Abstract
Background
Significant reductions in glucose control immediately post bariatric surgery in patients with longstanding poor glycemic control can lead to the paradoxical progression of diabetic retinopathy (DR) in susceptible individuals. Bariatric surgery results in dramatic and immediate diabetic control postoperatively. We aimed to systematically review the literature to assess the effect of bariatric surgery on DR.
Methods
A comprehensive search of electronic databases (e.g., MEDLINE, EMBASE, SCOPUS, Web of Science, and the Cochrane Library) was completed. All randomized controlled trials, non-randomized comparison study, and case series were included. Inclusion criteria included English-speaking studies, enrolling ≥5 patients, and contained ophthalmological data on outcome of DR pre- and post bariatric surgery. Two independently reviewers screened abstracts, reviewed full text versions of all studies classified, and extracted data. All comparison studies included in the meta-analysis were assessed independently by two reviewers for methodological quality using the Cochrane Risk of Bias (RoB) tools. Disagreements were resolved by re-extraction, or third-party adjudication. Where possible and appropriate, a meta-analysis was conducted.
Results
A total of 277 studies were identified using our search criteria for screening. Four primary studies (n = 148 patients) met our inclusion criteria and were included in the systematic review. These included no randomized controlled trials and four non-randomized case series.
Patients with no preoperative DR (n = 80), following bariatric surgery, an average of 92.5 ± 7.4 % remained disease free, while 7.5± 7.4 % of patients progressed to DR.
Patients with diabetic retinopathy preoperatively (n = 68), following bariatric surgery, an average of 57.4 ± 18.5 % of patients had no change, 23.5 ± 18.7 % of patients had progression, and 19.2 ± 12.9 % of patients had improvement in their disease.
Conclusions
Progression of diabetic retinopathy is a significant issue postoperatively following bariatric surgery. Patients with a diagnosis of DR prior to surgery are at increased risk of further progression in their disease and should receive adequate counseling and evaluation prior to undergoing a surgical procedure. However, the few primary studies in this systematic review limit any conclusion. Further studies are needed to further evaluate these results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obesity and its complications have a significant and increasing effect on global morbidity and mortality [1, 2]. In particular, many morbidly obese patients present with associated poor glycemic control and its resultant diabetic macrovascular and microvascular complications, including atherosclerotic disease, neuropathy, nephropathy, and retinopathy [3, 4]. Despite efforts to control obesity through pharmacological and lifestyle modifications, a significant percentage of patients will require bariatric surgery to control morbid obesity [5, 6].
Bariatric surgery is an effective option for treating obesity comorbidities. Postoperatively, glycemic levels achieve a dramatic and rapid response back to normal, essentially, resolving their diabetes [7–11]. Counterintuitively, however, the significant reduction in blood glucose for patients with longstanding poor glycemic control immediately following surgery has been reported to lead to paradoxical progression of diabetic retinopathy (DR) in certain individuals [12, 13]. This therefore can affect quality of life and should be a major consideration before offering bariatric surgery to morbidly obese patients with preexisting DR [14].
At this time, limited studies or guidelines exist to direct bariatric surgeons on how to proceed with bariatric surgery in patients with DR. The purpose of this paper is to systematically review and analyze the literature to assess the effect of bariatric surgery on the progression of diabetic retinopathy.
Methods
A comprehensive search of electronic databases (e.g., MEDLINE, EMBASE, SCOPUS, Web of Science and the Cochrane Library) using search terms “retino* OR retina* OR microvascular OR eye* OR ocular” AND “diabet*” AND “gastric bypass OR gastric band* OR sleeve gastrectomy OR bariatric” was completed, and conference abstracts were also searched. All randomized controlled trials, non-randomized comparison study, and case series were included. All human studies limited to English were included. The reference lists of included studies were also checked to identify missing studies in the primary search. Two independently reviewers (D.C. and N.S.) screened abstracts, reviewed full text versions of all studies classified, and extracted data. All comparison studies included in the systematic review were assessed independently by two reviewers (D.C. and N.S.) for methodological quality using the Cochrane Risk of Bias (RoB) tools. Disagreements were resolved by re-extraction, or third party adjudication.
