Abstract
Environmental gradients in a marine setting may have significant effects on morphological variations and evolutionary patterns, including sexual dimorphism variations within and between fish populations. We analyzed sexual shape and size dimorphism in accordance with Rensch and Bergmann’s rules in five coastal populations of the gobiid Bathygobius soporator along 4000 km of the Brazilian coastline. The populations differ significantly in sexual body shape dimorphism, with a tendency toward reduced intrapopulation dimorphism, increasing with latitude. Body size variation was significant between populations and population vs. sex, and inverse to Bergmann’s rule. Moreover, size dimorphism among populations of B. soporator does not follow Rensch’s rule. These data represent a rare example of inter and intrapopulation spatial variation in sexual dimorphism associated with latitude in marine fish. This suggests a complex and particularized scenario of biotic and abiotic interactions acting on local populations of B. soporator in extensive coastal areas of the Western Atlantic, with profound implications for species evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual dimorphism (SD) awakened the interest of Darwin, who, in 1871, considered it a result of sexual selection affecting differential mating success. Sexual selection is divided into two complementary processes, namely intrasexual selection, where same-sex individuals compete in different ways for access to the opposite sex, and intersexual or epigamic selection, involving the differential selection of members of the opposite sex (Darwin 1871; Clutton-Brock 2009; Fairbairn 2013). Sexual dimorphism is widely known in the animal kingdom (Shine 1989; Fairbairn 2013), the most commonly analyzed forms being behavioral, alimentary and morphological dimorphism, especially differences in size, appendages, tegumentum, color and body shape (e.g. Stuart-Fox and Ord 2004; Fairbairn et al. 2007; Fairbairn 2013).
Existing morphological variations between the sexes may correspond to the evolution of traits that influence mating success, increasing the mating chances of one or both sexes (sexual selection). The physical and ecological properties of the environment may also drive the evolution of dimorphism (natural selection). A set of environmental conditions during the initial postnatal development of males and females may prompt an increase or decrease in dimorphism in the adult phase (Badyaev 2011). Sensory biases in light conditions, food aspects and predation may also trigger a range of selective pressures in different types of SD (Tsuboi et al. 2012). Despite the vast repertoire of SD cases, whether morphological, behavioral or alimentary, little is known about interpopulation variation in sexual dimorphism (Bidau and Martí 2008; Lengkeek et al. 2008; Kitano et al. 2012).
Sexual selection is a powerful evolutionary force that can be in the opposite direction of natural selection. Knowing how the different sexual selection mechanisms act within variable environments is crucial to understanding evolutionary processes (Walsh and Reznick 2009; Ingleby et al. 2010). Morphological analyses in populations of different taxonomic groups commonly demonstrate a variation in dimorphism magnitude, based on body size and as a function of environmental conditions (Fernández-Montraveta and Moya-Larano 2007; Stillwell and Fox 2007; Karl and Fischer 2008; Bidau et al. 2012).
A number of ecogeographic rules describe macroscale patterns of body variation that may interfere in the expression of sexual dimorphism. The most charismatic of them, Bergmann’s rule (Bergmann 1847; Bidau 2014), states that organisms at higher altitudes or latitudes tend to be larger than those at lower ones. Despite having been intensively tested in endo- and ectotherms (Shelomi 2012; Gohli and Voje 2016), its association with sexual dimorphism has been largely neglected. Intraspecific patterns found clines most frequently than those examining interespecific patterns. In fact, the variation among species within the clades frequently renders interspecific studies unhelpful (Shelomi 2012). Thus, with reduced phylogenetic effects, intraspecific studies reveal the action of environmental conditions on a same gene pool.
The difference in body size between males and females, denominated sexual size dimorphism (SSD), led to the proposal of a presumably widespread pattern known as Rensch’s rule (Rensch 1950). This rule states that in interspecies comparisons, SSD increases with body size when males are larger than females, but declines with an increase in body size when females are larger. Despite evidence of Rensch’s rule in some comparative studies of insects, reptiles, birds and mammals (Blanckenhorn et al. 2006; Raihani et al. 2006), no evidence of the pattern was found in several groups (e.g., Bidau et al. 2013; Martinez and Bidau 2014; Martinez et al. 2014; Bidau and Martinez 2016) and converse patterns are not uncommon (Webb and Freckleton 2007). Furthermore, fish are under-represented in comparative studies of SSD, Rensch’s rule (Lengkeek et al. 2008) and sexual shape dimorphism (SShD), despite the known variability of SSD in this group (Parker 1992). Thus, reflecting their evolutionary importance, there is a growing number of studies on fish involving population differences in SShD (Langerhans et al. 2004; Hendry et al. 2006), the relationship between SSD, SShD and latitudinal variation (Kelly et al. 2013) and intraspecific tests of Rensch’s rule (Lengkeek et al. 2008; Herczeg et al. 2010).
A number of marine fish species are particularly favorable models to analyze the action of evolutionary forces. One of these is the gobiid Bathygobius soporator, which exhibits traits closely associated with local adaptive processes. This species, considered sedentary benthic and coastal residents (Demartini 1999), displays wide geographic distribution that extends from Southeastern USA to Southern Brazil (Lima-Filho et al. 2016), inhabiting tide pools in the intertidal zone (Gibson and Yoshiyama 1999). Population analyses have demonstrated that this species exhibits different morphological patterns across a north–south geographic distribution on the Brazilian coast (Lima-Filho et al. 2012).
