Abstract
Background
This study evaluated the early postoperative benefits of laparoscopic sleeve gastrectomy (LSG) on the left ventricular (LV) function and left atrial (LA) structural, mechanical, and electrical functions in severely obese patients.
Methods
Thirty-two patients with severe obesity who were consecutively scheduled for LSG and 30 healthy controls were included in the study. LV global longitudinal strain (LGS), peak atrial longitudinal strain (PALS), and strain rates (S-Sr, E-Sr, and A-Sr) of the lateral and septal LA walls, and intra- and interatrial dyssynchrony periods for all subjects were evaluated using strain echocardiography. The measurements were repeated in patients 1 month after surgery.
Results
LGS of the LV was significantly depressed in the patient group compared with the control group (p < 0.001). LA peak septal and lateral wall strain values were significantly lower in patients than in controls (both p values <0.001). LA intra- and interatrial dyssynchrony periods were longer in patients than in controls (p = 0.012 and p = 0.004, respectively). LGS significantly improved after LSG (p < 0.001). Significant reductions were noted in the LA antero-posterior diameter (p < 0.001), LA volume index (LAVI, p = 0.001), and in the mitral velocity to the early diastolic velocity of the mitral annulus ratio (E/e’ ratio, p = 0.046). The PALS of the septal and lateral LA walls significantly increased (p = 0.001 and p < 0.001, respectively). S-Sr, E-Sr, and A-Sr values of the septal LA wall (p = 0.049, p < 0.001, and p = 0.001, respectively) and the lateral LA wall (p = 0.009, p = 0.007, and p = 0.002, respectively) significantly improved postoperatively. Intra- and interatrial dyssynchrony significantly decreased (p = 0.001 and p < 0.001, respectively). Weight loss positively correlated with changes in LGS (R = 0.39, p = 0.039), LAVI (R = 0.39, p = 0.034), intra-atrial dyssynchrony (R = 0.45, p = 0.021), interatrial dyssynchrony (R = 0.42, p = 0.038), septal LA wall peak strain (R = 0.44, p = 0.027), lateral LA wall peak strain (R = 0.46, p = 0.017), septal LA wall A-Sr (R = 0.43, p = 0.028), and lateral LA wall A-Sr (R = 0.46, p = 0.019). The comparison of postoperative findings of the patients with controls revealed that the LA diameter, both LA volume and volume index (LAVI), E/e’ ratio, S-Sr and E-Sr of both lateral and septal LA walls, intra- and interatrial LA dyssynchrony of the patient group became similar to the control group (all p value >0.05). Postoperative A-Sr values of both LA walls (both p value <0.001) were higher in patients than controls.
Conclusion
The benefits of LSG on LV and LA function may be observed even in the early postoperative phase. The resulting weight loss correlates with LV and LA reverse remodeling in severely obese patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obesity is an increasingly prevalent health care problem that is associated with high mortality and morbidity rates [1, 2]. It is an independent risk factor for hypertension (HT), myocardial ischemic disease, and heart failure [2, 3]. Obesity was also shown to prompt atrial fibrillation (AF) development [4]. Ventricular and atrial functions are both affected by increased body weight [5–7], and the adverse effects on cardiac function contribute to the devastating consequences of obesity [8, 9]. Weight loss strategies include physical activity, diet, and pharmacologic treatment. However, when these options fail, laparoscopic sleeve gastrectomy (LSG) serves as an effective option for weight loss [10]. This treatment strategy reduces mortality and comorbidities associated with obesity while improving the quality of life [11].
Evaluation of left atrial (LA) function is important because deterioration in atrial function is related to impaired functional capacity in various cardiac disorders [12, 13] and AF development [14, 15]. New ultrasound technology provides better assessment of LA function. LA mechanical function has three phases during the cardiac cycle: reservoir, conduit, and contractile. First, the LA behaves as a “reservoir,” collecting blood from the pulmonary veins. Second, when the mitral valve opens, it then functions as a “conduit” to transfer blood into the left ventricle (LV) via a pressure gradient. Third, the “contractile” function augments the LV stroke volume by approximately 20–30 % [16–19]. LA strain and strain rate, which are measures of LA deformation and the proportion of deformation, respectively, can be calculated using tissue Doppler strain imaging [20]. These indices provide vital information about LA mechanical function [21].
