Abstract
Background
Gastric stenosis (GS) is a potential adverse event post-laparoscopic sleeve gastrectomy (LSG). Endoscopic management is preferred; however, there is significant variation in therapeutic strategies with no defined algorithm. This study aims to describe the safety and efficacy of a predefined step-wise algorithm for endoscopic management of GS post-LSG.
Methods
Consecutive patients with symptomatic GS post-LSG, presenting between July 2015 and August 2016, were subjected to a predefined treatment algorithm of serial dilations using achalasia balloons, followed by a fully covered self-expanding metal stent (FCSEMS) if dilations were inadequate. Patients who did not respond or opted out of ongoing endoscopic therapy were offered revision Roux-en-Y gastric bypass (RYGB).
Results
Total of 17 patients underwent a median of 2 (range 1–4) balloon dilations. Twelve patients (70.6%) reported clinical improvement with balloon dilation alone, while 3 (17.6%) required subsequent FCSEMS placement. One patient suffered a tear to the muscularis propria with balloon dilation, which was managed conservatively. Overall, 15 (88.2%) reported clinical improvement with endoscopic management. PAGI-SYM scores revealed that the strongest response to therapy, based on mean reduction of score ± SD, was in the following items: nausea (3 ± 1.9, P < 0.001), heartburn during day (2.8 ± 1.5, P = 0.003), heartburn on lying down (3.4 ± 1.4, P < 0.001), reflux during day (2.8 ± 1.9, P < 0.001), and reflux on lying down (3.0 ± 1.9, P < 0.001). Two (11.8%) patients failed endoscopic therapy and underwent RYGB.
Conclusions
Endoscopic management of GS using the described algorithmic approach is safe and effective post-LSG. Patients with severe stenosis or helical stenosis are likely to require revision RYGB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laparoscopic sleeve gastrectomy (LSG), a relatively new surgical option for morbidly obese patients, has gained popularity in recent years, with several studies showing post-LSG weight loss comparable to that post Roux-en-Y gastric bypass (RYGB) [1, 2]. Still, LSG is prone to certain serious adverse events, such as staple line leakage, bleeding, and gastric stenosis (GS) [3, 4]. The last of these complications, GS, occurs in 0.7 to 4% of patients that undergo LSG and often causes nausea, vomiting, epigastric pain, and reflux, and if untreated, can lead to dehydration and malnutrition [4, 5].
GS occurs as a result of progressive rotation of the staple line and scarring of the sleeve in a kinked fashion, or due to imbrications of the staple line and over-retraction of the greater curvature during stapling. The stenosis site is most often at the incisura angularis or gastro-esophageal junction [6]. Diagnosis is commonly made by X-ray examination after ingestion of radio-opaque contrast or esophagogastroduodenoscopy (EGD) with or without injection of contrast under fluoroscopic guidance.
Despite encouraging results from case reports and series utilizing various treatment methods, there are no clear guidelines on the management of post-LSG stenosis. Current endoscopic treatment modalities include balloon dilation and stenting [7, 8]. Recent reports have shown the use of an achalasia balloon to be successful in 71.4 to 86.6% of cases, and self-expanding metal stents (SEMS) have been used successfully in some cases in which dilation failed [5, 9]. However, the present literature is scant, and there are no guidelines on when to abort one strategy and try another. Surgical treatment options include revision sleeve gastrectomy, seromyotomy, and RYGB [10, 11]. However, the rate of adverse events after these surgical procedures is high, and the success rates are not encouraging [12].
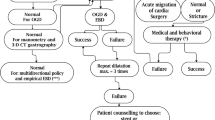
In this retrospective study, we present the results of our standardized approach to treatment in a series of consecutive patients that presented with symptomatic GS post-LSG. We aim to describe the efficacy and safety of endoscopic therapy for GS post-LSG based using a predefined treatment algorithm (Fig. 1).