The morphometric traits observed in B. soporator along the Brazilian coast make it particularly favorable for the analysis of sexual dimorphism variation patterns among widely distributed populations, and the investigation of ecological and evolutionary factors that act on SD differentiation in marine fish. Thus, analyses of intra and interpopulation SD variation based on body shape (SShD) and size (SSD) were conducted in five populations of B. soporator, distributed along the Brazilian coast, using traditional and geometric morphometrics techniques.
Materials and Methods
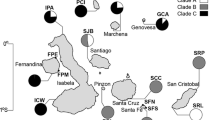
A total of 321 injury-free Bathygobius soporator (Valenciennes 1837) adults from the northeastern, southeastern and southern coasts of Brazil were used in morphometric analyses. On the northeastern coast, sampling points included the states of Maranhão (MA) (2°27′50.89ʺS, 44°12′18.58ʺW; N = 58; ♀ 34; ♂ 24), Rio Grande do Norte (RN) (6°0′31.12ʺS, 35° 6′17.86ʺW; N = 72; ♀ 39; ♂ 33), and Bahia (BA) (13°0′37.29ʺS, 38°31′28.32ʺW; N = 61; ♀ 33; ♂ 28); in the southeastern, the coast of São Paulo (SP) (23°59′44.88ʺS, 46°15′2.89ʺW; N = 71; ♀ 31; ♂ 40); and on the southern coast, samples were obtained in Santa Catarina (SC) state (27°34′21.14ʺS, 48°25′16.10ʺW; N = 59; ♀ 35; ♂ 24), (Fig. 1a). The sex of each specimen was determined by observing the urogenital papilla (Miller 1961) and analyzing fresh gonad fragments between the slide and coverslip, under optical microscope at ×200 magnification.
Sexual Shape Dimorphism (SShD)
Geometric morphometrics analyses were used to identify sexual shape dimorphism (SShD), based on the configuration of anatomical points, with a reduction in the effects inherent to size (Monteiro et al. 2002; Parsons et al. 2003). Their effectiveness in discriminating shape in B. soporator has been demonstrated in population studies and recognition of cryptic species in this genus (Lima-Filho et al. 2010, 2012, 2016).
The individuals were photographed in the left lateral view alongside a ruler graduated in millimeters, using a Sony H10, 8.1 pixel camera connected to a tripod, at standard distance and position (250 mm), without the use of optical or digital zoom. A total of 10 landmarks were selected and digitized onto the images using TPSdig v2.16 software (Rohlf 2006) (Fig. 1b). The images were arranged in a single TPS file using tpsUtil software (Rohlf 2013).
Landmark configurations were submitted to Generalized Procrustes Analysis (GPA) (Dryden and Mardia 1998), which collects shape information not related to variations due to specimen position, size and rotation (Rohlf and Slice 1990). Allometric correction, according to procedures proposed by Sidlauskas et al. (2011) and Alencar et al. (2014), was used to compare intra and interpopulation shape variations, controlling the effects of static and ontogenetic allometry. To that end, the residuals of a multivariate regression of Procrustes shape coordinates on size (centroid size) were used for subsequent statistical analyses and assessment of shape variation. The definition of static and ontogenetic allometry used in this study was based on Cock (1966). Thus, ontogenetic allometry refers to size as a result of covariation between body parts during the growth of a specimen, while static allometry refers to size as a result of the variation of individuals in the same population and age group.
Numerical residual data generated (with no static or ontogenetic allometry effect) were analyzed applying Canonic Variable Analysis (CVA) and Discriminant Function Analysis (DFA). The former was used to obtain a general view of sex vs. population variations. The first canonic variable (greater data variation in an ordination) was employed to obtain warped outlines in order to clearly identify the displacement vectors of deformation grids between sexes within their respective populations, whereas DFA was used to generate shape variations between males and females. Moreover, Procrustes (Proc) and Mahalanobis distance (D 2) values obtained in comparisons of DFA body shape between sexes were analyzed. Further investigations on SShD were conducted with Procrustes distances. The Procrustes distance was favored over the Mahalanobis distance because the former accounts for the absolute variation in shape, while the latter explains the relative variation in shape (Klingenberg and Monteiro 2005). Shape variations obtained from CVA and DFA were visualized using outline drawing, whose alternative representation of body shape facilitates interpretation of shape changes based on consensual shape (average shape) (Alencar et al. 2014).
Generalized Procrustes Analysis, multivariate regression, CVA and DFA multivariate analyses and outline drawing were conducted using MorphoJ 1.02b® software (Klingenberg 2011).
Sexual Size Dimorphism (SSD) and Rensch’s Rule
Initially, standard length (SL) and height (H) data were obtained from the digital images (Fig. 1c) using TPSdig v2.16 software (Rohlf 2006), logarithmized (log10) and tested for normality and homoscedasticity, respectively, applying the Shapiro–Wilk (Shapiro and Wilk 1965) and Levene tests (1960). Next, SSD was calculated as the logarithmized difference between mean male and female size (Smith 1999) for SL and H (Table 1). Rensch’s rule was tested by applying the Reduced Major Axis (RMA) regression method with logarithmized population means of female size minus male size for SL and H. The RMA technique (type II regression; Sokal and Rohlf 1985) was adjusted because common linear regression (type I regression) fits data to a straight line using the minimum squares method of residues in the direction of the independent variable (X), whereas the dependent variable (Y) is conditioned, that is, its effect is fixed (Kendall and Stuart 1973; Sokal and Rohlf 1985). According to Warton et al. (2006), the RMA regression method is based on the calculation of a standardized major axis, equivalent to the first major component, which minimizes the sum of squares of the shortest distances of the samples to the axis. An important factor in selecting the RMA method is the potential presence of an error in both morphometric variables (Warton et al. 2006) meaning that a single fitting line defines a symmetric relationship between the variables (Smith 2009). Furthermore, the null hypothesis of the slope line =1 (sexual isometry) was tested by calculating 95% confidence intervals. Finally, analysis of variance (ANOVA) was applied for SL and H, separately, and the Tukey–Kramer test as a posteriori verification of whether there were any size differences in relation to sex and population.
