Abstract
Background
Bariatric surgery (BS) procedures are increasing but few studies have investigated their influence on medical management and costs in France.
Methods
The “Echantillon Généraliste des Beneficiaires” (EGB) is a 1/97 representative sample (n = 520,000 in 2011) of a national claims database covering about 80 % of the population. Adult patients treated for the first time with BS from January 2007 to December 2009 were identified, and a cohort including 350 patients was constituted with a 2-year follow-up before and after this primary procedure date (T). All items of reimbursed medical consumption and comorbidities over this period were identified. A comparison on the consumed resources and costs of BS was performed over time using multivariate models.
Results
The annual per capita reimbursed health expenses evolved from 2633 € (±3124) in year (T − 2) to 3557 € (±3380) in (T − 1), to 4240 € (±3840) in (T + 1) (excluding procedure cost), and to 3755 € (±5037) in (T + 2) with differences according to the type of surgery. In 39 % of patients, the evolution of those costs between (T − 2) and (T + 2) decreased by 5 %. In multivariate models, the significant factors were the presence of diabetes or hypertension medications before the procedure. Most items of medical consumption increased over the period pre- and post-procedure and started to decrease in (T + 2).
Conclusions
Although this series contains mostly gastric bandings, which were less likely to affect comorbidities, the workup for preparing BS was probably an opportunity to benefit from a general clinical assessment which has generated extra short-term medical consumption and expenses began decreasing without allowing return on investment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery (BS) is a treatment for obesity that has demonstrated healthcare benefits [1–3] and survival improvement over time [4]. Few studies have investigated the impact of BS on medical management before and after surgery. The number of BS procedures performed in France has been rapidly increasing over the last 5 years with more than 30,000 patients treated in 2011 and a growth rate of 16 % per year (http://www.ameli.fr/fileadmin/user_upload/documents/cnamts_rapport_charges_produits_2014.pdf) [5]. The increase of the obesity rate among the population is far from being the main explanation of this trend. The 2012 Obepi national survey [6] estimated obesity prevalence at 15 % of the French population (nearly 7 million people) of which 3.1 % had a body mass index (BMI) in the range 35–39.9 and 1.2 % above 40 kg/m2. During this period, the number of bariatric procedures has increased dramatically. According to current French guidelines (http://www.has-sante.fr/portail/upload/docs/application/pdf/2011-10/reco2clics_obesite_adulte_chirurgie.pdf), BS may be considered as a second-line treatment after 6 months of a medical management in patients with a BMI ≥40 or ≥35 kg/m2 with associated comorbidities. The choice of use of BS techniques has rapidly evolved over the last decade. The reversible gastric banding (GB) technique was representing only 25 % of procedures in 2011, whereas other new techniques such as gastric bypass (GBP) and especially sleeve gastrectomy (SG) were expanding rapidly (+65 % per year over the period 2005–2011 for SG).
Recently, a representative sample of a national claims database [7] was made available for researchers by the main French National Sickness Fund (“Caisse Nationale d’Assurance Maladie des travailleurs Salariés,” CNAMTS) covering about 80 % of the whole population of all ages. It gives access to a large representative cohort from 2003 onwards.
The aim of this study was to investigate in this database the 2-year impact before and after BS on medical management and healthcare costs of obese patients according to the type of surgical technique.
Materials and Methods
The EGB Database
The “Echantillon Généraliste des Beneficiaires” (EGB) database, is a permanent random 1/97 sample of the French statutory healthcare insurance system database covering a population of approximately 50 million affiliates, which is regularly updated. The EGB includes basic demographic data and has prospectively collected reimbursed medical expenses since 2003, except for acute care hospitalizations that were only collected from 2005 onwards. Up to 2011, the EGB was restricted to the population covered by the main National Scheme (CNAMTS) composed of salaried workers and their relatives representing approximately 80 % of the whole French population (self-employed and individuals working in some other specific activities are covered by other schemes).
Available Data
The EGB contains data on all reimbursed services, procedures (coded according to a reference list “Classification Commune des Actes Médicaux” or CCAM), drugs (CIP codes), and devices (coded according to the list “Liste des Produits et Prestations Remboursables”—LPPR) at whatever co-payment level, including dates of prescription, dispensing, and quantities dispensed. Information on hospitalizations is extracted from a specific diagnosis related groups (DRG) database used for funding hospital stays (PMSI). Data available include main and secondary diagnoses (ICD-10 codes), procedures performed during the stay, length of stay (total and in ICU), and expensive medications and medical devices used which are funded on top DRG tariffs.