Material and Methods
This study includes all patients who, over a 13-month period between July 2015 and August 2016, presented to our medical institution for treatment of GS post-LSG. A statewide referral base was developed in July of 2015, following the addition of a gastroenterologist with expertise in management of adverse events post-bariatric surgery (VK) to our hospital. Electronic medical records were searched for all EGD reports associated with the provider VK in the abovementioned time period. EGD reports were reviewed for the keywords “LSG,” “sleeve gastrectomy,” and “stenosis” to identify patients with a history of LSG that underwent EGD for management of gastric stenosis.
Inclusion criteria consisted of primary or secondary LSG followed by symptoms of GS. GS was diagnosed in patients presenting with nausea, vomiting, or dysphagia at any time post-LSG based on evidence of a narrowed segment of the stomach in an upper gastrointestinal swallow study or visual appearance of stenosis during an EGD. Patients having undergone RYGB or duodenal switch, those with achalasia, concomitant gastric leak or fistula, any previous therapy for gastric stenosis apart from through-the-scope balloon dilation, and those lost to follow-up were excluded.
Treatment Algorithm
Our predefined, step-wise treatment algorithm for the endoscopic management of GS post-LSG consisted of serial dilations with an achalasia balloon, followed by long-term placement of a fully covered self-expanding metal stent (FCSEMS) if dilations failed.
Endoscopic dilation was performed under monitored anesthesia care (MAC). All stent deployments and removals were performed under general anesthesia. For balloon dilation, a Savary Guidewire (Cook Medical, Boomington, Indiana) was introduced through the scope into the fourth part of the duodenum. After removing the endoscope, an achalasia balloon (Rigiflex™ II Single-Use Achalasia Balloon Dilators, Boston Scientific, Natick, MA, USA) was inserted over the wire, situated with the middle of the balloon across the stenotic segment, and dilated. Dilation began using a balloon with a diameter of 30 mm and progressed to a balloon with a diameter of 35 mm if needed, at the subsequent endoscopy. The 35-mm balloon was used to a maximum of three times. A 40 mm dilation balloon was not used in our algorithm due to the potential risk of perforation. Dilations were performed at 2-week intervals until either the symptoms resolved or significantly improved as assessed by a single expert provider. The achalasia balloons were dilated under endoscopic guidance to achieve 20 psi pneumatic pressure maintained for 1 min. While the balloon was inflated, relative tissue ischemia at the site of the stenosis confirmed (1) that the stenosis did in fact exist and (2) that the balloon was positioned appropriately.
Dilation was deemed a failure if after four attempts of dilations, there was no significant clinical improvement or, after any attempts, there was no improvement in symptoms. If dilation failed, a Wallflex™ 23 mm × 120 mm esophageal (Boston Scientific, Natick, MA) FCSEMS was placed. The WallFlex™ stent was only kept in situ for a short period, as it quickly became apparent that it resulted in mucosal erosions. It was replaced with an 18 mm × 60 mm Niti-S (Taewoong, Seoul, South Korea) FCSEMS, with a plan to review and exchange it every 6 months. As this stent is much softer and when used previously did not cause significant mucosal injury [13].
The stent was secured in place using an endoscopic suturing system (OverStitch, Apollo Endosurgery, Austin, Texas, USA). In each patient, four 2–0 Prolene (non-absorbable) sutures were used to secure the stent. Sutures were placed in the following pattern: a full thickness bite using the tissue helix through the gastric wall, then a bite through the stent, followed by another full thickness bite using the tissue helix through the gastric wall. The suture was then cinched. In total, four sutures were placed equidistant from one another.