Finally, logarithmized Procrustes distance (from SShD assessed in each population) was also type II regressed with latitude data (logarithmized decimal degrees) from each sample location to evaluate the latitudinal inference on sexual shape dimorphism. The null hypothesis that regression slope =0 was also tested by calculating 95% confidence intervals.
Analyses were carried out using R software (R Development Core Team 2012) and the “smatr” package for RMA analyses (Warton et al. 2012). A 5% significance level was adopted for all tests (Zar 2010).
Results
Sexual Shape Dimorphism (SShD)
Geometric morphometry revealed the highest contributions in intrapopulation and interpopulation sexual differentiation for canonical variables 1, 2 (Fig. 2), 3, 4 and 5. These variables jointly corresponding to 94.29% of total shape variation.
Distribution of morphometric data of B. soporator in CV1 and CV2 (a) for populations from MA (open square ♂; Filled square ♀), RN (open circle ♂; filled circle ♀), BA (open triangle ♂; filled triangle ♀), SP (open plus ♂; filled plus ♀) and SC (open star ♂; filled star ♀). The larger symbols indicate the morphometric mean of males (hollow symbols) and females (solid symbols). Taken together, CV1 = 37.39% and CV2 = 20.75% account for the shape variation obtained (58.15%). Warped outlines generated from CV1 among males (black line) and females (grey line) of each population
Intrapopulation and interpopulation comparison between sexes, by quantifying morphological variation using the Procrustes distance, indicated greater morphological distinction between the sexes of the MA population (Proc = 0.0260, D 2 = 3.13), located in the north at a lower latitude, with a tendency to a progressive decline in SShD at higher latitudes (SC, Proc = 0.0124, D 2 = 1.95). The RMA regression of SShD and latitude showed a significant negative correlation, indicating that sexual dimorphism is less pronounced at higher latitudes (Fig. 3).
Latitudinal inference on sexual shape dimorphism (SShD) in Bathygobius soporator populations based on Procrustes distance. R2—RMA Regression coefficient; pRMA—p-value for tests against the hypothesis of RMA slope = 0; b = slope of line; p (b = 1)—p-value tests against the hypothesis of RMA slope = 1. Populations: MA—Maranhão, RN—Rio Grande do Norte, BA—Bahia, SP—São Paulo, SC—Santa Catarina
The comparison of same-sex individuals between neighboring populations exhibited greater morphological variation among males (MA-RN, Proc = 0.0368, D 2 = 4.40; RN-BA, Proc = 0.0405, D 2 = 3.63; BA-SP, Proc = 0.0191, D 2 = 3.10; SP-SC, Proc = 0.0300, D 2 = 3.08). For females, the variation among adjacent populations was less than that observed for males, but also significant (MA-RN, Proc = 0.0363, D 2 = 3.47; RN-BA, D 2 = Proc = 0.0300, 2.68; BA-SP, Proc = 0.0184, D 2 = 2.86; SP-SC, Proc = 0.0254, D 2 = 2.88) (Fig. 4; Table 2).
Sexual size dimorphism (SSD) based on mean values of standard length (a) and height (b) of the Bathygobius soporator specimens. R2—RMA Regression coefficient; pRMA—p-value for inter-variable correlation; b = slope of the line; p (b = 1)—p-value tests against the hypothesis of RMA slope = 1; bold highlighted line slope of the line (b = 1); grey line slope of the line estimated by the RMA model. Populations: MA—Maranhão, RN—Rio Grande do Norte, BA—Bahia, SP—São Paulo, SC—Santa Catarina
Outline drawings, created from comparative deformation grids of the first canonical variable, indicated substantially different morphotypes between sexes at the intrapopulational level with regard to body height, anterior region and set of dorsal fins. The main intrapopulational morphological variation between sexes was related to height and body shape, which decrease progressively with increasing latitude. In fact, the northernmost population (MA) shows greater variation between the sexes regarding body shape and fin size. By contrast, the southernmost population (SC) displayed a markedly smaller variation in these morphological variables (Fig. 2).
Sexual Size Dimorphism (SSD) and Rensch’s Rule
Males showed a latitudinal cline in body size. Analyses of variance (ANOVA) for SL and H were significant for population and for the interaction between population and sex. No differences between sexes were observed in any of the ANOVAs conducted. Interpopulational comparisons using the Tukey–Kramer test revealed significant differences for H, except between SC and RN populations (Table 3). Analyses of interpopulation SSD exhibited an equally divergent pattern in size, where the lowest values were between RN and BA and the highest between SP and MA.
