Abstract
Introduction
Internal herniation (IH) probably is the most elusive complication of laparoscopic Roux- en-Y gastric bypass (LRYGB) surgery. This study provides a definition for IH, a diagnosing algorithm, and information on several factors influencing IH formation.
Method
Baseline characteristics, laboratory findings, imaging studies, operative findings, and follow up data of 1583 patients that underwent LRYGB at our bariatric facility between 2007 and 2013 were recorded. Follow up varied between 3 and 76 months, and 85 % of the data was available for analysis at 12 months. Our surgical technique was standardized. Intermesenteric spaces were not closed until July 2012, where after they were closed. To facilitate comparison, IH cases were matched with controls.
Results
Forty patients (2.5 %) had an IH during re-laparoscopy. The modal clinical presentation is acute onset epigastric discomfort, often crampy/colicky in nature. Additional examinations included laboratory testing, abdominal X-ray, abdominal ultrasound, and abdominal CT scanning. Patients who developed an IH lost a significantly higher percentage of their total body weight than their matched controls at every time point. IH incidence was higher in the non-closure group than the closure group.
Conclusion
The large variation in reported IH incidence is due to the large variation in IH definition. To gain more uniformity in reporting IH prevalence, we propose the use of the AMSTERDAM classification. Post-LRYGB patients with acute onset crampy/colicky epigastric pain should undergo abdominal ultrasound to rule out gallbladder pathology and offered re-laparoscopy with a low threshold. IH incidence is highest among patients with rapid weight loss and non-closure of intermesenteric defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic Roux-en-Y gastric bypass (LRYGB) is the most performed bariatric procedure for the management of morbidly obese patients [1]. After LRYGB, several defects remain in the intermesenteric spaces. A defect on both sides of the jejunal mesentery remains at the jejunojejunostomy between the alimentary limb and the biliary limb (JJ defect). The defect that remains between the alimentary limb and the mesocolon is generally addressed as Petersen’s space. In retrocolic positioning of the alimentary limb, an extra defect is created in the mesocolon. These three spaces are potential hernia sites in which bowel can migrate, leading to an internal herniation (IH).
IH is probably the most elusive complication of LRYGB surgery for several reasons. First, we have no knowledge of the exact prevalence of IH. IH incidence varies largely in the literature: 0–14.4 % [2–16]. This is remarkable for a condition that will affect many LRYGB patients. Life table analysis from a large Norwegian cohort revealed that up to 11 % of all LRYGB patients will develop an IH within 5 years [7]. Second, the presentation of IH is as diverse as the reported incidence rates. Patients present with complains ranging from vague abdominal discomfort to acute small bowel obstruction (SBO). The incidence of SBO after LRYGB is 0.6–9.7 % [17]. IH has been said to account for 42–55 % of all SBO cases [15, 17].
Third, IH is difficult to diagnose. Computer tomography (CT) has a reported sensitivity of 63–92 % [18–20], with the so-called mesenteric swirl being the most sensitive sign [21, 22]. CT might not be suited for detecting intermittent IH. A lack of a clear definition of IH might be responsible for the large incidence range reported in the literature. Furthermore, it might cause underdiagnoses and treatment delay of this potentially fatal complication.
Finally, we have very little knowledge of which factors contribute to or prevent IH formation. The antecolic or retrocolic positioning of the alimentary limb, closure or non-closure of the intermesenteric defects, and postoperative rapid weight loss are factors that have been postulated in the literature, but without unanimous results.
This study has three aims. First, to expose how the lack of a clear diagnosis of IH can lead to multiple incidence rates; second, to extract the predominant clinical features of IH from our own LRYGB cohort and comment on the role of laboratory and radiodiagnostic findings in the diagnosing process; and third, to investigate how several factors influence IH formation.