To cover healthcare expenses that are not managed by the compulsory public scheme, individuals have the possibility of taking out supplementary insurance with a mutual fund or insurance company. Persons on low incomes are entitled to supplementary Universal Health Insurance Coverage (“CMU complémentaire” or CMUc), which covers all costs. This last information is the only one available in the database to qualify individuals with a low socioeconomic status.
Comorbidities
A list of 30 severe chronic diseases including, for example, diabetes and cardiovascular diseases has been designated by the healthcare insurance as “Affection de Longue Durée” (ALD), which provides eligibility for full coverage of all medical and pharmaceutical expenses related to the disease. General comorbidity was identified here as combining data from diagnosis recorded during acute care hospitalization in the DRG database and diagnosis belonging to the ALD list of diseases making patients eligible for full coverage. The EGB database also includes the date of death if any.
Obese Patient Identification
Obese patients were identified in the database as those hospitalized for BS. Only in that case, the DRG database used for reimbursement of acute care hospitalization requests a specific coding of patients’ BMI according to the following groups (E66 ICD-10 codes of obesity): 30–39.9, 40–49.9, and above 50 kg/m2. This coding was made compulsory as primary diagnosis by health authorities in order to allow monitoring the appropriateness of surgery regarding French clinical guidelines (http://www.has-sante.fr/portail/upload/docs/application/pdf/2011-10/reco2clics_obesite_adulte_chirurgie.pdf). Aside from this situation, the ICD-10 coding of obesity can be recorded as an associated diagnosis in case of hospitalization for any cause but at a physician’s discretion. In this context, constituting a control group of obese patients not having benefited from any BS was not attempted here due to potential biases.
Study Period
We used a “before and after” approach consisting in comparing patients’ utilization of healthcare over a 2-year period before and after the date of the primary BS from January 2007 to December 2009. The medical management of this population and its associated costs were described over four subsequent 1-year periods. The first 1-year period started 2 years before the BS date, the second one was the year before surgery, the third one was the year after surgery, and the last one was the year starting 1 year after surgery.
Selection Criteria for the Study Population
The following selection criteria were used to identify the study population: age ≥18 the day of surgery; present in the database 2 years before and after the date of surgery; with a primary BS procedure performed during the period 2007–2009 recorded with the following main ICD-10 diagnoses: E660 (obesity due to excess calories), E662 (extreme obesity with alveolar hypoventilation), E668 (other obesity), or E669 (obesity, unspecified).
To identify BS procedures, we used the two types of codes currently used in France within the DRG database for reimbursement either of procedures (CCAM) or of devices (LPP) in case of GB. Procedures consisting in changing or removing GB had specific codes (HFKC001, HFKA002, HFMC008, HFMA011, HFKA001, and HFMA002) and could then be used to identify non-primary BS procedures, and the corresponding patients were excluded from the analysis.
Patients were categorized according to the surgical technique: GB (codes HFMC007, HFMC005, HFMA006, and HFMA009), GBP (codes HFCC003 and HFCA001), SG (codes HFMC006, HFMA010, HFFC018, and HFFA011), and biliopancreatic diversion (BPD; codes HFFC004, HFFA001, HGCC027, and HGCA009).
Obesity-Associated Comorbidity
Five specific comorbidities were analyzed through the consumption of tracking drugs or devices: diabetes mellitus, dyslipidemia, hypertension, depression, and sleep apnea.
-
1.
Antidiabetics: A10 class “drugs used in diabetes”
-
2.
Statins or fibrates: C10AA class “HMG CoA reductase inhibitors” or C10BX class “HMG CoA reductase inhibitors, other combinations” or C10AB class “fibrates”
-
3.
Antihypertensive drugs: C02 class “antihypertensive” or C03 class “diuretics” or C07 class “beta-blocking agents” or C08 class “calcium channel blockers” or C09 class “agents acting on the rennin-angiotensin system”
-
4.
Antidepressants: N06A class “antidepressants”
-
5.