No significant correlations were found for SL (R2 = 0.421, slope = 1.37, CI 0.441–4.279, H0: correlated variables p-value = 0.235) or H variables (R2 = 0.545, slope = 1.41, CI 0.050–4.010, H0: correlated variables p-value = 0.153), in the evaluation of Rensch’s rule. Moreover, in the comparison test against a regression slope line equal to 1, the estimated lines for SL and H indicated isometry (SL; p = 0.515; H; p = 0.427) (Fig. 5a, b).
Rensch’s Rule based on mean values of standard length (a) and height (b) of the Bathygobius soporator specimens. R2—RMA Regression coefficient; pRMA—p-value for inter-variable correlation; b = slope of the line; p(b = 1)—p-value tests against the hypothesis of RMA slope = 1; bold highlighted line slope of the line (b = 1); grey line slope of the line estimated by the RMA model. Populations: MA—Maranhão, RN—Rio Grande do Norte, BA—Bahia, SP—São Paulo, SC—Santa Catarina
Discussion
Clinal Variation in Sexual Shape Dimorphism (SShD)
The family Gobiidae contains around 2000 described species, is the most speciose in the marine environment (Nelson 2006) and is found primarily on tropical reefs (Thacker 2003). A number of its species display extensive geographic distribution. These include Bathygobius soporator, a benthic species found in extremely mutable and variable intertidal regions in long geographic transects. In contrast to pelagic species capable of seeking favorable environmental conditions (Mora and Ospína 2001), the low vagility of adult B. soporator subjects its populations to differential selective pressures particular to different habitats (Lima-Filho et al. 2012).
Gradual continuous geographic variations in morphological traits are known as clines. Clinal variation has been identified for morphometric characters in a number of marine fish on the coast of Brazil (Molina et al. 2006), which seem to exhibit adaptive norms to gradually variable environments. However, clinal variations in body shape, such as those observed in the populations of B. soporator analyzed here, are very rare. This species displays a clear variation in intra and interpopulation sexual dimorphism. Indeed, spatial comparisons of sexual dimorphism values among populations indicate a clinal gradient that progressively declines with increasing latitudes (North–South).
Rensch’s rule proposes that SSD increases when males are larger than females and decreases when females are larger. This rule has been debated and frequently refuted (Webb and Freckleton 2007; Bidau and Martinez 2016). Interestingly, the morphometric data of B. soporator populations indicate that in areas where males are larger than females, SShD variation was significantly higher. The possible association of SShD with Rensch’s rule is unusual and may represent a new and promising perspective for testing the extension and implications of this ecogeographic/evolutionary rule.
The B. soporator populations analyzed are distributed along 4000 km of the coast, extending from the North to the South of Brazil. Thermal regimes differ considerably between the northernmost and southernmost distribution points. The northernmost population (MA), with a mean temperature of 28.3 °C, is influenced by the Guiana Current, which flows north from the South Equatorial Current, branching into an offshore region perpendicular to the area occupied by the RN population, with an average temperature of 28.2 °C (Lumpkin and Garzoli 2005). The Brazil current flows southward parallel to the BA coast in the northeast, with a mean temperature of 25.5 °C, as far as SP in the southeast. The SC population, the most southerly, with a mean temperature of 16.9 °C, is influenced by the Falklands/Malvinas Current (Stramma and England 1999).
Considerable evidence suggests the influence of environmental factors in the increase of sexual shape dimorphism. In zebrafish (Danio rerio), experimental data indicate a significant temperature effect on body shape variation, with different consequences for both sexes (Georga and Koumoundouros 2010), a condition also identified in other organisms (Fairbairn 2005). Temperature may also play an important role in sexual dimorphism, since it is an important adaptive environmental factor for marine organisms (Somero 2002; Hughes et al. 2003). Its influence on the environment can cause changes in the physiology of cells and organs (Hochachka and Somero 2002; Forster et al. 2011), thereby acting on growth, as well as reproduction and mortality rates (Brey 1995). In light of the adaptive patterns, it affects the size and geographic distribution of populations (Grove 1985; Arntz and Fahrbach 1996) and, on a macroscale, the structure of communities and ecosystems (Glynn 1988).
Morphological differences in the body shape of males in neighboring populations showed greater variation (MA-RN, Proc = 0.0368, D 2 = 4.40; RN-BA, Proc = 0.0405, D 2 = 3.63; BA-SP, Proc = 0.0191, D 2 = 3.10; SP-SC, Proc = 0.0300, D 2 = 3.08) than in females (MA-RN, Proc = 0.0363, D 2 = 3.47; RN-BA, Proc = 0.0300, D 2 = 2.68; BA-SP, Proc = 0.0184, D 2 = 2.86; SP-SC, Proc = 0.0254, D 2 = 2.88). The higher interpopulation variation in morphology among males may be associated with reproductive behavioral factors and habitat characteristics, which promote sexual selection. In B. soporator, males are selected by females for reproduction after preparing the shelter for egg laying and courtship behavior in the form of vigorous undulations of the body, and pectoral and caudal fins, which produce shallow depressions in the sand (Tavolga 1950).
An open question in the study of B. soporator is the extent to which the variation of SShD can be attributed to genetic differences or phenotypic plasticity (Lima-Filho et al. 2012). Even in cases where sexual selection is the main driving force of sexual dimorphism, other factors such as selective pressures or restrictions derived from predation, locomotion, nutrition and sensory perception may influence its evolutionary trajectory and magnitude (Boughman 2002; Langerhans et al. 2005; Hendry et al. 2006).