Methods
All 1583 patients that underwent a primary or revisional LRYGB at our bariatric facility between December 2007 and September 2013 were included in this study, allowing for a follow up of at least 1 year. In order to minimize the chance of missing IH cases, patients were searched in duplicate. Forward, every LRYGB patient was checked if they had undergone a re-laparoscopy. Backward, every laparoscopy patient was checked for LRYGB in their medical history. All laparoscopic procedures in this period were searched for patients that had undergone a LRYGB. Baseline characteristics, laboratory findings, imaging studies, operative findings, and follow up data were recorded in a database. Information on clinical symptoms was extracted from electronic patient charts made on the emergency department and/or the outpatient clinic. Special attention was given to clinical or radiologic signs of SBO: abdominal distention, vomiting, and dilated bowel loops on imaging studies. All radiologic reports of conducted imaging studies were searched for signs related to IH: the swirling of the mesenteric veins (whirlpool sign), dilation of bowel, and/or fluid levels within the bowel. From the surgical reports of the re-intervention, details of the IH were extracted: location of the IH, the management of the IH, and if there were any sign of SBO preoperatively (dilated bowel loops, intra-abdominal fluid, and questionable viability of bowel). Postoperative weight loss is presented as percentage of preoperative total body weight (%TBW). Nadir weight was the lowest recorded weight reported in the medical record. To compare the patients that did develop an IH to those who did not, a case–control analysis was performed. Cases were matched to controls by age, preoperative BMI, surgeon, year of surgery, and operative technique (closure or non-closure of the intermesenteric defects during primary LRYGB).
Surgical Technique
All gastric bypass procedures were performed in a standardized way with a 30–50 ml gastric pouch (approximately 4 × 8 cm), an antecolic/antegastric alimentary limb of 150 cm, a side to side 30 mm linear stapled gastrojejunostomy, closed with an absorbable unidirectional barbed 3–0 V-Loc™ suture (Covidien, Dublin, Ireland), and a side to side jejunojejunostomy with a 50-cm biliary limb. The gastrojejunal anastomosis is tested for leakage with methylene blue through the orogastric tube. All surgeries were performed by one of the four surgeons working at our facility. All surgeons were trained at our facility and applied the same surgical technique. A full description of our technique and modifications that were made over time is available elsewhere [23]. The mesenteric defects and Peterson’s space were left open until July 2012. Closure of the intermesenteric spaces (both at primary LRYGB and re-laparoscopy) was accomplished with an EndoHernia stapler (Covidien, Dublin, Ireland). An illustrated description of this device is provided elsewhere [7]. It was our practise to close open mesenteric spaces at re-laparoscopy, even if no IH was present.
Statistics
Patient characteristics are presented as a mean (±SD) or median, depending on the normality of the distribution. Between-group comparisons were performed with the Mann–Whitney U test or the independent sample t test in case of continuous data, depending on parametric distribution. Categorical between-group comparisons were made with the chi-square test or Fisher’s exact test when the expected frequencies were less than 5. The level of significance was set at p < .05. All statistical analyses were performed with SPSS version 21 (IBM corporation, New York, USA). Kaplan–Meier time-to-event curves were made with GraphPad version 6 for Mac (GraphPad, La Jolla, California, USA).
Results
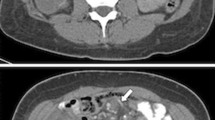
Out of the 1583 LRYGB patients, 40 (2.5 %) had an IH at re-laparoscopy. Thirty-three patients underwent re-laparoscopy for a clinical suspicion of IH. In the seven remaining patients, IH was found co-existent with gallbladder pathology, during a scheduled cholecystectomy. Eight patients were re-operated because of a clinical suspicion of IH, but IH was not present at re-laparoscopy. In five of these eight cases, other pathology was found (adhesions). In the three remaining patients, no other pathology was found. The intermesenteric spaces were closed, and these patients were all symptom-free for a minimum of 3 months postoperatively (see Fig. 1). Patients had a mean age of 40 years and a median preoperative BMI of 42 kg/m2. Most patients who developed an IH had no closure of the intermesenteric spaces on LRYGB. There were no statistically significant differences between the cases and the matched controls (see Table 1). Follow up varied between 3 to 76 months, and 85 % of all patient data was available for analysis at 12 months.
Clinical Symptoms
Median time between LRYGB and first onset of IH symptoms was 9 months (range 0–32). Ninety percent of all IH developed within 20 months (see Fig. 2). Most patients presented with complaints at regular check-ups at the outpatient clinic (60 %). All patients presented with abdominal complaints, mostly with an acute onset (80 %), cramping/colicky nature (65 %), and located in the epigastrium (45 %) (see Table 2).