Sleep apnea: if patient had at least one hospitalization with an ICD-10 diagnosis Z991+1 (dependence on respirator: nasal ventilator) or Z991+8 (dependence on respirator: other procedures) or at least one medical device recorded for sleep apnea.
Economic Analysis
The perspective of costing was societal but restricted to direct costs. All items of healthcare consumption eligible for reimbursement and associated costs were assessed for each study period at current prices. These costs comprised the reimbursed part as well as the co-payment. This co-payment varies according to the item of consumption and severity of condition and is generally partly covered by supplemental private insurance. Inpatient care costs were derived from the DRG coding currently used for acute care hospital funding. Full unit costs per DRG were used for cost estimation. Those values were issued from the 2010 “Etude Nationale de Coûts à méthodologie Commune” (ENCC cost study), which is used as the reference for hospital tariff definition in France (http://www.atih.sante.fr/?id=000370000AFF). All consumptions of services, devices, or drugs not eligible for reimbursements by public insurance (OTC drugs, for example) are not recorded in the database, and costs could thus not be estimated.
Statistical Analysis
Data management and statistical analysis were carried out using SAS® V9.2 (NC, USA) software. Analysis was based on the defined patient groups according to the nature of BS. Student’s t test, chi2 test, or Fisher’s exact test was performed according to the type of variable. A p value below 0.05 was considered significant.
We modeled the trends in healthcare expenses per patient by ATC classes and comorbidity drug-derived markers using generalized estimating equation (GEE) models in order to take into account intrasubject correlations. Statistical significance of time trends was determined based on fractional polynomial models of the time trends across the four periods. A multivariate analysis was also performed (generalized linear model) to determine factors linked with the evolution of the mean total healthcare expenses per patient between −2 and −1-year periods. The following explanatory variables were added to the model: age, gender, BMI, year of surgery, presence of severe comorbidity, low economic status, and planned BS technique. A reference group (most frequent characteristics of patient) was defined as female patients, less than 30 in age, with a BMI of 30–40, with no severe comorbidity, and with a planned GB surgery. In this group, the mean per patient increase in healthcare expenditures before surgery corresponds to the intercept of the model.
Results
Description of the Study Population
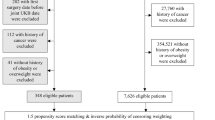
A total number of 667 procedures were first identified over the inclusion period (January 1, 2007, to December 31, 2009) corresponding to 591 adult patients. Further exclusions concerned 180 patients readmitted for gastric bypass or balloon change or removal, 54 patients covered by other insurance schemes during the whole study period, and 7 patients who had previous procedures in the years 2005 and 2006. The final population sample comprised 350 evaluable patients (Fig. 1).
Among the 350 obese patients who underwent primary BS over the period 2007–2009, 62.5 % had a GB, 20 % a GBP, 16.5 % a SG, and only 1 % (n = 4) a biliopancreatic diversion (BPD). Globally, 83.4 % of the population was female. Patients were aged in the range 18–66 years with a mean of 38.9 years (±11.3). The BMI was between 30 and 40 kg/m2 in 27.4 %, between 40 and 50 kg/m2 in 69.7 %, and over 50 kg/m2 in 2.9 % of patients. Female patients were younger (37.9 ± 11.0 years) than male patients (44.0 ± 11.7 years) and were more frequently benefitting from CMUc between 2005 and 2011 (30.1 versus 20.7 %), indicating a lower economic status.
The characteristics of the subgroups according to the type of surgery are described in Table 1. The percentage of women was slightly lower among patients with a GBP (76.8 %), but the difference did not reach statistical significance (p = 0.30). Patients who benefitted from a SG or a BPD were 43 years old, so about 4 or 5 years older than patients with a GB (37 years) or patients with a GBP (40 years) (p = 0.002). No statistical difference in the proportion of BMI categories was found between the four bariatric surgery groups (p = 0.20). The proportion of patients with full reimbursement of expenses (because of presence of any severe comorbidity) was higher, even if borderline significant, in the GB (22.4 %) and the GBP (24.6 %) groups and lower in the SG group of patients (15.5 %) (p = 0.05). The frequencies of comorbidities were as follows: 0.6 % of patients had HIV, 1.4 % rheumatoid arthritis, 3.1 % cancers, 9.4 % psychiatric disorders, 9.7 % asthma and respiratory diseases, 13.1 % diabetes, and 18.5 % hypertension.