While the body characteristics of males and females are perceptibly less divergent in the southernmost population (SC), females in the northernmost population (MA) have greater body height, shortened bodies and smaller fins. The males, in turn, have significantly larger fins and are slim, making them propitious to an increase in body size during ontogenesis. These variations indicate complex sexual dimorphism, resulting from the joint action of SSD and SShD, whose expression is differential along the geographic transect analyzed.
Due to low vagility and tendency to population fragmentation, variations in these characters may be related to adaptive environmental and local ecological conditions (Lima-Filho et al. 2012). The interaction between natural selection and sexual pressures becomes more complex when both are considered. The evolution of sexually selected traits may be driven by sexual competition within one sex, as well as by differential choice by members of the opposite sex or intersexual selection (Leese et al. 2009). The sex differences promoted by sexual selection make individuals of the more conspicuous sex (in terms of size, coloration or sexual ornamentation) more susceptible to predation and competition, albeit compensated by greater reproductive success (Samia et al. 2015). Thus, sexual dimorphism is prone to variations and may differ among populations in relation to the intensity of sexual selection (Delph and Bell 2008). However, it can be difficult to measure these two mechanisms separately, since the same characters that are important for competition between males may play a role in the choice of partners (Wong and Candolin 2005; Hunt et al. 2009).
Analyses of fish populations belonging to the family Goodeidae indicate greater genetic differences in more dimorphic populations, resulting in lower gene flow and accelerated differentiation (Ritchie et al. 2007). It has been suggested that sexual isolation evolves more rapidly in species susceptible to strong sexual selection (Darwin 1871; Lande 1982; Gavrilets 2000). Likewise, the marked sexual dimorphism observed in B. soporator likely contributes to the genetic structuring of this species along the Brazilian coast (Lima et al. 2005).
Ecogeographic Rules and SSD
Sexual size dimorphism (SSD), as identified in B. soporator, may have important influences on ecology, behavior and population dynamics. Latitude can have differential effects on the sexes depending on life history and different reproductive efforts mediated by climatic changes. Indeed, many interspecific interactions, such as predation, show latitudinal clines (Díaz et al. 2013). Risk of predation is one of the factors capable of impacting the selection of different body sizes between sexes (Hernandez-Jimenez and Rios-Cardenas 2012). SSD can also be influenced by latitude due to the greater availability of resources that can affect the potential for polygyny (Isaac 2005).
A number of ecogeographic rules, such as Bergmann’s rule (Bergmann 1847; Bidau 2014), seek to describe macroscale patterns of body variation, and may interfere in the expression of sexual dimorphism. A pattern inverse to that of Bergmann’s rule was found in geographical samples of male B. soporator: body size decreases toward southern latitudes instead of increasing, as predicted by the rule. Although initially described for endothermic organisms, Bergmann’s rule is not universal (Ashton et al. 2000). As in B. soporator, many ectotherms follow the converse Bergmann’s rule, in which body size tends to decline in colder climates (Ashton and Feldman 2003). Seasonality and temperature reduce growth potential at higher latitudes due to restricted foraging, growth and developmental time with which body size is associated (Blanckenhorn et al. 2006). A number of these conditions, in association or individually, could contribute to progressively smaller B. soporator body sizes in the colder regions of its distribution, in addition to affecting sexual dimorphism.
Rensch’s rule was originally formulated to describe interspecific variation in sexual dimorphism. However, it may also apply to interpopulation variation in dimorphism (Lengkeek et al. 2008). Though rare, tests on intrapopulation dimorphism patterns are important in confirming the emergence of macroevolutionary models from microevolutionary processes (Blanckenhorn et al. 2006). Despite differing from a number of other fish species that exhibit a latitudinal version of Rensch’s rule (Herczeg et al. 2010), more extensive analyses have indicated that intraspecific SSD variation in a large number of taxa contradicts Rensch’s rule (Blanckenhorn et al. 2006).
It is important to emphasize that B. soporator shows isometric growth (Lima-Filho et al. 2010), excluding allometric changes through individual ontogeny that can compromise the identification of Rensch’s rule (Liao 2013). Analysis of previous studies in different taxa (arthropods, reptiles, fish, birds and amphibians) showing females being larger than males revealed that Rensch’s is an exception rather than a rule (Webb and Freckleton 2007; Liao and Chen 2012).
Intraspecific SSD patterns that corroborate Rensch’s rule can be explained by local genetic adaptation to the intensity of male sexual selection (Fairbairn and Preziosi 1994), or as a product of males with greater phenotypic plasticity of body size than in females. Lengkeek et al. (2008) suggest that phenotypic plasticity may underlie interpopulation variation in blennies (Blenniidae) from the Mediterranean. The causes of this pattern remain unclear and likely stem from multiple factors across the distribution of a taxon (Fairbairn 1997, 2005). Thus, significant interpopulation phenotypic variation in B. soporator, either genetic or due to phenotypic plasticity (Lima-Filho et al. 2012), suggests that complex factors may act on their populations.
Comparative analysis of sexual dimorphism in different B. soporator populations shows a variation in latitudinal SShD, which is minimized in populations on the southern Brazilian coast. This condition, associated with the establishment of favorable sexual characters, may play an important role in reducing gene flow between its populations and consequently in initial diversification processes within the species. This provides new insight in the search for a better understanding of the ecological and evolutionary factors that act on vicariant processes, which led to the wide diversity of the family Gobiidae and other groups of marine fish.
