Abstract
Background
Bariatric surgery is effective at achieving sustained weight loss and improving the control and resolution of obesity-related co-morbidities. Most studies that have demonstrated co-morbidity resolution in patients undergoing laparoscopic sleeve gastrectomy (LSG) only follow patients for the short term (less than 1 year) or follow a relatively small cohort (<100 patients) for the intermediate or long term (more than 5 years). We report our experience following a large cohort of morbidly obese patients who underwent LSG with intermediate-term follow-up.
Methods
We retrospectively reviewed 435 consecutive patients who underwent LSG from January 2004 to November 2013. Co-morbidities investigated included diabetes mellitus (DM), hypertension (HTN), and hyperlipidemia (HL). A co-morbidity was determined to be resolved if the patient was no longer taking any medication to treat that specific co-morbidity.
Results
Mean follow-up was 26 ± 25 months (range = 1–112). Mean postoperative total weight loss (%TWL) at 6, 12, 24, 36, 48, 60, and 72 months were 23.6, 29.9, 29.5, 25.2, 26.7, 25.4, and 24.3 %, respectively. The incidence of all three co-morbidities was found to be significantly lower at the last patient follow-up. The resolution rates for DM, HTN, and HL were 59, 31, and 50 %, respectively. In patients who continued to have co-morbidities, the mean numbers of medications for DM (1.2 ± 0.7 vs. 0.5 ± 0.7, p < 0.0001), HTN (1.8 ± 1.1 vs. 1.3 ± 1.2, p < 0.0001), and HL (0.9 ± 0.7 vs. 0.6 ± 0.6, p < 0.0001) postoperatively were all significantly less.
Conclusions
LSG is effective at achieving significant and sustained weight loss, improvement in co-morbidity profiles, and a reduction in poly-pharmacy for these conditions over intermediate-term follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is effective at achieving sustained weight loss, but more importantly, it improves the control and resolution of obesity-related co-morbidities [1–6]. These claims are supported by prospective randomized controlled trials [1, 2, 7]. The most frequently evaluated obesity-related co-morbidities in these studies include diabetes mellitus (DM), hypertension (HTN), and hyperlipidemia (HL). Other co-morbidities studied include gastro-esophageal reflux disease (GERD) and obstructive sleep apnea (OSA).
Laparoscopic sleeve gastrectomy (LSG) has gained popularity as the preferred surgical option for patients with morbid obesity. There have been several recent studies that have sought to demonstrate co-morbidity resolution in patients undergoing LSG [3, 8–13]. Most of these studies, however, only follow patients for the short term (less than 1 year) [2, 5, 9, 11–15] or follow a relatively small cohort (<100 patients) for the intermediate [1, 3, 6–8] or long term (more than 5 years) [10, 16–21].
In this paper, we report our experience following a large cohort of morbidly obese patients who underwent LSG at our institution with intermediate-term follow-up. Specifically, we focused on the resolution or improvement of DM, HTN, and HL in this patient population.
Materials and Methods
The records of all patients undergoing LSG were reviewed using our institutional review board-approved database. We retrospectively reviewed 435 consecutive patients who underwent LSG for morbid obesity from January 2004 to November 2013. We included patients who previously had laparoscopic adjustable gastric band placement and subsequent removal that were revised to LSG. A total of four surgeons specializing in bariatric surgery performed all procedures. Our technique has been previously described both with and without omentopexy [22, 23].
Preoperative parameters included age, gender, race, body mass index (BMI), American Society of Anesthesiologists (ASA) score, and co-morbidities. Co-morbidities investigated included DM, HTN, and HL. To better characterize DM disease severity, we recorded preoperative hemoglobin A1c (HgbA1c) values, number of oral diabetic medicines, and whether the patients required insulin therapy. We similarly recorded the number of medications patients were taking preoperatively for HTN and HL.
Surgical parameters included total operative time, estimated blood loss (EBL), conversion rate, intraoperative complications, postoperative complications, length of stay (LOS), leak rate, stenosis rate, and 90-day readmission rate. All postoperative complications were graded using the modified Clavien-Dindo system [24] and were further classified by organ system. Grade 1 and 2 complications were regarded as minor, and grades 3–5 were regarded as major.
Postoperative weights were tracked every 3 months for the first year and every 6 months thereafter in accordance with the standard postoperative visit schedule. At the time of the last follow-up, the patient’s last available HgbA1c, number of oral hypoglycemic medications, need for insulin therapy, and total number of medications used to treat HTN and HL were recorded. A co-morbidity was determined to be resolved if the patient was no longer taking any medication to treat that specific co-morbidity.