Assessment of Study Eligibility
We systematically reviewed each study according to the following criteria: (1) There were no study format restrictions for the systematic review due to the limited evidence available, (2) the study reported data on blood glucose and DR as primary outcomes; (3) patients had undergone primary bariatric surgery; (4) if any disparity existed between the conference proceedings and a later published article, the initial presented results were used.
Outcomes of Interest
The primary outcome of interest was DR status (progression, no change, regression) following bariatric surgery. Unfortunately, due to heterogeneity across data sets, severity of diabetic retinopathy including arteriolar-to-venular diameter ratio (AVR) and retinal images could not be assessed.
Statistical Analysis
Meta-analysis was not feasible in our review as only four studies were identified, most of which were either not a control study or with different comparison group. Heterogeneity also could not be accessed because of the scarcity of the available evidence. Only summarized weighted mean or percentages were presented.
Results
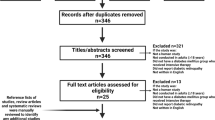
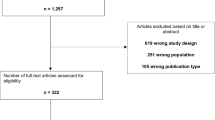
A total of 522 studies were identified using our search criteria for screening (Fig. 1). Of 522 studies screened on their titles and abstracts after an assessment according to our eligibility criteria, 506 did not meet the basic data requirements on DR outcomes following bariatric surgery and were excluded. Of 16 remaining for review, 12 were excluded based on insufficient primary outcomes and follow-up. Thus, a total of four primary studies (148 patients) were identified that met our inclusion criteria for the systematic review and meta-analysis and were assessed by full manuscript. These included four case series, no controlled studies or randomized controlled trials [15–18]. The duration of follow-up following bariatric surgery ranged between 6 and 18 months postoperatively.
Only two studies reported patient age (average 51/50.2 years old) and male percentage (average 45/33 %). Study patients were broken in two groups: (1) without preoperative DR and (2) with preoperative DR. In total, 80 patients had no preexisting disease and 68 patients had prior DR before bariatric surgery. Across all articles, the average patient was 50.5 years old, female, had a pre-op BMI of 48.8, and pre-op hemoglobin A1c (HbA1c) of 8.0. The balance of patient demographics across the three groups could not be accessed due to a lack of original information. These characteristics are summarized in Table 1.
For patients with no pre-operative DR (n = 80), following bariatric surgery, an average of 92.5 ± 7.4 % remained disease free, while 7.5 ± 7.4 % of patients progressed to DR. In contrast, for patients with diabetic retinopathy preoperatively (n = 68), an average of 57.4 ± 18.5 % of patients had no change, 23.5 ± 18.7 % of patients experienced progression, and 19.2 ± 12.9 % of patients had improvement in their disease following bariatric surgery. These findings are summarized in Table 2.
Furthermore, in patients with progression to DR, patients were found to be significantly more likely to have had previous existing DR (odds ratio 2.77, confidence interval (CI) 1.10–6.99, p = 0.03). These findings are summarized in Table 3.
Discussion
Multiple studies have reported that bariatric surgery is beneficial in the control of diabetic-related microvascular complications including nephropathy and neuropathy [7, 8, 19]. However, a number of recent reports have indicated a trend towards progression of diabetic retinopathy following bariatric surgery, and this presents a clinical challenge for bariatric surgeons [12, 13, 16].
Studies have shown long-term, optimal diabetic control is correlated with improved outcomes in diabetic retinopathy [20, 21]. After bariatric surgery, the correction of weight loss outcomes, hemoglobin A1c (HbA1c) values, and subsequent tight glucose control is rapid and the majority of patients achieve a normal, diabetic-free physiologic state quickly after surgery, leading to potential complications such as DR progression [22]. Similarly, other aggressive forms of glycemic control including glucagon-like peptide-1 (GLP-1) that rapidly lower blood glucose levels have also been associated with progression of DR in the short term [23]. Interestingly, continued treatment with GLP-1 beyond 2 years has been reported to reverse this effect, but no similar studies have been reported for bariatric surgery [24].