Characteristics of Hospitalization for Primary Procedures
The comparison of mean in-hospital length of stay (LOS) for the primary procedure (Table 1) indicates marked differences according to the techniques from 3.1 days in average for GB to 8.2 days for SG. The median full hospital costs were in the range 3568–6384 € according to the techniques with the highest value observed for GB. These costs were derived from the various DRGs used to cover the hospital stay including procedure as well as medical fees and overhead.
Healthcare Consumption Over Time: Physician Visits and Specific Medication Uptake
We analyzed the percentage of patients who had at least one visit to physicians of different specialties each year of the study period (users) and the mean annual number of visits per patient (Table 2). The number of GP visits slightly increased over time from the first year pre-surgery and then remained steady. Two years pre-surgery, a low proportion of patients were consulting cardiologists, diabetologists, gastroenterologists, and psychiatrists, whereas in the year pre-surgery, those proportions increased dramatically. After surgery, the frequencies of specialist visits were decreasing.
In the whole population, the mean annual specific expenses per patient were decreasing significantly over time for antidiabetics and antihypertensive drugs and remained steady for statins and antidepressants whereas an increase was observed for sleep apnea treatment, although none reaching statistical significance, due to the low numbers of patients involved (Fig. 2). These results reflected mostly the proportions of patients using the corresponding drugs or devices.
These trends were different according to the BS techniques. In the most numerous group of patients, i.e., treated by GB, the only significant modification in proportion of users concerned sleep apnea where it almost doubled from 4.6 to 7.8 % over the 4-year period. The other proportions of users remained steady over time (7–8 % for antidiabetics, 8–10 % for statins/fibrates, 18 % for antihypertensive drugs, and 16 % for antidepressants). The reduction of antidiabetics and antihypertensive drug use was driven by the subgroups of patients with SG and GBP (Table 3). In these two groups, the drops in the proportion of drug users were significant with an absolute reduction of a magnitude of 10 % before and after surgery. Concerning sleep apnea, the data suggested that this condition was only identified and treated in a large proportion of patients at the occasion of the pre-surgery workup, with a doubling of the number of patients treated. Then, a moderate decline of users was observed post-surgery.
Overall Healthcare Cost Evolution
Figure 3 describes the evolution of overall healthcare costs in the whole study population. The medical costs increased markedly before surgery during the second year as compared to the first. This increase was observed in all cost categories, inpatient as well as outpatient cares. Immediately after surgery, costs continued to increase but were mainly driven by outpatient care with a small decrease in inpatient care costs (excluding primary surgery). In the second year post-surgery, costs were decreasing and this was driven by the lower outpatient care costs and by a small increase in inpatient care costs. The mean cost associated with readmissions related to BS complications or failures (192 €) was not sufficient to explain this last increase. These evolutions were similar in all categories of surgery (Table 4).
Healthcare Expense Increase Pre-surgery
In multivariate analysis, age, gender, severe comorbidities, and type of surgery remained significantly related with healthcare expense increase (Table 5). A reference group chosen arbitrarily was defined as female patients, less than 30 in age, with a BMI of 30–40, with no severe comorbidity, and with a planned GB surgery. In this group, the mean per patient increase in healthcare expenditures before surgery was 1628 €.
Discussion
In this representative sample of obese patients referred for BS, healthcare resource consumption increased rapidly before surgery and decreased after surgery but remained at a higher level than before the BS procedure. This evolution of costs was observed whatever the type of surgery performed.