Electrical conduction in a normal heart begins in the sinus node, which is located high in the right atrium (RA), near the superior vena cava. From there, electrical activation spreads throughout the low RA and atrioventricular junction and to different regions of the LA. The time required to excite another region within the same atrium is termed intra-atrial conduction delay, which could apply to both the RA and the LA (RA dyssynchrony or LA dyssynchrony). The time required for a signal starting in the RA to pass through different conduction tracts and reach the lateral wall of LA is termed the interatrial conduction delay or dyssynchrony. Intra- and interatrial conduction characteristics can be assessed non-invasively using color tissue Doppler imaging. Atrial dyssynchrony periods become prolonged as LA size increases [22], leading to the development of atrial arrhythmias [23, 24]. Obese patients exhibit prolonged intra- and interatrial dyssynchrony [7]; however, the effect that weight loss has on atrial conduction has not been examined.
This study investigated the early effects of LSG on LV function and LA structural, mechanical, and electrical functions in severly obese patients.
Materials and Methods
Study Population
Patients were weighed wearing light clothing without shoes using a G-TECH®(capacity up to 200 kg) scale with an accuracy of 50 g. Height was measured using a stadiometer with an accuracy of 0.1 cm. Body mass index (BMI) was calculated using the conventional formula of weight in kilograms divided by the square of height in meters. Blood pressure was measured using an appropriate-sized cuff after at least a 30-min interval free from caffeine or cigarette consumption. Two blood pressure measurements separated by a 5-min interval were obtained, and the mean value of the two measurements was recorded. A detailed medical history was obtained from all participants. Patients were questioned for the presence of the following symptoms to rule out obstructive sleep apnea syndrome (OSAS): nocturnal choking or gasping, morning headache, apnea, excessive daytime sleepiness, and snoring. First-degree relatives and/or home mates of the severely obese patients were also questioned as to whether any had witnessed the patient having an apneic episode while sleeping. An oropharyngeal examination was performed on all patients. Patients with a previous diagnosis of OSAS, any symptoms consistent with OSAS, or OSAS signs identified on oropharyngeal examination were excluded.
Sixty severely obese patients with a BMI >40 kg/m2 who were consecutively scheduled for LSG in Karşıyaka State Hospital were prospectively evaluated. Those with diabetes mellitus (n = 4), HT (n = 7), history of coronary artery disease (CAD) (≥50 % stenosis of any major epicardial vessel; n = 2), OSAS (n = 3), or those with poor image quality rejected by the software (n = 12) were excluded. A detailed medical history was obtained and all patients underwent treadmill electrocardiography to rule out CAD. Ultimately, 32 patients with severe obesity (7 men, 25 women) and a normal sinus rhythm without bundle branch block at the time of enrollment were included in the study. The percentage of weight loss at 1 month following the surgery was calculated by dividing the difference in body weight between the weight at the first echocardiographic study (prior to surgery) and the weight 1 month postoperatively by the initial body weight multiplied by 100 % [25]. The baseline findings in the patient group were compared with healthy controls. The control group was formed by the subjects having all the following criteria: (1) aged between 18 and 50 years; (2) body mass index between 18.5 and 24.99 kg/m2; (3) presence of a normal electrocardiogram; (4) presence of a normal echocardiogram; (5) absence of a history and/or physical examination findings of any systemic, endocrinologic, or pulmonary disorder that could affect cardiac functions; (6) detection of a normal coronary angiogram within 6 months before enrollment in those aged ≥40 years; and (7) absence of signs or symptoms of OSAS with a normal oropharyngeal examination. The study was approved by the institutional review board of Katip Celebi University. Informed consent was obtained from all subjects.
Echocardiographic Study
All patients and controls underwent two-dimensional (2D) transthoracic echocardiographic (TTE) evaluation, tissue Doppler, tissue strain, and 2D strain imaging prior to and 1 month following LSG. A commercially available ultrasound machine, Vivid S5 (GE-Vingmed Ultrasound AS, Horten, Norway), equipped with a 2.5–3.5 MHz transducer, was used for all echocardiographic examinations. Standard 2D echocardiography was performed according to the American Society of Echocardiography/European Association of Echocardiography recommendations [26]. The LV ejection fraction (LVEF) was measured using the biplane Simpson method, and the LA diameter was measured according to guideline criteria [26]. Echocardiographic cine loops were obtained by recording three consecutive heart cycles. All studies were performed by a single experienced echocardiographer. Offline analyses were performed by two experienced echocardiographers blinded to all other patient data and using a dedicated software package (Echo PAC PC; GE Healthcare, Waukesha, WI, USA).
2D Strain Echocardiography and Tissue Doppler Strain Imaging
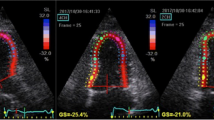
To measure global LV strain parameters, digital cineloops were acquired at end-expiration at 50–90 frames/s from the peak of the R-wave and stored in optical disks for offline analysis. The software tracked endocardial contour on an end-diastolic frame, automatically generating a region of interest divided into segments. The quality of myocardial tracking was visually assessed. The process was repeated by adjusting the region of interest or manually correcting the contour to ensure optimal tracking in instances of unsatisfactory tracking. Graphics of deformation parameters for each segment were automatically produced. LV global longitudinal strain (LGS) consists of the segmental value average obtained from apical four-chamber, apical two-chamber, and apical three-chamber views.