Diagnostic Tools
Seventy-eight percent of all IH patients underwent laboratory testing, which was normal in the majority of the cases. An abdominal X-ray was taken in 30 % of the IH patients. In five patients, bowel distension or fluid levels were visible on abdominal X-ray. An abdominal ultrasound was performed in 72.5 % of the IH patients, which revealed cholecystolithiasis or cholecystitis in the majority of the cases. Abdominal CT scanning was done in 60 % of the IH patients. The whirlpool sign was visible on CT in six patients. Upper endoscopy was not performed in any of the IH patients. Seven patients presented with clinical or radiological signs of SBO (see Table 3).
Findings During Re-laparoscopy
Median time between the onset of IH symptoms and re-laparoscopy was 1 month (range 0–31). Ninety percent of all patients with clinical suspected IH were re-operated within 14 months (see Fig. 3). Seventy-five percent of IH’s were located at the jejunojejunostomy. The vast majority of the IH’s were managed by reduction and closure of the intermesenteric defects. Resection of bowel due to questionable viability was needed in two patients. There were four conversions (10 %) to an open procedure because the bowel could not be mobilized laparoscopically. Peroperative 14 patients (35 %) had signs of SBO (see Table 4).
Postoperative Weight Loss
Patients that developed an IH had lost significantly more %TBW than their matched controls at 3 (18 versus 14 %, p = .008), 6 (26 versus 21 %, p = .028), and 12 months (35 versus 28 %, p = .001) follow up. Nadir %TBW loss was also highest in IH patients compared to matched controls (36 versus 28 %, p = .001) (see Fig. 4) (Table 5).
Closure of Mesenteric Defects
In 1028 cases of the 1583 LRYGB procedures, the intermesenteric spaces were left open. Five hundred fifty-five patients had their intermesenteric spaces closed during LRYGB. Of the group with open intermesenteric defects, 35 patients (3.4 %) developed an IH. In the group with closed intermesenteric defects, five patients (0.9 %) developed an IH. This difference is significant (p = .013). We did not encounter any perioperative or short-term complications of our stapling technique. In the five patients that were re-operated for IH whom previously underwent closure of their intermesenteric spaces with the hernia stapler, no long-term complications of the stapling technique were found.
Discussion
The most common definition of IH is ‘IH being present on re-laparoscopy.’ According to this definition, our IH incidence is 2.5 %, well within the range of the reported literature [2–16]. There are three characteristics of IH that are not accounted for in this definition. First of all, IH is well known to present in an intermittent fashion [15, 24–26]. After a period of entanglement (and concomitant subacute clinical symptoms), peristaltic movements can cause the bowel to slip into place again. Consequently, no IH can be found during re-laparoscopy (bearing in mind that a vague abdominal discomfort is not characterized as a surgical emergency some delay will be common). In our series, 3 of the 41 patients that underwent re-laparoscopy under clinical suspicion of IH did not have an IH and did not have any other anomaly present. Surprisingly enough, they were symptom-free for at least 3 months after closure of the intermesenteric spaces. These patients might have suffered from intermittent IH and should be added to the count, bringing our IH incidence up to 2.7 %. Secondly, IH can be accompanied by other pathological findings that mimic the clinical presentation of IH (gallbladder pathology, SBO caused by adhesions, etc.). In our series, IH was found as a coincidental finding in seven patients during laparoscopic cholecystectomy. Since the clinical symptoms of IH have some resemblance to those of gallbladder pathology, it remains elusive, which of the two caused the symptoms. This has implications for the definition on IH: when we only count the cases in which we can certainly say that IH caused the symptoms, our IH incidence count drops to 2.1 %. Another inconsistency in the current definition is the resolution of complaints after re-laparoscopy and closure of the intermesenteric defects. Nine of the 40 patients were not symptom-free afterwards, which does not only raise the question of closure patency but also of the diagnosis. When only patients that were symptom-free after re-laparoscopy and closure of the intermesenteric spaces are taken into account, the incidence decreases to 2 %. In our opinion, these factors should be addressed in any study investigating the incidence and etiology of IH.