Statistical analyses were performed using Graphpad Prism software version 5.03 (GraphPad Software, Inc. La Jolla, CA) and MYSTAT version 12 (SYSTAT Software, Inc. Chicago, IL). Categorical variables were compared using Fisher’s exact test or chi-square test when appropriate, whereas continuous variables were compared using the Kruskal-Wallis test or Mann-Whitney U test (two-tailed). Univariate binary logistic regression analysis was utilized to identify predictors of conversion, intraoperative complications, and postoperative complications. Those parameters with a p < 0.05 as well as BMI were included in the multivariable analysis. Multivariate logistic regression analysis was used to identify independent predictors of conversion, intraoperative complications, and postoperative complications. Both forward and backward stepwise regression analyses were utilized removing parameters with a p > 0.15. All results are expressed as mean ± SD, unless specified otherwise. The null hypothesis was rejected when α < 0.05.
Results
Preoperative Patient Characteristics
All preoperative parameters are listed in Table 1. The mean (±SD) age of our cohort was 44 ± 13.3 years. The median BMI was 48.3 kg/m2 (range = 31.0–95.1). Approximately 74 % of patients had American Society of Anesthesiology (ASA) scores >2.
Perioperative Outcomes
The mean operative time and EBL were 118 ± 39.7 min and 80 mL ± 43.0, respectively. There was no conversion to open surgery in our cohort. Our intraoperative complication rate was 2 % and total 30-day postoperative complication rate was 11 %. Our 30-day leak rate was 1.1 %. Our 90-day readmission rate was 5.7 % and mean follow-up was 26 ± 25 months (range = 1–112) (Table 2).
Weight Loss
Mean postoperative total weight loss (%TWL) at 6, 12, 24, 36, 48, 60, and 72 months were 23.6, 29.9, 29.5, 25.2, 26.7, 25.4, and 24.3 %, respectively (Fig. 1).
Mean postoperative total weight loss (%TWL) over time. The box and whisker plots represent the 10th, 25th, 50th (median), 75th, and 90th percentiles. Outliers are depicted as dots on the graph. The x-axis is the postoperative time in months while the y-axis represents the percent total weight change
Resolution of Co-morbidities
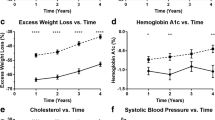
The incidence of all three co-morbidities was found to be significantly lower at the last patient follow-up (mean = 26 months). The incidence of DM was 30 % preoperatively and 12 % postoperatively (p < 0.0001) representing a 59 % resolution rate. The incidence of HTN was 56 % preoperatively and 39 % postoperatively (p < 0.0001) representing a 31 % resolution rate. The incidence of HL was 45 % preoperatively and 22 % postoperatively (p < 0.0001) representing a 50 % resolution rate (Table 3). Linear regression analysis demonstrated no correlation between HgbA1c levels and time following LSG (p = 0.22) (Fig. 2).
Hemoglobin A1c change over time. The linear regression analysis is depicted in the graph above. The x-axis represents the postoperative time. The y-axis represents the total percent Hemoglobin A1c change. There was no significant correlation between postoperative time and percent Hemoglobin A1c change (r 2 = 0.01; p = 0.22)
Reduction in Medication Usage
In patients who continued to have co-morbidities, the mean numbers of medications for DM (1.2 ± 0.7 vs. 0.5 ± 0.7, respectively; p < 0.0001), HTN (1.8 ± 1.1 vs. 1.3 ± 1.2, respectively; p < 0.0001), and HL (0.9 ± 0.7 vs. 0.6 ± 0.6, respectively; p < 0.0001) postoperatively were all significantly less (Fig. 3).
Co-morbidity improvement profiles. The bar graphs depict the mean number of co-medications and the error bars depict the standard deviation. There was a significant improvement in co-morbidities with respect to diabetes mellitus (a), hypertension (b), and hyperlipidemia (c) at the last postoperative follow-up
Discussion
Our data demonstrate that LSG is effective at achieving significant and sustained weight loss, decreased incidence of DM, HTN, and HL, and significant reduction in poly-pharmacy for these conditions. There have been many studies documenting weight loss and improvement in co-morbidities over the short term (less than 1 year) [2, 5, 9, 11–15], but there are fewer studies investigating these outcomes at intermediate- [1, 3, 6–8] and long-term follow-up [10, 16–21]. Our study represents an important addition to the literature given the follow-up period and cohort size.
Resolution rates of DM vary in the literature. Gill et al. [4] performed a systematic review of studies investigating patients with DM who underwent LSG. They found a mean improvement rate of 66.2 %, ranging anywhere from 14 to 100 %. Sieber et al. [18] performed a retrospective analysis of a prospective cohort of 68 patients undergoing LSG from 2004 to 2007 with minimum follow-up of 5 years. Of all patients requiring medical treatment for DM, 62.5 % no longer required medical therapy.