Tissue Doppler Strain Imaging
Color tissue Doppler strain images assessing LA function were obtained from the apical four-chamber view and recorded at >110 frames/s. The narrowest image sector angle (usually 30°) was used for the maximum color Doppler frame rate, and the atrial wall was aligned parallel to the Doppler beam. The sample volume was placed in the basal segments of the septal and lateral LA walls in the apical four-chamber view. The peak systolic strain of the lateral and septal LA walls during late LV systole was measured during the conduit phase corresponding to LA diastasis (Fig. 1a) [27]. The strain rate (Sr) of the LA septal and lateral walls was measured in LV systole (S-Sr), early diastole (E-Sr), as well as late LV diastole (A-Sr). S-Sr was considered an index of LA reservoir function, while E-SR and A-SR were indices of LA conduit function and LA contractile function, respectively (Fig. 1b) [28]. Three cine loops were recorded for each patient and saved digitally on an optical disk for later analysis. Offline measurements were performed using a dedicated software package (Echo PAC PC; GE Healthcare, Waukesha, WI, USA). All echocardiographic measurements were calculated as averages from three beats.
Two examples of tissue Doppler imaging recordings. a Left atrial (LA) strain curve recorded within the LA wall. The peak systolic strain (Peak S) was measured. b LA strain rate (SR) curve recorded within the lateral LA wall. SR was measured during left ventricular (LV) systole (S-SR), early diastole (E-SR), and late LV diastole (A-SR)
Atrial Dyssynchrony
Time-velocity curves were measured below the tricuspid and mitral annulus on the right atrial (RA) free wall, the interatrial septum (IAS), and the LA lateral wall (Fig. 2) using digitally stored color tissue Doppler images. The A wave on the color tissue Doppler curve was defined as the second negative deflection. The time difference from the onset of the P wave to the peak of the A wave was measured at the right atrium (P-RA), the septal wall (P-SA), and the lateral LA wall (P-LA). Intra-atrial dyssynchrony was the difference between the P-LA and P-SA (LA dyssynchrony). Interatrial dyssynchrony was defined as the difference between the P-LA and P-RA.
An example of LA dyssynchrony assessed by color tissue Doppler imaging of the atria. Regional myocardial velocity curves were obtained at the right atrium (RA) (red), the interatrial septum (IAS) (green), and the LA (yellow) just below the level of the tricuspid and mitral annulus. Arrows the A wave peak
Surgical Intervention
The operation was performed with the patient supine, with open legs in the reverse Trendelenburg position (inclined) on an operating table with a 30° angle. To prevent falls and poor positioning, patients were attached to the operating table with special braces on the abdomen and lower limbs. The abdomen was insufflated with CO2 to achieve a 14 mmHg pneumoperitoneum. A LigaSure device (Covidien, Dublin, Ireland) was used to dissect the greater curvature of the stomach, starting 5 cm from the pylorus. A 34-F bougie was introduced and placed along the lesser curvature. Gastric resection was performed with the Endo GIA Universal Stapler (Covidien) device using green and blue loads, directed toward the angle of His. The resected side of the stomach was ligated with multiple hemoclips. A leakage test was performed with methylene blue. The resected stomach was extracted by extending the 12-mm trocar incision into the supraumbilical midline by 2–3 cm. No intra- or postoperative complications or death were recorded.
Reproducibility
Observer reliability for LA strain and strain rate (SR) was assessed in 25 patients using post-HD data sets, and were averaged from three lateral LA wall measurements. These data sets were analyzed for interobserver variability by two echocardiographers (KT and SYT). Intraobserver measurements were randomly performed at least 10 days apart by a single echocardiographer (KT).
Statistical Analyses
All analyses were performed using SPSS for Windows, version 15.0 (SPSS Inc., Chicago, IL). Results are presented as the means ± standard deviations for continuous data or as numbers with percentages for categorical data. Normality tests were performed to determine the distribution of continuous variables. Student’s t test was used to determine the significance of the difference between severely obese patients and controls for normally distributed continuous variables. Otherwise, the Mann-Whitney U test was used. To compare postoperative findings of patients with controls, Student’s t test was performed. Categorical data and proportions were analyzed using the X 2 test. A paired t test was performed to compare preoperative and postoperative findings of the patients. The associations between percentage of weight loss and changes in LGS, LA strain, and strain rate indices were performed using Pearson correlations or the Spearman correlation test, as appropriate. Reliability was tested using the intra-class correlation coefficient (ICC) by Cronbach’s α to determine both the intra- and interobserver variability. Bland-Altman analyses were also performed [29]. Two-sided p values <0.05 were considered significant. The sample size of the control group and the patient group was checked using a retrospective power analysis. As such, we estimated that the sample size needed in this study (power of 80 %, alpha = 0.05) is 17 individuals in both two groups. The retrospective power of the study was 0.994.