References
Alencar, C. E. R., Lima-Filho, P. A., Molina, W. F. F., & Freire, A. M. (2014). Sexual shape dimorphism of the mangrove crab Ucides cordatus (Linnaeus, 1763) (Decapoda, Ucididae) accessed through geometric morphometric. The Scientific World Journal, doi:10.1155/2014/206168.
Arntz, W., & Fahrbach, E. (1996). El Niño, experiment climático de la naturaliza. Mexico: Fondo de la Cultura Económica.
Ashton, K. G., & Feldman, C. R. (2003). Bergmann’s rule in non- avian reptiles: turtles follow it, lizards and snakes reverse it. Evolution, 57, 1151–1163.
Ashton, K. G., Tracy, M. C., & Queiroz, A. (2000). Is Bergmann’s rule valid for mammals? The American Naturalist, 156, 390–415.
Badyaev, A. V. (2011). Origin of the fittest: Link between emergent variation and evolutionary change as a critical question in evolutionary biology. Proceedings of Royal Society, Biological Sciences, 278, 1921–1929.
Bergmann, C. (1847). Ueber die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Gottinger studien, 3, 595–708.
Bidau, C. J. (2014). Some historical aspects of ecogeographic rules: Bergmann’s rule as an emblematic case. Entomology, Ornithology, Herpetology, 2, 1–10.
Bidau, C. J., Marti, D. A., & Castillo, E. R. (2013). Rensch’s rule is not verified in melanopline grasshoppers (Acrididae). Journal of Insect Biodiversity, 1, 1–14.
Bidau, C. J., & Martí, D. A. (2008). Geographic and climatic factors related to a body-size cline in Dichroplus pratensis Bruner, 1900 (Acrididae, Melanoplinae). Journal of Orthoptera Research, 17, 149–156.
Bidau, C. J., Miño, C. I., Castillo, E. R., & Martí, D. A. (2012). Effects of abiotic factors on the geographic distribution of body size variation and chromosomal polymorphisms in two neotropical grasshopper species (Dichroplus : Melanoplinae: Acrididae). Psyche: A Journal of Entomology. doi:10.1155/2012/863947.
Bidau, C. J., & Martinez, D. A. (2016). Sexual size dimorphism and Rensch’s rule in Canidae. Biological Journal of the Linnean Society, 119, 816–830.
Blanckenhorn, W. U., Stillwell, R. C., Young, K. A., Fox, C. W., & Ashton, K. G. (2006). When Rensch meets Bergmann, does sexual size dimorphism change with latitude? Evolution, 60, 2004–2011.
Boughman, J. W. (2002). How sensory drive can promote speciation. Trends in Ecology and Evolution, 17, 571–577.
Brey, T. (1995). Temperature and reproductive metabolism in macrobenthic populations. Marine Ecology Progress Series, 30, 159–166.
Clutton-Brock, T. H. (2009). Sexual selection in females. Animal Behaviour, 77, 3–11.
Cock, A. G. (1966). Genetical aspects of metrical growth and form in animals. Quarterly Review of Biology, 41, 131–190.
Darwin, C. (1871). The descent of man, and selection in relation to sex. London: John Murray.
Delph, L. F., & Bell, D. (2008). A test of the differential-plasticity hypothesis for variation in the degree of sexual dimorphism in Silene latifolia. Evolutionary Ecology Research, 10, 61–75.
Demartini, E. E. (1999). Intertidal spawning. In M. H. Horn, K. L. M. Martin & M. A. Chotkowski (Eds.), Intertidal fishes: Life in two worlds (pp. 143–164). San Diego: Academic Press.
Díaz, M., Møller, A. P., Flensted-Jensen, E., Grim, T., Ibáñez-Álamo, J. D., Jokimäki, J., Markó, G., & Tryjanowski, P. (2013). The geography of fear: A latitudinal gradient in anti-predator escape distances of birds across Europe. PLoS ONE, 8, e64634.
Dryden, I. L., & Mardia, K. V. (1998). Statistical shape analysis. New York: John Wiley & Sons.
Fairbairn, D. J. (1997). Allometry for sexual size dimorphism: Pattern and process in the coevolution of body size in males and females. Annual Review of Ecology, Evolution and Systematics, 28, 659–687.
Fairbairn, D. J. (2005). Allometry for sexual size dimorphism: testing two hypotheses for Rensch’s rule in the water strider. Aquarius remigis. The American Naturalist, 116, 69–84.
Fairbairn, D. J. (2013). Extraordinary differences between the sexes in the animal Kingdom. Princeton: Princeton University Press.
Fairbairn, D. J., Blanckenhorn, W. U., & Székely, T. (2007). Sex, size and gender roles: evolutionary studies of sexual size dimorphism. New York: Oxford University Press.
Fairbairn, D. J., & Preziosi, R. F. (1994). Sexual selection and the evolution of allometry for sexual size dimorphism in the waterstrider, Aquarius remigis. The American Naturalist, 144, 101–118.
Fernandez-Montraveta, C., & Moya-Larano, J. (2007). Sex-specific plasticity of growth and maturation size in a spider: Implications for sexual size dimorphism. Journal of Evolutionary Biology, 20, 1689–1699.
Forster, J., Hirst, A. G., & Woodward, G. (2011). Growth and development rates have different thermal responses. The American Naturalist, 178, 668–678.