In our study, the preoperative incidence of DM was 30 % which is slightly higher than that observed by most studies, including, for example, Fuks et al. [15] (24 %), Ruiz-Tovar et al. [10] (26 %), and Zhang et al. [13] (28.9 %). We noted a 59 % reduction of DM, which is slightly below the mean seen by Gill et al. [4] (66.2 %) and Sieber et al. [18] (62.5 %) and well below that seen by Albanopoulos et al. [8] (94 %). A possible explanation for this difference is the much larger cohort in our study, possibly reflecting a sicker patient population and poorly controlled DM. Since the most dramatic decrease in DM has been seen within the first year in other studies, it is unlikely that our follow-up period of 26 months compared to 36 months would change the observed reduction in incidence of DM. It is difficult to compare reduction rates between study groups given the lack of data about the preoperative duration of DM as well as variable definition of “resolution” as each of these issues has been shown to influence remission rates [3].
Improvement in DM control has been demonstrated not only in observational studies but also in randomized controlled trials as well. Schauer et al. enrolled 140 patients in a RCT comparing intensive medical therapy (IMT) alone, IMT plus roux-en-y gastric bypass (RYGB), and IMT plus LSG [2]. They found significantly improved glycemic control in each surgical group as compared to IMT alone, as measured by percentage of patients with HgbA1c <6.0 %, improved glycemic control overall, reduction in oral diabetic agents per day, as well as reduction in usage of insulin. These outcomes were only followed for 12 months postoperatively and only 50 patients underwent LSG.
Only a few studies have primarily assessed resolution or improvement of HTN and HL, although there have been many articles secondarily reporting resolution or improvement of each of these co-morbidities. Sarkhosh et al. [11] performed a systematic review of the impact of LSG on resolution or improvement in HTN. They report an average resolution rate of HTN, defined as cessation of antihypertensive medication, of 58 % of patients at 1-year follow-up with a range of 10–93 %. In contrast to the short-term results presented earlier, Ruiz-Tovar et al. [10] prospectively studied 50 patients undergoing LSG for morbid obesity and analyzed mean excess weight loss as well as remission of co-morbidities at 1, 2, and 5 years after surgery. Thirty percent of patients carried a diagnosis of HTN preoperatively, and resolution was seen in 67 % of patients with partial improvement seen in an additional 13 % of patients. In our study, we observed a 31 % reduction in incidence of HTN, falling within the range of previously reported values, although notably below the mean resolution rate reported by Sarkhosh et al. [11] (58 %) and Ruiz-Tovar et al. [10] (67 %). Our cohort had a preoperative incidence of HTN of 56 %, which is notably larger than that seen in Ruiz-Tovar et al. [10] (30 %), again possibly reflecting a more heterogeneous patient population.
Various studies have demonstrated improvements in lipid profiles following LSG. Our group has previously [13] retrospectively analyzed changes in lipid profiles in 45 morbidly obese patients 1 year after undergoing LSG. They found significantly increased high-density lipoprotein cholesterol (HDL) levels and decreased triglyceride (TG) levels 1 year postoperatively. Less patients were taking lipid-lowering medications 1 year postoperatively; however, this did not reach statistical significance, likely due to the small cohort size and short-term follow-up. In our cohort, we found a HL resolution rate of 50 %. Albanopoulos et al. [8] similarly report a resolution rate of HL of 52 % 3 years after LSG. Similarly, D’Hondt et al. [12] reported a HL resolution rate of 69 % at 12 months follow-up. While our preoperative HL rate was 45 %, rates noted by D’Hondt et al. [12] and Albanopoulos et al. [8] were 43 and 26.4 %, respectively.
We found a mean %TWL of 29.9, 29.5, 25.2, and 24.3 % at 1, 2, 3, and 6 years follow-up. In comparison, Albanopoulos et al. [8] report a %TWL of 35.7, 39.1, and 38.1 % at 1, 2, and 3 years follow-up. Our data are consistent in that weight loss is most pronounced between 1 and 2 years postoperatively with a plateau effect and slight weight regain thereafter.
There are limitations to our study. It is a retrospective analysis of a retrospectively maintained database that comes with inherent biases. This is reflected in our variable follow-up range. Second, given our study design, we were unable to perform a deeper data collection regarding disease severity to better characterize our patient population. Third, the majority of our patients underwent LSG over the last 5 years of the study time period as the operation became more popular and insurance approval became common. Nevertheless, this is one of the largest LSG cohorts in the literature and the study has significant intermediate-term follow-up.