Results
The mean patient age was 33.6 ± 8.6 years (range, 17–49 years), and 21.9 % were males. The comparison of baseline characteristics before and after LSG is presented in Table 1. Body weight significantly decreased, with the mean BMI changing from 43.95 ± 3.75 to 38.92 ± 3.56 kg/m2 (p < 0.001). There was an insignificant decrease in systolic and diastolic blood pressure 1 month following surgery. The comparison of baseline and echocardiographic characteristics between patients and controls is displayed in Table 2. No differences were found with respect to age or gender between the two groups. Both preoperative LA diameter and LA volume were larger in the patient group (both p value <0.001). Preoperative LVEF value was significantly lower in patients compared with controls, although both groups were within the normal range (p < 0.001). Preoperative LGS of the LV was significantly depressed in the patient group compared with the control group (p < 0.001). LA peak septal and lateral wall strain values were significantly lower in patients than in controls (both p value <0.001) before the operation. Preoperative LA intra- and interatrial dyssynchrony periods were longer in patients than in controls (p = 0.012 and p = 0.004, respectively). Comparison of postoperative baseline and echocardiographic findings of patients with controls is also shown in Table 2. Body surface area (p < 0.001) and body mass index (p < 0.001) were still larger in patients than in controls. LVEF (p = 0.01), LGS of the LV (p < 0.001), lateral LA wall peak strain (p = 0.09) values were lower in patients than in controls. The antero-posterior LA diameter, both LA volume and volume index (LAVI), E/e’ ratio, S-Sr and E-Sr of both lateral and septal LA walls, intra- and interatrial LA dyssynchrony of the patient group became similar to the control group. Postoperative A-Sr values of both LA walls (both p value <0.001) became significantly higher in patients compared to control subjects. Table 3 demonstrates the changes in echocardiographic variables before and after the operation. There was a slight increase in LVEF which remained insignificant (p = 0.21); however, LGS significantly improved from −14.46 ± 3.15 to −15.91 ± 2.75 % (p < 0.001). A significant reduction was detected in the antero-posterior LA diameter (p < 0.001), LAVI (p = 0.001), and E/e’ ratio (p = 0.046). The peak strain of the septal and lateral LA walls significantly increased (19.39 ± 5.06 to 23.64 ± 3.66, p = 0.001 and 18.52 ± 4.76 to 22.85 ± 5.04, p < 0.001, respectively). The S-Sr, E-Sr, and A-Sr from the LA septal wall (p = 0.049, p < 0.001, and p = 0.001, respectively) and lateral LA wall (p = 0.009, p = 0.007, and p = 0.002, respectively) significantly improved after LSG. Intra-atrial dyssynchrony decreased from 21.11 ± 8.91 to 13.46 ± 7.45 ms (p = 0.001), while interatrial dyssynchrony decreased from 28.52 ± 18.1 to 17.31 ± 16.13 ms (p < 0.001) (Table 3 and Fig. 3). The correlation analysis is presented in Figs. 4 and 5. The percentage of weight loss was positively correlated with changes in LGS (R = 0.39, p = 0.039), LAVI (R = 0.39, p = 0.034), intra-atrial dyssynchrony (R = 0.45, p = 0.021), interatrial dyssynchrony (R = 0.42, p = 0.038), LA septal wall peak strain (R = 0.44, p = 0.027), lateral LA wall peak strain (R = 0.46, p = 0.017), LA septal wall A-Sr (R = 0.43, p = 0.028), and lateral LA wall A-Sr (R = 0.46, p = 0.019).
Bar graphs demonstrate the effect of laparoscopic sleeve gastrectomy on echocardiographic parameters. Significant changes in all parameters were observed postoperatively. Pre preoperative, Post postoperative, LGS LV global strain, LAVI LA volume index, SASr septal left atrial wall A-SR, LASr left atrial lateral wall A-SR, intraAD: intra-atrial dyssynchrony, interAD interatrial dyssynchrony
The intra-class correlation coefficients (ICCs) of peak strain and SR of the lateral LA wall for the intra- and interobserver measurements are shown in Table 4. All measurements showed excellent or good agreement. Bland-Altman plots are presented in Fig. 6.