The pre-surgery workup generates multiple visits and procedures yielding to a substantial increase in healthcare expenses that may continue over the couple of years post-surgery for all conditions that have not been resolved or alleviated in the short term by the procedure. Multivariate analysis showed that pre-surgery cost increase was higher in patients operated by SG and GBP than in those operated by GB. Although the choice of surgical technique depends more often on surgeon’s experience and expertise than on scientific validation, GB is often proposed to younger patients with less comorbidity while SG and GBP have been proven to be more efficient in severely ill patients [8]. Looking at particular drug consumptions in diabetes and hypertension, declines of uptake appeared to be differentiated according to the type of surgery with significant results only for GBP and to a minor extent for SG. These data are in accordance with the literature as it is now widely accepted that GB has the lowest impact on weight loss and comorbidities in comparison with GBP and SG [3]. In our model, everything else being unchanged, the mean per patient healthcare expenditures before surgery would have been increased by 1827 € in case of SG, by 2007 € in the presence of a severe comorbidity, and by 1600 € for a patient >50 of age. Interestingly, those were decreased in patients with higher BMI. This may indicate that these patients were already well medically managed prior to 2 years before surgery. The variable indicating the existence of a low economic status became non-significant in the multivariate analysis, indicating its probable multiple correlations with the other variables analyzed. Due to the very low numbers of intragastric balloon placement and BPD, multivariate analyses did not take them into account.
French guidelines for BS have been issued in 2009 (http://www.has-sante.fr/portail/upload/docs/application/pdf/2011-10/reco2clics_obesite_adulte_chirurgie.pdf). They contain recommendations about the indications of BS in adult patients and the appropriate medical assessment and management before surgery. It is clear that the implementation of those recommendations translates into a series of visits to specialists, biological and clinical tests, and procedures that probably take place during the first year pre-surgery, leading to substantial increase of healthcare expenses per patient. This increase was higher in patients with GBP and SG that were more frequently performed at the end of our inclusion period. This finding may be interpreted in the perspective of a higher compliance with guidelines in general or consequently of the use of more invasive surgical procedures. However, our data were not precise enough as to check how French physicians complied with these recommendations.
Several economic studies were performed in other countries but with contrasting results. Most of them used also claims databases. In several studies, it was attempted to build control groups based on dedicated algorithms because of a lack of information on BMI. This probably indicates why other authors preferred to compare the expenses pre- and post-surgery. If some authors showed a significant decrease in healthcare expenses with a return on investment over 3 to 7 years [9–15], their results appeared to be highly dependent on the patients’ population selection, such as a selected group of diabetic or elderly patients, not representative samples (Table 6). Most studies were performed in the USA and included mostly patients complying with the Medicare recommendations. Operated patients presented with high levels of BMI, mainly over 50, and appeared to have a different profile as compared to our population. Other authors described an immediate increase in healthcare expenditures after surgery and a return to initial level in the long term [16–18]. All authors agreed that some medication uptakes were highly decreasing, especially for agents used in diabetes, dyslipidemia, and hypertension [19]. But these results were highly dependent on the mix of surgical techniques performed, on the time horizon, and on the characteristics of patients.
The principal strength of this study was the representativeness of the sample allowing extrapolation to the whole French healthcare system. Completeness in cost information was another advantage of the EGB database. The fact that we used specific drugs or devices as tracking variables for comorbidities led us to validate, in a representative real-life cohort study, the impact of BS on comorbidities such as diabetes and hypertension, especially with some types of surgery (GBP and SG). Moreover, the mix of procedures has drastically changed recently, with a vast majority of procedures being now GBP and SG. The methodological constraint of the before and after approach is to use data with a minimum of 2 years of follow-up post-surgery. In a situation of rapid change of practices, this method implies that the mix of surgical techniques observed is not reflecting the present situation. In fact, the landscape of bariatric surgery is changing, with GB now accounting for less than 25 % of cases. It is the reason why the analysis was performed according to each technique but remains representative when analyzed by type of surgery. At this stage, it may let us think that the impact of BS on comorbidities is today more important than in our sample of patients. But this needs to be confirmed with an update of this study on more recent data.
The lengths of stay are much longer in French acute care hospitals as compared to those in the USA. These results reflected not only the procedure itself but probably, in some cases, some pre-op workup and the management of early complications. Furthermore, although newly adopted in other countries, ambulatory bariatric procedures are still not routinely performed in France [20].
The weaknesses were mainly related to the limited sample size with only 350 patients and with the relative short duration of follow-up. Another limitation of the study was that BMI was only captured systematically in the French system in patients with BS, and therefore, selecting a control group appeared to be hazardous. Further, the cost assessment only went out for 2 years. However, at the time when the analysis was performed, the data used were the most recent ones available. Considering one extra year of follow-up would be possible but would change marginally the conclusion of our work if any. In terms of long-term follow-up, a significant duration would be 5 years. Finally, the impact of BS on weight loss and comorbidity resolution is well demonstrated [4]. However, in this 4-year French claims database, only 15–24 % of the patients undergoing BS (depending on the procedure) had major comorbidities. This fact would have led to artificially reduced global procedure effects.