Measured Outcomes
The Patient Assessment of Upper Gastrointestinal Symptoms (PAGI-SYM) questionnaire was developed and validated for the evaluation of symptom severity and treatment responsiveness in upper gastrointestinal conditions, namely Gastroesophageal Reflux Disease (GERD), dyspepsia, and gastroparesis. The 20-item PAGI-SYM has six subscales: heartburn/regurgitation, fullness/early satiety, nausea/vomiting, bloating, upper abdominal pain, and lower abdominal pain [14]. Each item is on a scale of 0–5, with 0 indicating no symptoms and 5 corresponding with the most severe symptoms. Revicki et al. showed that 0.30–0.55 points was the recommended minimally important difference in response to treatment for heartburn/regurgitation scores in patients with GERD. Higher scores demonstrated clear clinical significance [15].
Each patient was asked the 20-item PAGI-SYM questionnaire prior to commencement of treatment and again after his or her final endoscopic intervention. The questionnaire was administered over the phone by the same clinician at both instances. The difference in PAGI-SYM score for the relevant items before and after endoscopic management was calculated.
Technical success was defined by balloon dilation achieving 20 psi pneumatic pressure, maintained for 1 min, or successful deployment and fixation of FCSEMS. Clinical success was assessed by a single provider (VK) and was further defined by at least 1 point reduction in one or more items of PAGI-SYM. Severity of adverse events was defined according to the ASGE Lexicon by Cotton et al. [16].
Statistics
Statistical analysis was performed using SPSS Statistics v19. Pre- and post-treatment PAGI-SYM item scores were compared using paired sample t tests. Statistical significance was defined as p < 0.05.
Results
Patient Characteristics
During the study period, 17 patients (16 females) presented with symptoms of GS, with stenosis confirmed via a barium swallow study and/or an EGD. The mean age was 42.7 ± 12.8 years and the mean BMI was 32.9 ± 7.7 kg/m2. GERD and nausea or vomiting were the most common presenting symptoms (Table 1). Median follow-up after index EGD with dilation was 11 months (range 3–14 months).
Gastric Stenosis
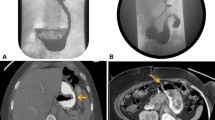
Sixteen patients underwent first-line LSG procedures and one underwent a second-line LSG procedure (gastric banding removal and LSG in the same surgical procedure) before presenting a mean of 17.7 ± 27.7 months after LSG. All stenoses were located at the incisura angularis (Fig. 2).
a Fluoroscopy showing contrast in proximal stomach with stenosis along with kink at incisura angularis. b Endoscope passed through the stenotic segment showing marked kinking. c Savary wire passed under fluoroscopic guidance followed by dilation of achalasia balloon. d After dilation, endoscope is able to pass freely with resolution of stenosis
Balloon Dilation
All patients (n = 17) underwent achalasia balloon dilation with the 30-mm balloon at the index endoscopy by a single endoscopist (VK) (Fig. 3). Dilations were technically successful in all (17/17, 100%) patients. One patient did not experience adequate dilation with the 30-mm balloon (no ischemia at the site of stenosis was noted during the balloon dilation process), and thus, a 35-mm balloon was used in the first procedure for this patient, which was technically successful. Seven patients (41%) underwent dilation with only 30-mm achalasia balloons, while the remainder required a 35 mm balloon. The median number of balloon dilation procedures per patient was 2 (1–4) (Table 2).
a Healed staple line visible to the left leading to the site of stenosis. b Site of gastric stenosis. c Achalasia balloon prior to inflation, passed over the guidewire. d Dilated balloon to 30 mm. e Ischemia at the center of the balloon confirming optimal positioning. f Re-inspection showing improvement in stenosis
One patient suffered a deep tear during the 35 mm achalasia balloon dilation, exposing the muscularis propria (Fig. 4). The tear, which measured 25 mm × 15 mm in the posterior aspect of lesser curvature, appeared to have occurred as the patient coughed, while the balloon was fully inflated. This resulted in a mild ooze of blood, which was self-limited. The patient was subsequently intubated and contrast injection directly over the tear showed no extravasation on fluoroscopy. Post-procedure, the patient was admitted to the surgical service for observation. The patient’s hemoglobin dropped from 9.7 to 5.3 mg/dL, which was attributed to ooze from the site of the tear. Accordingly, two units of packed red blood cells were administered and hemoglobin remained stable at follow-up. A computerized tomography (CT) scan with oral contrast revealed no leak or perforation. The patient was discharged after a four-night hospitalization and subsequently achieved an adequate clinical response. Based on ASGE lexicon, this was a moderate severity adverse event.