Discussion
The present study showed that LA structural, mechanical and electrical functions as well as LV functions were depressed in severely obese patients without common comorbidities (HT, diabetes mellitus, CAD and OSAS, etc.) compared to controls, suggesting that “severe obesity,” itself, is a disease affecting cardiac functions. We also evaluated postoperative LA and LV functional changes of the patients to determine the benefit of surgical weight loss. A significant improvement was observed in LV functions of patients 1 month postoperatively. LA volume significantly decreased, and LA mechanical functions, including all reservoir, conduit, and contractile functions, significantly improved after 1 month. Moreover, intra- and interatrial dyssynchrony significantly decreased. The improvements in LA structural, mechanical, and electrical functions, as well as LV function, were related to the percentage of weight loss. The comparison of postoperative echocardiographic findings of the patients with healthy controls displayed that the preoperative significant differences between two groups in the antero-posterior LA diameter, both LA volume and LAVI, E/e’ ratio, S-Sr and E-Sr of both lateral and septal LA walls disappeared. Moreover, atrial contractility (A-Sr) increased to an amount that it became significantly higher in patients than in controls following the first month of the surgery, displaying that LSG has impressive benefits on atrial functions.
In a study examining LV function in 92 severely obese patients with controlled HT or diabetes but without evidence of cardiovascular disease, patients had lower indices of LV systolic and diastolic function. This suggests that these patients had subclinical LV dysfunction despite having normal LVEF values [30]. In another study investigating the effect of LSG on cardiac function, the LV mass and arterial elasticity of 34 obese patients were evaluated 0, 6, and 12 months after the procedure and significant improvements in aortic elasticity and LV diastolic function were noted at 6 months and were maintained 12 months postoperatively. The LV hypertrophy also favorably evolved. The patients in this study had diabetes mellitus and HT, and, during follow-up, the blood pressure and glucose values also markedly improved [5]. Comorbidities that might accompany obesity such as DM, HT, CAD, and OSAS were previously shown to affect atrial conduction properties and impair atrial function even in non-obese patients [31–36]. The current study evaluated 60 severely obese patients, but 16 were excluded for HT, diabetes mellitus, CAD, and OSAS, so as to evaluate the sole effect of surgical weight loss on cardiac function using new echocardiographic techniques. We first compared baseline echocardiographic indices between the patients and healthy controls and found that LV function was depressed in patients, which may indicate that excess weight gain decreases cardiac function. Our findings are novel in that they show that surgical treatment of severe obesity improves LV function in patients without comorbidities, and that the positive effects of weight loss on cardiac function are evident in the early postoperative phase.
Recent findings support obesity as a risk factor for AF development [37]. It was reported in a meta-analysis that obese individuals have a 49 % greater risk of AF [4]. Moreover, the risk of AF increased with increasing BMI [4]. Increased LV mass, increased LA volume, and impaired LV diastolic function contribute to the development of AF in obese patients [38]. LA enlargement is an independent risk factor for AF in the general population [39, 40]. Obese patients have a greater LA diameter and volume even when they are in sinus rhythm [38]. In the current study, baseline LA volume and diameter were larger in patients than in controls, which is similar to findings of previous reports [38]. In the severely obese patients, LA reverse remodeling, corresponding to a decreased LA diameter and LAVI, was observed 1 month after surgery. The postoperative reverse structural remodeling effect of LSG was so prominent that the LA diameter and volume in patients became similar to those of controls. LA volume reflects an average of LV filling pressures over time, making it a good indicator of LV diastolic function [41]. The E/e’ ratio, which non-invasively estimates the LV filling pressure, also decreased after LSG. The decrease in LA diameter and LAVI in the current study population may be related to the decreased E/e’ ratio. The similar E/e’ ratios in patients during the first month of postoperative phase and controls also support this finding.
LA phasic function provides important information about atrial remodeling in various cardiac conditions [12, 42, 43], which may account for its value in predicting AF [14, 44]. However, the predictive value of LA function in AF development has not yet been determined in obese patients. In a study investigating LA function in 35 obese patients (BMI ≥ 30 kg/m2), conventional 2D echocardiography demonstrated that these patients had an increased LA active emptying fraction but a decreased passive emptying fraction [45]. The current study showed improvements in all LA phasic functions 1 month after surgery. The improvement in LA function might be due to the decreased LV filling pressure, which may also contribute to reverse atrial structural remodeling. One interesting finding of the study that should be also emphasized is that postoperative atrial contractility of the patients represented as A-Sr even exceeded the average value of the control group. It is probable that decrease in LA volume and LV filling pressure contributed to this change.