Despite these limitations, our results are in line with published data collected over the same time period with the exception of a positive return on investment that has been observed rapidly in some of the publications. The clinical procedures and visits prior to BS were probably an opportunity for most patients to benefit from a general checkup that has generated extra short-term medical consumption.
Conclusion
In conclusion, the workup for preparing bariatric surgery was probably an opportunity, in a large fraction of those patients, to benefit from a general clinical assessment which has generated extra short-term medical consumption and expenses that began decreasing without allowing a return on investment. Other studies with longer-term follow-up will be needed to capture the long-term economic benefit of bariatric surgery.
References
Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308:1122–31.
Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–62.
Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934.
Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52.
Basdevant A, Paita M, Rodde-Dunet MH, et al. A nationwide survey on bariatric surgery in France: two years prospective follow-up. Obes Surg. 2007;17:39–44.
Charles MA, Eschwege E, Basdevant A. Monitoring the obesity epidemic in France: the Obepi surveys 1997–2006. Obesity (Silver Spring). 2008;16:2182–6.
Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286–90.
Chevallier JM, Paita M, Rodde-Dunet MH, et al. Predictive factors of outcome after gastric banding: a nationwide survey on the role of center activity and patients’ behavior. Ann Surg. 2007;246:1034–9.
Makary MA, Clark JM, Shore AD, et al. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery. Arch Surg. 2010;145:726–31.
Sampalis JS, Liberman M, Auger S, et al. The impact of weight reduction surgery on health-care costs in morbidly obese patients. Obes Surg. 2004;14:939–47.
Sussenbach SP, Padoin AV, Silva EN, et al. Economic benefits of bariatric surgery. Obes Surg. 2012;22:266–70.
Cremieux PY, Buchwald H, Shikora SA, et al. A study on the economic impact of bariatric surgery. Am J Manage Care. 2008;14:589–96.
Klein S, Ghosh A, Cremieux PY, et al. Economic impact of the clinical benefits of bariatric surgery in diabetes patients with BMI >/=35 kg/m(2). Obesity (Silver Spring). 2011;19:581–7.
Mullen DM, Marr TJ. Longitudinal cost experience for gastric bypass patients. Surg Obes Relat Dis. 2010;6:243–8.
Myers VH, McVay MA, Adams CE, et al. Actual medical and pharmacy costs for bariatric surgery: 6-year follow-up. South Med J. 2012;105:530–7.
Maciejewski ML, Livingston EH, Smith VA, et al. Health expenditures among high-risk patients after gastric bypass and matched controls. Arch Surg. 2012;147:633–40.
Neovius M, Narbro K, Keating C, et al. Health care use during 20 years following bariatric surgery. JAMA. 2012;308:1132–41.
Weiner JP, Goodwin SM, Chang HY, et al. Impact of bariatric surgery on health care costs of obese persons: a 6-year follow-up of surgical and comparison cohorts using health plan data. JAMA Surg. 2013;148:555–62.
Segal JB, Clark JM, Shore AD, et al. Prompt reduction in use of medications for comorbid conditions after bariatric surgery. Obes Surg. 2009;19:1646–56.
Kraft K, Mariette C, Sauvanet A, et al. Indications for ambulatory gastrointestinal and endocrine surgery in adults. J Visc Chir. 2011;148:69–74.
Funding
This study was supported by a grant from Johnson & Johnson to Cemka-Eval.
Conflict of Interest
The authors have declared no conflicts of interest.
Statement of Informed Consent
For this administrative anonymous claims database based study, informed consent was not required.
Statement of Human and Animal Rights
For this type of study, formal consent is not required.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Czernichow, S., Moszkowicz, D., Szwarcensztein, K. et al. Impact of Bariatric Surgery on the Medical Management and Costs of Obese Patients in France: an Analysis of a National Representative Claims Database. OBES SURG 25, 986–996 (2015). https://doi.org/10.1007/s11695-014-1488-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1488-3