Stent Placement and RYGB
Three patients underwent Wallflex™ FCSEMS placement (Fig. 5). One patient developed abdominal pain after stent insertion. The stent was removed after 8 days, and there was a clean based ulcer at site of stent placement. The stenosis was widely patent on stent removal; thus, no further stent was placed. Symptoms resolved and did not recur after stent removal. Of the other two, the dwell time of Wallflex™ stent was 43 and 28 days, respectively. One showed mucosal erosions at site of stent placement and other sowed a clean-based ulcer at stenosis site. They underwent subsequent Niti S-Taewoong FCSEMS placement.
Of the 17 patients treated for GS, two had to undergo RYGB. In these two cases, one patient had very sharp angle (severe) stenosis unresponsive to two 35 mm dilations. The patient did not want to continue further endoscopy therapy. The second patient had a helical shaped stenosis as well as an excessively high Bravo® pH measurement post-dilation despite being on PPI. Thus, they were referred for RYGB before completion of our algorithm. In both, RYGB resulted in excellent resolution of symptoms.
PAGI-SYM Scores
Fifteen (88.2%) out of 17 patients were successfully treated solely with endoscopic management. All 15 patients reported improvement in symptoms. Fourteen patients completed PAGI-SYM questionnaires. The strongest response, based on mean reduction of score ± SD, as seen in Table 3, was in the items of nausea (3 ± 1.9, P < 0.001), heartburn during day (2.8 ± 1.5, P = 0.003), heartburn on lying down (3.4 ± 1.4, P < 0.001), reflux during day (2.8 ± 1.9, P = 0.001), and reflux on lying down (3.0 ± 1.9, P = 0.001) (Table 3). Post-intervention scores were reduced in two or more of the above items in all the patients apart from one, who showed a significant reduction in score for only one item. Further, the patient for whom PAGI-SYM scores were unavailable showed clinical improvement at follow-up.
Discussion
The number of bariatric surgeries performed in the USA alone has increased by more than 20% in the past 4 years, from 158,000 in 2011 to 196,000 in 2015 [17]. Of these surgeries, LSG is becoming an increasingly popular option, in part due to its quick recovery time and lower morbidity than other surgical routes. Moreover, this relatively new procedure has shown satisfactory weight loss and resolution of comorbidities in the short- and medium-term [18].
Nausea, vomiting, dysphagia, reflux, and abdominal pain post-LSG should warrant an investigation for GS post-LSG. Radiological examination, such as a barium swallow study and upper endoscopy, is indicated. GS can sometimes be missed on endoscopy, as there may be easy passage of the endoscope; therefore, contrast injection under fluoroscopic guidance may be helpful in suspected cases. Functional GS develops due to kinking of the incisura angularis, resulting in increased intragastric pressure [19, 20]. Therefore, patients may present with GS days to months after LSG. Initial imaging post-LSG does not predict development of future GS, as initial normal appearance of the sleeve is frequently observed [21].
It is hard to predict which patients are at higher risk of developing GS; however, studies suggest that surgical technique is likely a factor in incidence of this adverse event. From a technical perspective at the time of surgery, it is unclear if the size of the bougie used has an effect on formation of gastric stenosis after LSG [22]. Musella et al. observed that suturing of the staple line by means of a continuous suture increased incidence of GS [23]. Interestingly, a study in which the clinicians used a mechanical vise when applying continuous sutures did not observe any GS at 1 year follow-up [24]. Stenosis may also be a result of formation of a functional valve due to torsion of the sleeve along its long axis [4]. Also of note, the use of consecutive staplers or manual sutures is associated with increased risk of stenosis [25].