To help future authors present their data on IH incidence rates, we have developed the AMSTERDAM classification. This tool classifies patients with signs and symptoms of IH in to six scales of likeliness that their symptoms are caused by IH. Class I–III includes patients with IH being present during re-laparoscopy, but sets apart patients with other comorbidity present. Class IV includes patients with no IH present, but remission of their clinical symptoms after closure of their intermesenteric spaces (so-called intermittent IH). Class V and VI is reserved for patients with no IH during re-laparoscopy, but were it is doubtful that IH has caused their clinical symptoms. We suggest that when reporting on IH incidence, class I to IV is included. We realize that the clinical use for such a scale is limited, but we aim to narrow future estimation on IH incidence rate.
The most common clinical presentation of LRYGB patients with IH was acute onset, crampy/colicky abdominal pain, mostly located in the epigastrium. Laboratory studies were done in the majority of the cases, but did not reveal any major pathology. Plain abdominal X-rays were only performed in 12 patients and revealed bowel distension or/and fluid levels in 5 of them. Abdominal ultrasound was the most performed radio diagnostic study and revealed gallbladder pathology in 16/29 patients. This illustrates that like IH, gallbladder pathology is a very common finding in patients that underwent LRYGB surgery. An abdominal CT scan was performed in 24 patients. The whirlpool sign was present in six patients. Unfortunately, we lack information about the timing of the CT scanning to the complaints, because we suspect that the chances of finding an anomaly on CT scanning will increase if patients undergo the scan while experiencing abdominal symptoms. Taking clinical symptoms and radio diagnostic findings together, almost 18 % of the patients showed signs of SBO. Surprisingly, signs of SBO were found in twice as many patients during re-laparoscopy. This suggests that clinical symptoms are an ill representation of the actual pathology. We find that the work up for LRYGB patient presenting with abdominal complaints should be as follows: patients presenting with acute signs of SBO (vomiting, acute abdomen) should be considered a surgical emergency and require immediate re-laparoscopy. Subacute presenting patients should undergo an abdominal ultrasound to assess whether or not gallbladder pathology is present. A low threshold for elective re-laparoscopy should be set for these patients to prevent SBO. The algorithm presented by Agaba et al. included an abdominal CT scan. Of the 75 patients who were examined for abdominal complaints, 40 revealed signs of IH in that study. Thirty-nine (98 %) of them did have an IH on re-laparoscopy. Of the 35 patients that did not have any anomaly on CT scanning, 20 (69 %) turned out to have an IH at re-laparoscopy [27]. Garza et al. examined 1000 LRYGB patients for signs of IH. Of the 34 patients that had an IH, 22 (64 %) had signs of IH on their CT scan [26]. In our study, 6/24 (40 %) of the IH patients had signs of an IH on CT scanning. From the results above, we conclude that a negative CT scan does not rule out an IH, which makes us uncertain about making CT scanning a routine part of our IH work up.
Rapid weight loss has been associated with the formation of IH. In theory, rapid ‘melting’ of intra-abdominal fat could cause enlargement of the intermesenteric spaces that can lead to the formation of IH [15, 28]. When compared to 40 matched controls, we found that patients with IH had more total body weight loss (percent) within the first year after LRYGB. Our finding is supported by Schneider et al., who defined ‘rapid weight loss’ as an excess weight loss above the 90th percentile of the group average in 934 post LRYGB patients. They found that patients who developed an IH were 1.83 times more likely to have undergone one or multiple periods of rapid weight loss [29].
Another factor influencing the formation of IH is closure of the intermesenteric defects during LRYGB. The positive influence of closure on IH formation is a heavily debated subject. A recent meta-analysis of 31,320 patients revealed a lower IH incidence rate in an antecolic LRYGB patient group where the intermesenteric spaces had been closed in comparison to groups without closure [30]. Other authors warn that closure does not prevent IH formation [16, 28, 31], can in itself cause IH [2, 32], or can cause serious complications like bleeding by tearing of the mesentery [7, 13, 33, 34]. From July 2012, we started to close the intermesenteric spaces with a stapling device. The IH incidence dropped from 3.4 to 0.9 %. We are very well aware of the fact that this decrease in incidence rate may very well be the result of the shorter follow up duration in the closure group, as is advocated by several authors [14, 15, 24]. Ninety percent of all patients with IH herniation presented within 20 months after LRYGB surgery, indicating that a maximum follow up of 1 year and 2 months might not be enough time for all patients to present.