Conclusion
LSG is effective at achieving significant and sustained weight loss, improvement in co-morbidity profiles, and a reduction in poly-pharmacy for these conditions over intermediate-term follow-up. Further research is needed to better characterize patients who do not experience a resolution or improvement in these co-morbidities.
References
Kashyap SR, Bhatt DL, Schauer PR. STAMPEDE investigators. Bariatric surgery vs. advanced practice medical management in the treatment of type 2 diabetes mellitus: rationale and design of the Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently trial (STAMPEDE). Diabetes Obes Metab. 2010;12(5):452–4.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Capoccia D, Coccia F, Guida A, et al. Is type 2 diabetes really resolved after laparoscopic sleeve gastrectomy? Glucose variability studied by continuous glucose monitoring. J Diabetes Res. 2015:674268.
Gill RS, Birch DW, Shi X, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6(6):707–13.
Abbas M, Cumella L, Zhang Y, et al. Outcomes of laparoscopic sleeve gastrectomy and roux-en-Y gastric bypass in patients older than 60. Obes Surg. 2015.
Daigle CR, Andalib A, Corcelles R, et al. Bariatric and metabolic outcomes in the super-obese elderly. Surg Obes Relat Dis. 2015.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85.
Albanopoulos K, Tsamis D, Natoudi M, et al. The impact of laparoscopic sleeve gastrectomy on weight loss and obesity-associated comorbidities: the results of 3 years of follow-up. Surg Endosc. 2015.
Dillon C, Peddle J, Twells L, et al. Rapid reduction in use of antidiabetic medication after laparoscopic sleeve gastrectomy: The Newfoundland and Labrador Bariatric Surgery Cohort (BaSCo) Study. Can J Hosp Pharm. 2015;68(2):113–20.
Ruiz-Tovar J, Martinez R, Bonete JM, et al. Long-term weight and metabolic effects of laparoscopic sleeve gastrectomy calibrated with a 50-Fr Bougie. Obes Surg. 2015.
Sarkhosh K, Birch DW, Shi X, et al. The impact of sleeve gastrectomy on hypertension: a systematic review. Obes Surg. 2012;22(5):832–7.
D’Hondt M, Vanneste S, Pottel H, et al. Laparoscopic sleeve gastrectomy as a single-stage procedure for the treatment of morbid obesity and the resulting quality of life, resolution of comorbidities, food tolerance, and 6-year weight loss. Surg Endosc. 2011;25(8):2498–504.
Zhang F, Strain GW, Lei W, et al. Changes in lipid profiles in morbidly obese patients after laparoscopic sleeve gastrectomy (LSG). Obes Surg. 2011;21(3):305–9.
Cottam D, Qureshi FG, Mattar SG, et al. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20(6):859–63.
Fuks D, Verhaeghe P, Brehant O, et al. Results of laparoscopic sleeve gastrectomy: a prospective study in 135 patients with morbid obesity. Surgery. 2009;145(1):106–13.
Alexandrou A, Mantonakis E, Pikoulis E, et al. Robotic sleeve gastrectomy for morbid obesity: report of a 5 year experience. Int J Med Robot. 2015.
Sarela AI, Dexter SP, O’Kane M, et al. Long-term follow-up after laparoscopic sleeve gastrectomy: 8-9-year results. Surg Obes Relat Dis. 2012;8(6):679–84.
Sieber P, Gass M, Kern B, et al. Five-year results of laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(2):243–9.
Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–24.
Bohdjalian A, Langer FB, Shakeri-Leidenmuhler S, et al. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20(5):535–40.
Rawlins L, Rawlins MP, Brown CC, et al. Sleeve gastrectomy: 5-year outcomes of a single institution. Surg Obes Relat Dis. 2013;9(1):21–5.
Afaneh C, Costa R, Pomp A, et al. A prospective randomized controlled trial assessing the efficacy of omentopexy during laparoscopic sleeve gastrectomy in reducing postoperative gastrointestinal symptoms. Surg Endosc. 2015;29(1):41–7.
Moy J, Pomp A, Dakin G, et al. Laparoscopic sleeve gastrectomy for morbid obesity. Am J Surg. 2008;196(5):e56–9.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Jonathan S. Abelson and Cheguevera Afaneh contributed equally to this work.
Rights and permissions
About this article
Cite this article
Abelson, J.S., Afaneh, C., Dolan, P. et al. Laparoscopic Sleeve Gastrectomy: Co-morbidity Profiles and Intermediate-Term Outcomes. OBES SURG 26, 1788–1793 (2016). https://doi.org/10.1007/s11695-015-2002-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-2002-2