Two kinds of balloon dilations have been described in the management of GS. The first is through the scope (TTS) controlled radial expansion (CRE) balloon, and the second is an achalasia balloon, which is wire guided and provides more robust dilation. The success rate of TTS balloons described by Schnell et al. was 44% [26]. Interestingly, Ogra et al. found the success rate of TTS balloons to be 11% but, after switching to a 30-mm achalasia balloon, found the success rate to be 71.4% [9]. Rebibo et al. described an even more impressive success rate of 86.6% for achalasia balloon dilation in GS post-LSG in a series of 16 patients [5]. From our experience, we recommend using the achalasia balloon rather than the CRE balloon in initial therapy due to its superior outcomes and comparable safety profile.
Stenting has been described in some cases when dilation failed to provide adequate clinical response; however, stent migration is a concern [8, 27]. In the three patients who received FCSEMS in our study, there were no adverse effects. We prevented stent migration by suturing the stent to the gastric wall on deployment. We initially used the WallFlex™ stent but found that it was unnecessarily long and appeared to result in tissue hyperplasia and superficial ulceration. Therefore, we interchanged the shorter and more pliable Niti-S (Taewoong, Seoul, South Korea) stent, and it appeared to cause less mucosal injury. Accordingly, we will use the Niti-S stent as the first line of treatment after failed balloon dilation in the future. We posit that it is reasonable to consider FCSEMS after four attempts at dilation with persistent stenosis. However, if there is absolutely no response to 35 mm balloon dilation, then FCSEMS may be warranted earlier on. Failure with FCSEMS, or presence of complicated anatomy, would warrant consideration of surgical treatment.
Despite the mounting evidence supporting the safety of achalasia balloons, clinicians should be cautious so as to avoid perforations and deep mucosal tears, which are possible adverse events [20]. The one patient in our study who suffered a deep tear to the muscularis propria had to be hospitalized for gastrointestinal bleeding before recovering with conservative management. This highlights the need for monitored anesthesia care sedation, as movements, gagging, retching, and coughing can all transiently increase the effective intra-abdominal pressure during balloon inflation. Endoscopic assessment of any defect is crucial when a perforation is suspected, and if the perforation is amenable to endoscopic closure, it should be closed immediately in the same procedure. Upper gastrointestinal radiography with water-soluble contrast (e.g. Gastrografin) should be used to evaluate for a leak. Current endoscopic measures for the management of leaks includes fully covered stents, clips, suturing devices, and tissue sealants [28]. Large defects may need to be treated surgically [29]. Thus, it is beneficial for the provider to work in an environment where fluoroscopic support is available and be comfortable using endoscopic suturing devices to manage deep tears and perforations. A patient developed gastric stenosis at 2 weeks post-LSG. Despite three balloon dilations, the patient ended up getting a RYGB. However, we believe early dilation of gastric stenosis after LSG might be beneficial before more permanent changes such as fibrosis have developed.
Based on clinical response as assessed by a single provider, 15 (88.2%) out of 17 patients showed clinical improvement with endoscopic management. Our study is the first to attempt to objectively measure the benefit of endoscopic management of GS using the PAGI-SYM questionnaire, as well as to suggest an integrated approach of serial dilations using achalasia balloons with FCSEMS as second-line therapy. This study validates a predefined clinical algorithm for the management of GS post-LSG. The algorithm results in excellent clinical outcomes with a suitable safety profile. A sharp angle of stenosis (severe stenosis) or the stenosis being of helical shape is a poor prognostic marker of response to endoscopic treatment.