This is a retrospective study and therefore is subjected to all the potential flaws associated with this form of analysis. Loss to follow up and unavailability of patient data are well-known obstacles in any retrospective study involving bariatric patients. In our study, data of 85 % of the LRYGB patients was available for analysis after 1 year, but decreased thereafter. Given the fact that IH is a complication that can develop many years after LRYGB surgery, this is a major limitation in our study. In theory, it could be possible that IH patients were admitted and treated in another hospital, but we think this number is low (it is common practise to report complications in bariatric patients to the treating bariatric facility). However, the reported incidence of IH in our patients is probably underestimated. Only randomized prospective studies have sufficient methodological value to report on the true IH incidence. What does come forth very clearly from our data is the fact that there is a mismatch between clinical signs of SBO and peroperative signs of SBO in patients with IH. The risk of conversion during re-laparoscopy is many times higher than the reported rates of ~1 % [35, 36]. A low threshold for re-laparoscopy should be set for LRYGB patients presenting with intermittent abdominal complaints.
References
Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes. Surg. [Internet]. 2013 [cited 2013 Sep 17];23:427–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23338049
Madan AK, Lo Menzo E, Dhawan N, Tichansky DS. Internal hernias and nonclosure of mesenteric defects during laparoscopic Roux-en-Y gastric bypass. Obes. Surg. [Internet]. 2009 [cited 2013 Sep 4];19:549–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18931883
Cho M, Carrodeguas L, Pinto D, Lascano C, Soto F, Whipple O, et al. Diagnosis and management of partial small bowel obstruction after laparoscopic antecolic antegastric Roux-en-Y gastric bypass for morbid obesity. J. Am. Coll. Surg. [Internet]. 2006 [cited 2013 Sep 11];202:262–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16427551
Finnell CW, Madan AK, Tichansky DS, Ternovits C, Taddeucci R. Non-closure of defects during laparoscopic Roux-en-Y gastric bypass. Obes. Surg. [Internet]. 2007;17:145–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20143179
Han S, Gracia C, Mehran A. Improved outcomes using a systematic and evidence-based approach to the laparoscopic Roux-en-Y gastric bypass in a single academic institution. Am. … [Internet]. 2007 [cited 2014 May 6];73:955–8. Available from: http://www.ingentaconnect.com/content/sesc/tas/2007/00000073/00000010/art00004
Abasbassi M, Pottel H, Deylgat B, Vansteenkiste F, Van Rooy F, Devriendt D, et al. Small bowel obstruction after antecolic antegastric laparoscopic Roux-en-Y gastric bypass without division of small bowel mesentery: a single-centre, 7-year review. Obes. Surg. [Internet]. 2011 [cited 2013 Sep 4];21:1822–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21656166
Aghajani E, Jacobsen HJ, Nergaard BJ, Hedenbro JL, Leifson BG, Gislason H. Internal hernia after gastric bypass: a new and simplified technique for laparoscopic primary closure of the mesenteric defects. J. Gastrointest. Surg. [Internet]. 2012 [cited 2013 Sep 4];16:641–5. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3274684&tool=pmcentrez&rendertype=abstract
de la Cruz-Muñoz N, Cabrera JC, Cuesta M, Hartnett S, Rojas R. Closure of mesenteric defect can lead to decrease in internal hernias after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. [Internet]. Elsevier Inc.; 2011 [cited 2013 Sep 4];7:176–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21126922
Nelson LG, Gonzalez R, Haines K, Gallagher SF, Murr MM. Spectrum and treatment of small bowel obstruction after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. [Internet]. 2006 [cited 2013 Sep 11];2:377–83, discussion 383. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16925356