This initial worsening of diabetic retinopathy after rapid improvement of blood glucose control is well reported [25, 26]. Possible explanations for this worsening include a proposed “point of no return” that may be achieved early in the course of retinopathy, after which time disease progression is no longer influenced by a return to a euglycemic state [25, 27]. Another explanation for disease progression in patients with preexisting diabetic retinopathy could be due to a baseline deficiency in retinal perfusion coupled with the reduced nutrient substrate concentration delivered to retinal tissue once glucose levels are normalized [28]. Kowluru found that the initiation of euglycemia soon after induction of diabetes in rats reversed the increases in both retinal oxidative and nitrative stress [29]. However, Kowluru found that if the initiation of good glycemic control was delayed for 2 months or more, there was only a partial or no (>6 months) beneficial effect at reducing oxidative and nitrative stress [29]. The findings suggested that retinal proteins are oxidated or nitrosylated early (<2 months) in the disease course and are resistant to reversal even after a short duration of hyperglycemia, which may contribute to retinopathy progression after reinstitution of euglycemia [29]. These findings may explain the progression of diabetic retinopathy seen after bariatric surgery.
At this point, our systematic review and meta-analysis demonstrates that the effect of this rapid correction of the glycemia in diabetic retinopathy is still inconclusive. Although preexisting DR (versus no preexisting DR) appears to be a significant risk factor for progressive DR following bariatric surgery (odds ratio 2.77, CI 1.10–6.99, p = 0.03), a patient with preoperative disease is still likely to have an equal rate of improvement versus progression of their disease postoperatively in the short term (23.5 vs. 19.2 %, respectively). This hampers our ability to definitively recommend that patients with existing DR avoid or pursue bariatric surgery as management of their disease. Comparatively, patients with no preoperative DR had a minimal rate of progression to DR (7.5 ± 7.4 %) and will likely benefit from bariatric surgery.
Our recommendation is that the bariatric surgeon consider the following: (1) prudent identification of DR as a possible risk factor for progression and counsel patients appropriately, (2) consult ophthalmology and endocrinology in the assessment and presence of pre-operative DR; (3) ensure appropriate postoperative follow-up in a patient with DR; as well as prior established recommendations that surgeons, (4) involve allied health-care professionals including a dietician, and (5) perform operations in a tertiary care center such that complications can be limited and managed quickly and appropriately [30].
Clinically, the identification of the “ideal” bariatric surgery patient will be the most significant factor in order to stratify patients into low-, medium-, and high-risk groups based on their preoperative risk. Given the results from our systematic review and meta-analysis, the presence of and degree of pathology of DR should not be a standalone indication in favor of or against bariatric surgery. However, patients should be counseled that the complications of bariatric surgery could include adverse changes and paradoxical progression of their diabetic retinopathy despite resolution of the underlying disease process. In particular, one study reported that in cases of more severe DR, progression of DR was made increasingly likely; however, our study was unable to further quantify this risk due to the heterogeneity of primary studies [16]. In contrast, patients who have previously mild DR or no preexisting DR seem to demonstrate either no change or improvement following bariatric surgery. This suggests that this patient population with mild early stage disease has a greater ability to compensate for aggressive glycemic control. Consequently, bariatric surgery may be most beneficial early in the disease process, prior to any evidence of end-organ disease resulting from chronic hyperglycemia, to achieve the goal of diabetic remission without excess surgical risk of DR. Ultimately, counseling the diabetic patient will need to account for individual patient preference: on the one hand, bariatric surgery is an effective option in the management of the morbidly obese and its complications; however, the paradoxical progression of DR and its effect on patient quality of life is worrying.