We propose that all patients with symptomatic gastric stenosis should be managed initially with achalasia balloon dilation at 2-week intervals for up to four dilations. If serial dilations fail to resolve the stenosis, FCSEMS should be attempted. The long-term placement of FCSEMS is novel, and further study is required to confirm it as a long-term therapeutic option. At this stage, it is unclear if recurrent stenting will need to be performed every 6 months or so. Even if this is the case, it may be a better option than revision surgery for the selected patient. If there is no initial response to FCSEMS, then surgical laparoscopic seromyotomy or conversion to RYGB would be the next step in management [30, 31].
References
Silecchia G, Boru C, Pecchia A, et al. Effectiveness of laparoscopic sleeve gastrectomy (first stage of biliopancreatic diversion with duodenal switch) on co-morbidities in super-obese high-risk patients. Obes Surg. 2006;16(9):1138–44.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Moszkowicz D, Arienzo R, Khettab I, et al. Sleeve gastrectomy severe complications: is it always a reasonable surgical option? Obes Surg. 2013;23(5):676–86.
Parikh A, Alley JB, Peterson RM, et al. Management options for symptomatic stenosis after laparoscopic vertical sleeve gastrectomy in the morbidly obese. Surg Endosc. 2012;26(3):738–46.
Rebibo L, Hakim S, Dhahri A, et al. Gastric stenosis after laparoscopic sleeve gastrectomy: diagnosis and management. Obes Surg. 2016;26(5):995–1001.
Dapri G, Cadière GB, Himpens J. Laparoscopic seromyotomy for long stenosis after sleeve gastrectomy with or without duodenal switch. Obes Surg. 2009;19(4):495–9.
Burgos AM, Csendes A, Braghetto I. Gastric stenosis after laparoscopic sleeve gastrectomy in morbidly obese patients. Obes Surg. 2013;23(9):1481–6.
Eubanks S, Edwards CA, Fearing NM, et al. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206(5):935–8. discussion 938–9
Ogra R, Kini GP. Evolving endoscopic management options for symptomatic stenosis post-laparoscopic sleeve gastrectomy for morbid obesity: experience at a large bariatric surgery unit in New Zealand. Obes Surg. 2015;25(2):242–8.
Bellorin O, Lieb J, Szomstein S, et al. Laparoscopic conversion of sleeve gastrectomy to Roux-en-Y gastric bypass for acute gastric outlet obstruction after laparoscopic sleeve gastrectomy for morbid obesity. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2010;6(5):566–8.
Lacy A, Ibarzabal A, Obarzabal A, et al. Revisional surgery after sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2010;20(5):351–6.
Chouillard EK, Karaa A, Elkhoury M, et al. Intercontinental Society of Natural Orifice, endoscopic, and laparoscopic surgery (i-NOELS). Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for morbid obesity: case-control study. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2011;7(4):500–5.
Khashab MA, Besharati S, Ngamruengphong S, et al. Refractory gastroparesis can be successfully managed with endoscopic transpyloric stent placement and fixation (with video). Gastrointest Endosc. 2015;82(6):1106–9.
Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2004;13(10):1737–49.
Revicki DA, Rentz AM, Tack J, et al. Responsiveness and interpretation of a symptom severity index specific to upper gastrointestinal disorders. Clin Gastroenterol Hepatol. 2004;2(9):769–77.
Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71(3):446–54.
Estimate of Bariatric Surgery Numbers, 2011-2015 [Internet]. American Society for Metabolic and Bariatric Surgery. [cited 2016]. Available from: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers
Srinivasa S, Hill LS, Sammour T, et al. Early and mid-term outcomes of single-stage laparoscopic sleeve gastrectomy. Obes Surg. 2010;20(11):1484–90.
Zundel N, Hernandez JD, Galvao Neto M, et al. Strictures after laparoscopic sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2010;20(3):154–8.
Baretta G, Campos J, Correia S, et al. Bariatric postoperative fistula: a life-saving endoscopic procedure. Surg Endosc. 2015;29(7):1714–20.
Werquin C, Caudron J, Mezghani J, et al. Early imaging features after sleeve gastrectomy. J Radiol. 2008;89(11 Pt 1):1721–8.