Escalona A, Devaud N, Pérez G, Crovari F, Boza C, Viviani P, et al. Antecolic versus retrocolic alimentary limb in laparoscopic Roux-en-Y gastric bypass: a comparative study. Surg. Obes. Relat. Dis. [Internet]. 2007 [cited 2013 Sep 11];3:423–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17544923
Steele KE, Prokopowicz GP, Magnuson T, Lidor a, Schweitzer M. Laparoscopic antecolic Roux-en-Y gastric bypass with closure of internal defects leads to fewer internal hernias than the retrocolic approach. Surg. Endosc. [Internet]. 2008 [cited 2013 Sep 11];22:2056–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18270773
Rodríguez A, Mosti M, Sierra M, Pérez-Johnson R, Flores S, Dominguez G, et al. Small bowel obstruction after antecolic and antegastric laparoscopic Roux-en-Y gastric bypass: could the incidence be reduced? Obes. Surg. [Internet]. 2010 [cited 2013 Sep 4];20:1380–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20401758
Himpens J, Verbrugghe A, Cadière G-B, Everaerts W, Greve J-W. Long-term results of laparoscopic Roux-en-Y Gastric bypass: evaluation after 9 years. Obes. Surg. [Internet]. 2012 [cited 2013 Sep 4];22:1586–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22865194
Brolin RE, Kella VN. Impact of complete mesenteric closure on small bowel obstruction and internal mesenteric hernia after laparoscopic Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. [Internet]. Elsevier; 2013 [cited 2013 Sep 4];1–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23415691
Gandhi AD, Patel R a, Brolin RE. Elective laparoscopy for herald symptoms of mesenteric/internal hernia after laparoscopic Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. [Internet]. American Society for Metabolic and Bariatric Surgery; 2009 [cited 2013 Sep 4];5:144–9; discussion 149. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19249249
Ortega J, Cassinello N, Sánchez-Antúnez D, Sebastián C, Martínez-Soriano F. Anatomical basis for the low incidence of internal hernia after a laparoscopic Roux-en-Y gastric bypass without mesenteric closure. Obes. Surg. [Internet]. 2013 [cited 2013 Sep 4];23:1273–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23462859
Koppman JS, Li C, Gandsas A. Small bowel obstruction after laparoscopic Roux-en-Y gastric bypass: a review of 9,527 patients. J. Am. Coll. Surg. [Internet]. 2008 [cited 2014 Jan 10];206:571–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18308230
Blachar A, Federle MP. Internal hernia: An increasingly common cause of small bowel obstruction. Semin. Ultrasound, CT MRI [Internet]. 2002;23:174–83. Available from: http://linkinghub.elsevier.com/retrieve/pii/S088721710290003X
Yu J, Turner MA, Cho S-R, Fulcher AS, DeMaria EJ, Kellum JM, et al. Normal anatomy and complications after gastric bypass surgery: helical CT findings. Radiology. 2004;231:753–60.
Ahmed AR, Rickards G, Johnson J, Boss T, O’Malley W. Radiological findings in symptomatic internal hernias after laparoscopic gastric bypass. Obes. Surg. [Internet]. 2009 [cited 2013 Sep 4];19:1530–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19756892
Lockhart ME, Tessler FN, Canon CL, Smith JK, Larrison MC, Fineberg NS, et al. Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls. AJR. Am. J. Roentgenol. [Internet]. 2007 [cited 2014 May 6];188:745–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17312063
Iannuccilli JD, Grand D, Murphy BL, Evangelista P, Roye GD, Mayo-Smith W. Sensitivity and specificity of eight CT signs in the preoperative diagnosis of internal mesenteric hernia following Roux-en-Y gastric bypass surgery. Clin. Radiol. [Internet]. The Royal College of Radiologists; 2009 [cited 2014 May 6];64:373–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19264181
Geubbels N, de Brauw LM, Acherman YIZ, van de Laar AWJM, Wouters MWJM, Bruin SC. The Preceding Surgeon Factor in Bariatric Surgery: a Positive Influence on the Learning Curve of Subsequent Surgeons. Obes. Surg. [Internet]. 2014 [cited 2014 Dec 19]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25511752
Comeau E, Gagner M, Inabnet WB, Herron DM, Quinn TM, Pomp a. Symptomatic internal hernias after laparoscopic bariatric surgery. Surg. Endosc. [Internet]. 2005 [cited 2014 Jan 10];19:34–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15529196