Future Research
Bariatric surgery continues to be an exciting field with many research possibilities. Looking forward, it will be important to investigate further subgroup analysis including stratification according to the type of surgery performed and correlation with other patient risk factors for disease such as pre-op body mass index, hypertension, and hyperlipidemia. Identifying these possible predictors of DR progression would be especially valuable to the bariatric surgeon in counseling prospective patients. In particular, Miras et al. reported minimal progression of DR in their patients (one case, n = 67) indicating that their experience with bariatric surgery (primarily Roux-en-Y gastric bypass operations) may not necessarily accompany DR progression [15]. While we suspect that staggering two-step procedures may demonstrate even fewer adverse DR changes, further subgroup analysis by procedure will be required to correlate this hypothesis. Unfortunately, the current primary studies are insufficiently detailed and precludes further analysis of these risk factors at this time.
Furthermore, it will be interesting to extend the follow-up period of DR following bariatric surgery. Once more long-term studies are available, data regarding the long-term progression or regression after the transient short-term effects on DR can be studied to properly inform patients of the risks and benefits of bariatric surgery. In an analogous case, although aggressive glycemic control with GLP-1 therapy has been correlated with short-term DR progression, continued treatment has been reported to reverse this phenomenon in the long term [24]. Whether similar promising results can be achieved in bariatric surgery will require further studies.
Similarly, neoadjuvant and adjuvant dietary, exercise, and lifestyle recommendations will need to be studied to assess their impact on modulating the drastic postoperative drop in glycemic levels, and subsequently, DR progression. However, at this time, the evidence is inconclusive to use DR as an independent risk factor in the consideration of bariatric surgery in the morbidly obese patient.
Limitations of the Study
Our systematic review was limited by the quality and quantity of primary studies within this field. In aggregate, the existing studies enrolled a limited number of patients (n = 148) within a limited amount of primary studies (4). It therefore restricts the conclusions of our systematic review. No randomized controlled trials were done. The studies were heterogeneous with different follow-up intervals, total length of follow-up, and methods of reporting outcomes. At this time, there is relatively limited long-term data on DR after bariatric surgery.
Conclusion
To our knowledge, we are the first to systematically review the literature for the effect of bariatric surgery on diabetic retinopathy. Pooled data suggests that patients with a diagnosis of DR prior to surgery are at increased risk of further progression in their disease compared to those without preexisting DR (OR 2.77); however, the rate of progression remains similar to the rate of improvement in the retinopathy (23.5 vs. 19.2 % respectively). In those without preoperative disease, progression of DR was slight (7.5 %) and represents minimal risk for the patient undergoing bariatric surgery without preexisting disease. Patients should receive adequate counseling and evaluation prior to undergoing a surgical procedure, in particular those with preexisting DR. Moving forward, further long-term data will be necessary to guide the clinically correlation of diabetic retinopathy as a risk factor for complication following bariatric surgery.
References
Pi-Sunyer FX. The medical risks of obesity. Obes Surg. 2002;12 Suppl 1:6S–11.
Ghoorah K, et al. Obesity and cardiovascular outcomes: a review. Eur Heart J Acute Cardiovasc Care. 2014: p. [Epub ahead of print].
Petrovic MA et al. Correlation between metabolic controls and changes in retina in patients having diabetes. Med Pregl. 2014;67(1–2):49–54.
Gray SP, Jandeleit-Dahm K. The pathobiology of diabetic vascular complications–cardiovascular and kidney disease. J Mol Med (Berl). 2014;92(5):441–52.
Gloy VL et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934.
Lannoo M, Dillemans B. Laparoscopy for primary and secondary bariatric procedures. Best Pract Res Clin Gastroenterol. 2014;28(1):159–73.
Aminian A et al. Risk prediction of complications of metabolic syndrome before and 6 years after gastric bypass. Surg Obes Relat Dis. 2014;10(4):576–82.
Brethauer SA et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258(4):628–36. discussion 636–7.
