictive values. Stat Med. 1994;13:1737–45.
Acknowledgements
We extend our gratitude to Dr. Damien Van Gysel who provided data on length of hospital stay and Mr. Arnaud Serret Larmande for proofreading the final draft of the manuscript.
Author information
Authors and Affiliations
Contributions
Study conception and design: Marmuse, Dib.
Acquisition of data: Marmuse.
Analysis and interpretation of data: Marmuse, Dib.
Drafting of manuscript: Dib.
Critical revision: Hajage, Ribiero Parenti, Boutten.
Corresponding author
Ethics declarations
Funding
The authors received no specific funding for this work.
Ethics Statement
This research was conducted according to French Ethics law (Loi Huriet) which does not require formal ethical approval for this kind of studies. For this type of study, formal consent is not required. The data file was declared to the French national commission for computerized files and liberty (declaration no. 1995504). This study was reported in accordance with the guidelines from the Standards for Reporting of Diagnostic Accuracy statement.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Appendix 1
Preoperative assessment, surgical procedure and post-operative management. (DOCX 13.6 kb)
Appendix 2
Definitions of septic complications included in the outcome of interest. (DOCX 14.9 kb)
Appendix 3
Sensitivity analysis. (DOCX 12.5 kb)
Appendix 4
Post-operative changes in C-reactive protein in patients with and without septic complications. (TIFF 1877 kb)
Appendix 5
Post-operative changes in C-reactive protein in patients with and without a simultaneous procedure. (TIFF 1877 kb)
Appendix 6
Diagnostic performance of C- reactive protein levels on post-operative days 1, 3 and 5, for the detection of infectious complications occurring within 8 days of surgery. (DOCX 16.7 kb)
Appendix 7
Diagnostic performance of C-reactive protein levels on post-operative days 1, 3 and 5, for the detection of gastric leak. (DOCX 15.2 kb)
Rights and permissions
About this article
Cite this article
Dib, F., Parenti, L.R., Boutten, A. et al. Diagnostic Performance of C-Reactive Protein in Detecting Post-Operative Infectious Complications After Laparoscopic Sleeve Gastrectomy. OBES SURG 27, 3124–3132 (2017). https://doi.org/10.1007/s11695-017-2744-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2744-0