Atrial electrical remodeling plays a role in the initiation and maintenance of AF. Prolonged interatrial dyssynchrony contributed to chronicity in AF patients with non-valvular paroxysmal AF [23]. Progressive conduction abnormalities with slowing atrial conduction and increased conduction heterogeneity were shown in an animal obesity model [46]. A recent study showed that epicardial fat infiltration of the LA wall served as an AF substrate in an obese ovine model [47]. Prolongation of intra- and interatrial dyssynchrony has been demonstrated in obese patients, but the influence of surgical weight loss on atrial electrical properties has not been previously investigated [7, 45]. This study evaluated atrial electrical properties non-invasively using echocardiography and found that both intra- and interatrial dyssynchrony periods were prolonged in severely obese patients compared with controls, and there was a significant decline in both measures 1 month after LSG in the patients. Gaborit et al. demonstrated a significant reduction of epicardial fat volume in obese patients 6 months after bariatric surgery [25]. Atrial electrical reverse remodeling in patients 1 month after LSG may be a result of a reduction in epicardial fat. In several conditions, atrial dyssynchrony was related to LA structural remodeling [22]. A decrease in LA volume might also contribute to reverse atrial electrical remodeling. This hypothesis was also supported by our finding that both structural and electrical properties of patients were similar to those of controls at the end of the first month after the surgery. It may be concluded that LSG decreases atrial arrhythmogenesis in obese patients by reducing epicardial fat, decreasing LA volume and diameter, and improving diastolic function.
In a study investigating the long-term effect of weight loss on rhythm control in obese patients with AF, those with ≥10 % weight loss had a sixfold greater probability of arrhythmia-free survival compared to those with <10 % weight loss [48]. Interestingly, >5 % weight fluctuation in that cohort partially offset this benefit with a twofold increased risk of arrhythmia recurrence [44]. When cardiac function was evaluated in these patients, LAVI and E/e’ decreased during follow-up, which might have contributed to AF-free survival. In the current study, LA reverse remodeling was observed after LSG, and improvements in all LA structural, mechanical, and electrical indices were related to the percentage of weight loss.
Limitations
This study had several limitations, including its small sample size. Although 60 severely obese patients were screened, only 32 were enrolled so as to determine the effect of LSG weight loss on cardiac function without common comorbidities that could also affect cardiac parameters. These results need to be verified by further studies with a larger number of patients. Secondly, the presence of OSAS was not excluded using polysomnography. However, we carefully questioned patients and their relatives and/or home mates for the presence of symptoms consistent with OSAS, and performed routine oropharyngeal examination on all subjects. Third, although we intended to investigate whether surgical benefits are observed short-term, a longer follow-up period would provide more valuable information on the impact that surgical weight loss has on cardiac function. The presence of CAD was ruled out using non-invasive tests. Performing coronary angiography (CAG) would more accurately exclude CAD, but these patients were relatively young and did not have common comorbidities that would have contributed to CAD development. Moreover, many patients would not agree to routine CAG prior to surgery.
Conclusion
These findings suggest that LSG improves LA and LV function in obese patients who do not have comorbidities commonly accompanying obesity in the early postoperative phase. LA structural, mechanical, and electrical reverse remodeling was related to the percentage of weight loss 1 month following the procedure.
References
Finnucane MM, Stevens GA, Cowan MJ, et al. Global Burden of Metabolic Risk Factors of Chronic Disease Collaborating Group (body mass index): national, regional, and global trends in body-mass index since 1980—systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–67.
Contaldo F, Pasanisi F, Finelli C, de Simone G. Obesity, heart failure and sudden death. Nutr Metab Cardiovasc Dis. 2002;12(4):190–7.
Corry DB, Tuck AM. Obesity, hypertension and sympathetic nervous system activity. Curr Hypertens Rep. 1998;1:119–26.
Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of a metaanalysis. Am Heart J. 2008;155(2):310–5.
Iancu ME, Copăescu C, Şerban M, Ginghină C. Favorable changes in arterial elasticity, left ventricular mass, and diastolic function after significant weight loss following laparoscopic sleeve gastrectomy in obese individuals. Obes Surg. 2014;24(3):364–70.
Aggarwal R, Harling L, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. The effects of bariatric surgery on cardiac structure and function: a systematic review of cardiac imaging outcomes. Obes Surg. 2016;26(5):1030–40.
Yagmur J, Cansel M, Acikgoz N, Ermis N, Yagmur M, Atas H, Tasolar H, Karakus Y, Pekdemir H, Ozdemir R. Assessment of atrial electromechanical delay by tissue Doppler echocardiography in obese subjects. Obesity (Silver Spring). 2011;19(4):779–83.