Gavrilets, S. (2000). Rapid evolution of reproductive barriers driven by sexual selection. Nature, 403, 886–889.
Georga, I., & Koumoundouros, G. (2010). Thermally induced plasticity of body shape in adult zebrafish Danio rerio (Hamilton, 1822). Journal of Morphology, 271, 1319–1327.
Gibson, R. N., & Yoshiyama, R. M. (1999). Intertidal fish communities. In M. H. Horn, K. L. M. Martin, & M. A. Chotkowski (Eds.), Intertidal fishes: Life in two worlds (pp. 264–296). San Diego: Academic Press.
Glynn, P. W. (1988). El Niño-Southern Oscillation 1982–1983: nearshore population, community, and ecosystem responses. Annual Review of Ecology, Evolution and Systematics, 19, 309–345.
Gohli, J., & Voje, K. L. (2016). An interspecific assessment of Bergmann’s rule in 22 mammalian families. BMC Evolutionary Biology, 16, 222.
Grove, J. (1985). Influence of the the 1982–1983 El Niño en la esla Galápagos: el evento de 1982–1983. Quito: Fundación Charles Darwin.
Hendry, A. P., Kelly, M. L., Kinnison, M. T., & Reznick, D. N. (2006). Parallel evolution of the sexes? Effects of predation and habitat features on the size and shape of wild guppies. Journal of Evolutionary Biology, 19, 741–754.
Herczeg, G., Gonda, A., & Merila, J. (2010). Rensch’s rule inverted—female-driven gigantism in nine-spined stickleback Pungitius pungitius. Journal of Animal Ecology, 79, 581–588.
Hernandez-Jimenez, A., & Rios-Cardenas, O. (2012). Natural versus sexual selection: Predation risk in relation to body size and sexual ornaments in the green swordtail. Animal Behaviour, 84, 1051–1059.
Hochachka, P. W., & Somero, G. N. (2002). Biochemical adaptation: Mechanism and process in physiological evolution. New York: Oxford University Press.
Hughes, T. P., Baird, A. H., Bellwood, D. R., Card, M., Connolly, S. R., Folke, C., Grosberg, R., Hoegh-Guldberg, O., Jackson, J. B. C., Kleypas, J., Lough, J. M., Marshall, P., Nyström, M., Palumbi, S. R., Pandolfi, J. M., Rosen, B., & Roughgarden, J. (2003). Climate change, human impacts, and the resilience of coral reefs. Science, 301, 929–933.
Hunt, J., Breuker, C. J., Sadowski, J. A., & Moore, A. J. (2009). Male–male competition, female mate choice and their interaction: Determining total sexual selection. Journal of Evolutionary Biology. doi:10.1111/j.1420-9101.2008.01633.x.
Ingleby, F. C., Lewis, Z., & Wedell, N. (2010). Level of sperm competition promotes evolution of male ejaculate allocation patterns in a moth. Animal Behavior, 80, 37–43.
Isaac, J. (2005). Potential causes and life-history consequences of sexual size dimorphism in mammals. Mammal Review, 35, 101–115.
Karl, I., & Fischer, K. (2008). Why get big in the cold? Towards a solution to a life-history puzzle. Oecologia, 155, 215–225.
Kelly, C. D., Folinsbee, K. E., Adams, D. C., & Jennions, M. D. (2013). Intraspecific sexual size and shape dimorphism in an australian freshwater fish differs with respect to a biogeographic barrier and latitude. Journal of Evolutionary Biology, 40, 408–419.
Kendall, M. G., & Stuart, A. (1973). The Advanced Theory of Statistics. London: Charles Green.
Kitano, J., Mori, S., & Peichel, C. L. (2012). Reduction of sexual dimorphism in stream resident forms of three-spined stickleback Gasterosteus aculeatus. Journal Fish Biology, 80, 136–146.
Klingenberg, C. P. (2011). MorphoJ: An integrated software package for geometric morphometrics. Molecular Ecology Resources, 11, 353–357.
Klingenberg, C. P., & Monteiro, L. R. (2005). Distances and directions in multidimensional shape spaces: Implications for morphometric applications. Systematic Biology, 54, 678–688.
Lande, R. (1982). Rapid origin of sexual isolation and character divergence in a cline. Evolution, 36, 213–223.
Langerhans, R. B., Layman, C. A., & DeWitt, T. J. (2005). Male genital size reflects a trade-off between attracting mates and avoiding predators in two live-bearing fish species. Proceedings of the National Academy of Sciences of the United States of America, 102, 7618–7623.
Langerhans, R. B., Layman, C. A., Shokrollahi, A. M., & DeWitt, T. J. (2004). Predator-driven phenotypic diversification in Gambusia affinis. Evolution, 58, 2305–2318.
Leese, J. M., Snekser, J. L., Ganim, A., & Itzkowitz, M. (2009). Assessment and decision making in a Caribbean damselfish: Nest-site quality influences prioritization of court ship and brood defense. Biology Letters, 5, 180–198.
Lengkeek, W., Didderen, K., Côté, I. M., van der Zee, E. M., Snoek, R. C., & Reynolds, J. D. (2008). Plasticity in sexual size dimorphism and Rensch’s rule in Mediterranean blennies (Blenniidae). Canadian Journal of Zoology, 86, 1173–1178.
Levene, H. (1960). Robust tests for equality of variances. In I. Olkin (Ed.), Contributions to probability and statistics (pp. 278–292). California: Stanford University.