Blachar a, Federle MP, Brancatelli G, Peterson MS, Oliver JH, Li W. Radiologist performance in the diagnosis of internal hernia by using specific CT findings with emphasis on transmesenteric hernia. Radiology [Internet]. 2001;221:422–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11687686
Garza E, Kuhn J, Arnold D, Nicholson W, Reddy S, McCarty T. Internal hernias after laparoscopic Roux-en-Y gastric bypass. Am. J. Surg. [Internet]. 2004 [cited 2013 Sep 11];188:796–800. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15619502
Agaba E a, Gentles C V, Shamseddeen H, Sasthakonar V, Kandel A, Gadelata D, et al. Retrospective analysis of abdominal pain in postoperative laparoscopic Roux-en-Y gastric bypass patients: is a simple algorithm the answer? Surg. Obes. Relat. Dis. [Internet]. 2008 [cited 2013 Sep 11];4:587–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18226974
Ahmed AR, Rickards G, Husain S, Johnson J, Boss T, O’Malley W. Trends in internal hernia incidence after laparoscopic Roux-en-Y gastric bypass. Obes. Surg. [Internet]. 2007 [cited 2013 Sep 11];17:1563–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18004631
Schneider C, Cobb W, Scott J, Carbonell A, Myers K, Bour E. Rapid excess weight loss following laparoscopic gastric bypass leads to increased risk of internal hernia. Surg. Endosc. [Internet]. 2011 [cited 2013 Sep 4];25:1594–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21072668
Geubbels N, Lijftogt N, Fiocco M, van Leersum NJ, Wouters MWJM, de Brauw LM. Meta-analysis of internal herniation after gastric bypass surgery. Br. J. Surg. [Internet]. 2015;n/a – n/a. Available from: 10.1002/bjs.9738
Hope WW, Sing RF, Chen AY, Lincourt AE, Gersin KS, Kuwada TS, et al. Failure of mesenteric defect closure after Roux-en-Y gastric bypass. JSLS [Internet]. 2010 [cited 2013 Sep 4];14:213–6. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3043570&tool=pmcentrez&rendertype=abstract
Eckhauser A, Torquati A, Youssef Y, Kaiser JL, Richards WO. Internal hernia: postoperative complication of roux-en-Y gastric bypass surgery. [Internet]. Am. Surg. 2006. p. 581–4; discussion 584–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22874695
Iannelli A, Buratti MS, Novellas S, Dahman M, Amor I Ben, Sejor E, et al. Internal hernia as a complication of laparoscopic Roux-en-Y gastric bypass. Obes. Surg. [Internet]. 2007;17:1283–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22874695
Coleman MH, Awad ZT, Pomp A, Gagner M. Laparoscopic closure of the Petersen mesenteric defect. Obes. Surg. [Internet]. 2006;16:770–2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16756740
Stenberg E, Szabo E, Agren G, Näslund E, Boman L, Bylund A, et al. Early complications after laparoscopic gastric bypass surgery: results from the Scandinavian Obesity Surgery Registry. Ann. Surg. [Internet]. 2013 [cited 2014 Sep 23];00:1–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24374541
Finks JF, Kole KL, Yenumula PR, English WJ, Krause KR, Carlin AM, et al. Predicting risk for serious complications with bariatric surgery: results from the Michigan Bariatric Surgery Collaborative. Ann. Surg. [Internet]. 2011 [cited 2014 May 13];254:633–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21897200
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Grant Information
None of the authors have any grant information to disclose.
Conflict of Interest
None of the authors have any conflict of interests to report.
Ethical Rights
For this type of study, formal consent is not required.
Informed Consent
Informed consent for the anonymous use of patient data was obtained from all participants.
Rights and permissions
About this article
Cite this article
Geubbels, N., Röell, E.A., Acherman, Y.I.Z. et al. Internal Herniation After Laparoscopic Roux-en-Y Gastric Bypass Surgery: Pitfalls in Diagnosing and the Introduction of the AMSTERDAM Classification. OBES SURG 26, 1859–1866 (2016). https://doi.org/10.1007/s11695-015-2028-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-2028-5