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association scientific statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918.
Schunkert H. Obesity and target organ damage: the heart. Int J Obes Relat Metab Disord. 2002;26(4):15–20.
Borisenko O, Colpan Z, Dillemans B, Funch-Jensen P, Hedenbro J, Ahmed AR. Clinical indications, utilization, and funding of bariatric surgery in Europe. Obes Surg. 2015;25(8):1408–16.
Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, Ahlin S, Anveden Å, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lönroth H, Narbro K, Näslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65.
Tülüce K, Yakar Tülüce S, Yavuzgil O, İsayev E, Bilgin M, Akyıldız Akçay F, Nalbantgil S, Özerkan F. The left atrial phasic functions and the relationship with plasma N-terminal pro-B-type natriuretic peptide levels and symptomatic states in patients with hypertrophic cardiomyopathy. Anadolu Kardiyol Derg. 2014;14(8):719–27.
Van Beeumen K, Duytschaever M, Tavernier R, Van de Veire N, De Sutter J. Intra- and interatrial asynchrony in patients with heart failure. Am J Cardiol. 2007;99(1):79–83.
Tuluce K, Yakar Tuluce S, Kahya Eren N, Kocabas U, Akyildiz Akcay F, Gunduz R, Akyildiz ZI, Ergene O. Predictors of future atrial fibrillation development in patients with hypertrophic cardiomyopathy: a prospective follow-up study. Echocardiography. 2016;33(3):379–85.
Cho GY, Jo SH, Kim MK, Kim HS, Park WJ, Choi YJ, Hong KS, Oh DJ, Rhim CY. Left atrial dyssynchrony assessed by strain imaging in predicting future development of atrial fibrillation in patients with heart failure. Int J Cardiol. 2009;134(3):336–41.
Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47(12):2357–63.
Matsuda Y, Toma Y, Ogawa H, Matsuzaki M, Katayama K, Fujii T, Yoshino F, Moritani K, Kumada T, Kusukawa R. Importance of left atrial function in patients with myocardial infarction. Circulation. 1983;67(3):566–71.
Mitchell JH, Shapiro W. Atrial function and the hemodynamic consequences of atrial fibrillation in man. Am J Cardiol. 1969;23(4):556–67.
Prioli A, Marino P, Lanzoni L, Zardini P. Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. Am J Cardiol. 1998;82(6):756–61.
Leong DP, Penhall A, Perry R, Shirazi M, Altman M, Chong D, Bradley J, Joseph MX, Selvanayagam JB. Speckle-tracking strain of the left atrium: a transoesophageal echocardiographic validation study. Eur Heart J Cardiovasc Imaging. 2013;14(9):898–905.
Inaba Y, Yuda S, Kobayashi N, Hashimoto A, Uno K, Nakata T, Tsuchihashi K, Miura T, Ura N, Shimamoto K. Strain rate imaging for noninvasive functional quantification of the left atrium: comparative studies in controls and patients with atrial fibrillation. J Am Soc Echocardiogr. 2005;18(7):729–36.
Tuluce K, Ozerkan F, Yakar Tuluce S, Yavuzgil O, Gurgun C, Bilgin M, Kahya Eren N, Kocabas U, Nalbantgil S, Soydas CC. Relationships between P wave dispersion, atrial electromechanical delay, left atrial remodeling, and NT-proBNP levels, in patients with hypertrophic cardiomyopathy. Cardiol J. 2015;22(1):94–100.
Sakabe K, Fukuda N, Fukuda Y, Morishita S, Shinohara H, Tamura Y. Interatrial dyssynchrony on tissue Doppler imaging predicts progression to chronic atrial fibrillation in patients with non-valvular paroxysmal atrial fibrillation. Heart. 2009;95(12):988–93.
De Vos CB, Weijs B, Crijns HJ, Cheriex EC, Palmans A, Habets J, Prins MH, Pisters R, Nieuwlaat R, Tieleman RG. Atrial tissue Doppler imaging for prediction of new-onset atrial fibrillation. Heart. 2009;95(10):835–40.
Gaborit B, Jacquier A, Kober F, Abdesselam I, Cuisset T, Boullu-Ciocca S, Emungania O, Alessi MC, Clément K, Bernard M, Dutour A. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol. 2012;60(15):1381–9.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79–108.
Vieira MJ, Teixeira R, Gonçalves L, Gersh BJ. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr. 2014;27(5):463–78.
To AC, Flamm SD, Marwick TH, Klein AL. Clinical utility of multimodality LA imaging: assessment of size, function, and structure. JACC Cardiovasc Imaging. 2011;4(7):788–98.