Keidar A, Appelbaum L, Schweiger C, et al. Dilated upper sleeve can be associated with severe postoperative gastroesophageal dysmotility and reflux. Obes Surg. 2010;20(2):140–7.
Musella M, Milone M, Bellini M, et al. Laparoscopic sleeve gastrectomy. Do we need to oversew the staple line? Ann Ital Chir. 2011;82(4):273–7.
Paluszkiewicz R, Kalinowski P, Wróblewski T, et al. Prospective randomized clinical trial of laparoscopic sleeve gastrectomy versus open Roux-en-Y gastric bypass for the management of patients with morbid obesity. Wideochirurgia Inne Tech Maloinwazyjne Videosurgery Miniinvasive Tech. 2012;7(4):225–32.
Binda A, Jaworski P, Tarnowski W. Stenosis after sleeve gastrectomy--cause, diagnosis and management strategy. Pol Przegl Chir. 2013;85(12):730–6.
Shnell M, Fishman S, Eldar S, et al. Balloon dilatation for symptomatic gastric sleeve stricture. Gastrointest Endosc. 2014;79(3):521–4.
Jones M, Healey AJ, Efthimiou E. Early use of self-expanding metallic stents to relieve sleeve gastrectomy stenosis after intragastric balloon removal. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2011;7(5):e16–7.
Willingham FF, Buscaglia JM. Endoscopic Management of Gastrointestinal Leaks and Fistulae. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2015;13(10):1714–21.
Kumar N, Thompson CC. Endoscopic therapy for postoperative leaks and fistulae. Gastrointest Endosc Clin N Am. 2013;23(1):123–36.
Vilallonga R, Himpens J, van de Vrande S. Laparoscopic management of persistent strictures after laparoscopic sleeve gastrectomy. Obes Surg. 2013;23(10):1655–61.
Rosenthal RJ, International Sleeve Gastrectomy Expert Panel, Diaz AA, et al. International sleeve gastrectomy expert panel consensus statement: best practice guidelines based on experience of >12, 000 cases. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2012;8(1):8–19.
Author information
Authors and Affiliations
Contributions
The authors were involved in the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Manoel Galvao Neto is a consultant for Apollo Endosurgery, consultant and SAB member of GI Dynamics, consultant and SAB member of Fractyl Labs, consultant of GI Windows, consultant and SAB member of Ethicon EndoSurgery.
Mouen A Khashab is a consultant for Boston Scientific and Olympus America.
Vivek Kumbhari is a consultant for Boston Scientific and Apollo Endosurgery.
All other authors have no conflicts of interest to declare.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Funding Sources or Institutional or Corporate Affiliations
None
Electronic supplementary material
Initial endoscope being advanced through the gastro-esophageal junction with healed staple line visible to the left leading to the site of stenosis (0:10). Endoscope passed through stenotic segment and into the fourth part of duodenum. Savary Guidewire (Cook Medical, Boomington, Indiana) being introduced through the scope into the fourth part of the duodenum (0:30). Inspecting the deflated achalasia balloon (0:50). Achalasia balloon (Rigiflex™ II Single-Use Achalasia Balloon Dilators, Boston Scientific, Natick, MA, USA) being inserted over the wire, situated with the middle of the balloon across the stenotic segment (1:06). Balloon inflated to 20 psi pneumatic pressure which is maintained for 1 min. There is visible relative tissue ischemia at the site of the stenosis (1:24). Balloon deflated and withdrawn followed by re-inspection of stenosis segment (2:00). (MP4 232,436 kb)
Rights and permissions
About this article
Cite this article
Agnihotri, A., Barola, S., Hill, C. et al. An Algorithmic Approach to the Management of Gastric Stenosis Following Laparoscopic Sleeve Gastrectomy. OBES SURG 27, 2628–2636 (2017). https://doi.org/10.1007/s11695-017-2689-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2689-3