Heneghan HM et al. Effects of bariatric surgery on diabetic nephropathy after 5 years of follow-up. Surg Obes Relat Dis. 2013;9(1):7–14.
Johnson BL et al. Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg. 2013;216(4):545–56. discussion 556–8.
Leong WB, Taheri S. The role of bariatric surgery in the treatment of type 2 diabetes mellitus. J R Coll Physicians Edinb. 2012;42(3):194–8.
Silva RA, Morton JM, Moshfeghi DM. Severe worsening of diabetic retinopathy following bariatric surgery. Ophthalmic Surg Laser Imag Retina. 2013;44 Online(6): p. E11-4.
Mohite AAF, Shah S, Miras PR, et al. The effects of obesity surgery on diabetic retinopathy. Appetite. 2012;58(3):1171.
Zhang X, et al. Diabetic macular edema: new concepts in patho-physiology and treatment. Cell Biosci. 2014;4(27): p. [Epub ahead of print].
Miras AD et al. Bariatric surgery does not exacerbate and may be beneficial for the microvascular complications of type 2 diabetes. Diabetes Care. 2012;35(12):e81.
Thomas RL et al. Does bariatric surgery adversely impact on diabetic retinopathy in persons with morbid obesity and type 2 diabetes? A pilot study. J Diabetes Complications. 2014;28(2):191–5.
Varadhan L et al. Bariatric surgery and diabetic retinopathy: a pilot analysis. Obes Surg. 2012;22(3):515–6.
Leong WBTS. The progression of diabetic retinopathy post bariatric surgery. Diabetes. 2013;2013(62):A159.
Ros Ruiz S. Diabetic nephropathy: changes after diabetes surgery? Nutr Hosp. 2013;28 Suppl 2:57–65.
Hellgren KJ, Agardh E, Bengtsson B. Progression of early retinal dysfunction in diabetes over time: results of a long-term prospective clinical study. Diabetes. 2014;63(9):3104–11.
Fante RJ, Gardner TW, Sundstrom JM. Current and future management of diabetic retinopathy: a personalized evidence-based approach. Diab Manag (Lond). 2013;3(6):481–94.
Salehi M, D’Alessio DA. Effects of glucagon like peptide-1 to mediate glycemic effects of weight loss surgery. Rev Endocr Metab Disord. 2014;15(3):171–9.
Varadhan L et al. GLP-1 agonist treatment: implications for diabetic retinopathy screening. Diabetes Res Clin Pract. 2011;94(3):e68–71.
Varadhan L et al. The impact of improved glycaemic control with GLP-1 receptor agonist therapy on diabetic retinopathy. Diabetes Res Clin Pract. 2014;103(3):e37–9.
Dahl-Jorgensen K et al. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br Med J (Clin Res Ed). 1985;290(6471):811–5.
Wang PH, Lau J, Chalmers TC. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet. 1993;341(8856):1306–9.
Lauritzen T et al. Effect of 1 year of near-normal blood glucose levels on retinopathy in insulin-dependent diabetics. Lancet. 1983;1(8318):200–4.
Parving HH et al. Hemodynamic factors in the genesis of diabetic microangiopathy. Metabolism. 1983;32(9):943–9.
Kowluru RA. Effect of reinstitution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes. 2003;52(3):818–23.
Stefanidis D et al. Revisional bariatric surgery: perioperative morbidity is determined by type of procedure. Surg Endosc. 2013;27(12):4504–10.
Conflict of Interest
Douglas Cheung, Noah Switzer, David Ehmann, Christopher Rudnisky, and Xinzhe Shi have no disclosures to report. Shahzeer Karmali is a consultant for Ethicon and Covidien.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheung, D., Switzer, N.J., Ehmann, D. et al. The Impact of Bariatric Surgery on Diabetic Retinopathy: A Systematic Review and Meta-Analysis. OBES SURG 25, 1604–1609 (2015). https://doi.org/10.1007/s11695-014-1539-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1539-9