Bland JM, Altman DG. Statistical methods for assessing agreementbetween two methods of clinical measurement. Lancet. 1986;1(8476):307–10.
Barbosa MM, Beleigoli AM, de Fatima DM, Freire CV, Ribeiro AL, Nunes MC. Strain imaging in morbid obesity: insights into subclinical ventricular dysfunction. Clin Cardiol. 2011;34(5):288–93.
Bakirci EM, Demirtas L, Degirmenci H, Topcu S, Demirelli S, Hamur H, et al. Relationship of the total atrial conduction time to subclinical atherosclerosis, inflammation and echocardiographic parameters in patients with type 2 diabetes mellitus. Clinics. 2015;70(2):73–80.
Ermiş N, Açıkgöz N, Yaşar E, Taşolar H, Yağmur J, Cansel M, Ataş H, Pekdemir H, Ozdemir R. Evaluation of atrial conduction time by P wave dispersion and tissue Doppler echocardiography in prehypertensive patients. Turk Kardiyol Dern Ars. 2010;38(8):525–30.
Dilaveris PE, Gialafos EJ, Chrissos D, Andrikopoulos GK, Richter DJ, Lazaki E, Gialafos JE. Detection of hypertensive patients at risk for paroxysmal atrial fibrillation during sinus rhythm by computer-assisted P wave analysis. J Hypertens. 1999;17(10):1463–70.
Dilaveris PE, Andrikopoulos GK, Metaxas G, Richter DJ, Avgeropoulou CK, Androulakis AM, Gialafos EJ, Michaelides AP, Toutouzas PK, Gialafos JE. Effects of ischemia on P wave dispersion and maximum P wave duration during spontaneous anginal episodes. Pacing Clin Electrophysiol. 1999;22(11):1640–7.
Kaviraj B, Bai SC, Su L, Zheng XO, Huang R, Li TP, Xu DL. Effect of obstructive sleep apnea syndrome on serum C reactive protein level, left atrial size and premature atrial contraction. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(2):197–200.
Mondillo S, Cameli M, Caputo ML, Lisi M, Palmerini E, Padeletti M, Ballo P. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr. 2011;24(8):898–908.
Grundvold I, Bodegard J, Nilsson PM, Svennblad B, Johansson G, Östgren CJ, Sundström J. Associations of obesity and body fat distribution with incident atrial fibrillation in the biracial health aging and body composition cohort of older adults. Cardiovasc Diabetol. 2015;14:5.
Owan T, Avelar E, Morley K, Jiji R, Hall N, Krezowski J, Gallagher J, Williams Z, Preece K, Gundersen N, Strong MB, Pendleton RC, Segerson N, Cloward TV, Walker JM, Farney RJ, Gress RE, Adams TD, Hunt SC, Litwin SE. Favorable changes in cardiac geometry and function following gastric bypass surgery. J Am Coll Cardiol. 2011;57(6):732–9.
Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–61.
Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89(2):724–30.
Stefanadis C, Dernellis J, Toutouzas P. A clinical appraisal of left atrial function. Eur Heart J. 2001;22(1):22–36.
Tadic M, Ilic S, Cuspidi C, Ivanovic B, Bukarica L, Kostic N, Marjanovic T, Kocijancic V, Celic V. Left and right atrial phasic function and deformation in untreated patients with prediabetes and type 2 diabetes mellitus. Int J Card Imaging. 2015;31(1):65–76.
Fang NN, Sui DX, Yu JG, Gong HP, Zhong M, Zhang Y, Zhang W. Strain/strain rate imaging of impaired left atrial function in patients with metabolic syndrome. Hypertens Res. 2015;38(11):758–64.
Abhayaratna WP, Fatema K, Barnes ME, Seward JB, Gersh BJ, Bailey KR, Casaclang-Verzosa G, Tsang TS. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or =65 years of age. Am J Cardiol. 2008;101(11):1626–9.
Erdem FH, Ozturk S, Baltaci D, Donmez I, Alçelik A, Ayhan S, Yaz M. Detection of atrial electromechanical dysfunction in obesity. Acta Cardiol. 2015;70(6):678–84.
Abed HS, Samuel CS, Lau DH, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10(1):90–100.
Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, Finnie JW, Samuel CS, Royce SG, Twomey DJ, Thanigaimani S, Kalman JM, Sanders P. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol. 2015;66(1):1–11.
Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65(20):2159–69.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Statement of Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Tuluce, K., Kara, C., Tuluce, S.Y. et al. Early Reverse Cardiac Remodeling Effect of Laparoscopic Sleeve Gastrectomy. OBES SURG 27, 364–375 (2017). https://doi.org/10.1007/s11695-016-2301-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2301-2