Liao, W. B. (2013). Evolution of sexual size dimorphism in a frog obeys the inverse of Rensch’s Rule. Evolutionary Biology, 40, 293–299.
Liao, W. B., & Chen, W. (2012). Inverse Rensch-rule in a frog with female-biased sexual size dimorphism. Die Naturwissenschaften, 99, 427–431.
Lima, D., Freitas, J. E. P., Araújo, M. E., & Solé-Cava, A. M. (2005). Genetic detection of cryptic species in the frillfin goby Bathygobius soporator. Journal of Experimental Marine Biology and Ecology, 320, 211–223.
Lima-Filho, P. A., Cioffi, M. B., Bertollo, L. A. C., & Molina, W. F. (2012). Chromosomal and morphological divergences in Atlantic populations of the frillfin goby Bathygobius soporator (Gobiidae, Perciformes). Journal Experimental Marine Biology Ecology, 43, 63–70.
Lima-Filho, P. A., Martinez, P. A., & Molina, W. F. (2010). Dimorfismo sexual e padrão de variação ontogenética em Bathygobius soporator (Valenciennes,1837) (Gobiidae—Perciformes) a partir de análises por morfometria geométrica. 62ª Reunião Anual da SBPC. Resumo 4277.
Lima-Filho, P. A., Rosa, R. S., Costa G. W. W. F., Souza, A. S., Oliveira, C., & Molina, W. F. (2016). Evolutionary diversification of Western Atlantic Bathygobius species based on cytogenetic, morphologic and DNA barcode data. Reviews in Fish Biology and Fisheries. Doi:10.1007/s11160-015-9411-0.
Lumpkin, R., & Garzoli, S. L. (2005). Near-surface Circulation in the Tropical Atlantic Ocean. Deep-Sea Research, 52, 495–518.
Martinez, P. A., Amado, T. F., & Bidau, C. J. (2014). A phylogenetic approach to the study of sexual size dimorphism in Felidae and an assessment of Rensch’s rule. Ecosistemas, 23, 27–36.
Martinez, P. A., & Bidau, C. J. (2014). A re-assessment of Rensch’s rule in tuco-tucos (Rodentia: Ctenomyidae:Ctenomys) using a phylogenetic approach. Mammalian Biology, 81, 66–72.
Miller, P. J. (1961). Age, growth, and reproduction of the rock goby Gobius paganellus L., in the Isle of Man. Journal of the Marine Biological Association of the United Kingdom, 41, 737–769.
Molina, W. F., Shibatta, O. A., & Galetti-Jr, P. M. (2006). Multivariate morphological analyses in continental and island populations of Abudefduf saxatilis (Linnaeus) (Pomacentridae, Perciformes) of Western Atlantic. Pan-American Journal of Aquatic Sciences, 1, 49–56.
Monteiro, L. R., Diniz-Filho, A. F., Reis, S. F., & Araújo, E. D. (2002). Geometric estimates of heritability in biological shape. Evolution, 56, 563–572.
Mora, C., & Ospína, A. F. (2001). Tolerance to high temperatures and potential impact of sea warming on reef fishes of Gorgona Island (tropical eastern Pacific). Marine Biology, 139, 765–769.
Nelson, J. S. (2006). Fishes of the world. New Jersey: Willey.
Parker, G. A. (1992). The evolution of sexual size dimorphism in fish. Journal of Fish Biology, 41, 1–20.
Parsons, K. J., Beren, W. R., & Hrbek, T. (2003). Getting into shape: An empirical comparison of traditional truss-based morphometric methods with a newer geometric method applied to New World cichlids. Environmental Biology of Fishes, 67, 417–431.
R Development Core Team (2012) R: A language and environment for statistical computing. Vienna: Austria: R Foundation for Statistical Computing. Accessed April 25, 2015, from http://www.R-project.org/.
Raihani, G., Sze´kely, T., Serrano-Meneses, M. A., Pitra, C., & Goriup, P. (2006). The influence of sexual selection and male agility on sexual size dimorphism in bustards (Otididae). Animal Behavior, 71, 833–838.
Rensch, B. (1950). Die Abhangigkeit der relativen Sexualdifferenz von der Korpergrosse. Bonner Zoologische Beitrage, 1, 58–69.
Ritchie, M. G., Hamill, R. M., Graves, J. A., Magurran, A. E., Webb, S. A., & Macías, G. C. (2007). Sex and differentiation: Population genetic divergence and sexual dimorphism in Mexican goodeid fish. Journal Evolutionary Biology, 20, 2048–2055.
Rohlf, F. J. (2006). tpsDig version 2.10. Ecology and evolution. Suny at Stony Brook. Accessed June 15, 2015, http://life.bio.sunysb.edu/morph/.
Rohlf, F. J. (2013). Tps Series. Department of Ecology and Evolution, State University of New York, Stony Brook, New York. Accessed June 15, 2015, http://life.bio.sunysb.edu/morph/.
Rohlf, F. J., & Slice, D. E. (1990). Extensions of the procrustes method for the optimal superimposition of landmarks. Systematic Zoology, 39, 40–59.
Samia, S. M. D., Møller, A. P., Blumstein, D. T., Stankowich, T., & Cooper, W. E. (2015). Sex differences in lizard escape decisions vary with latitude, but not sexual dimorphism. Proceedings of the Royal Society B, doi:10.1098/rspb.2015.0050.
Shapiro, S. S., & Wilk, M. B. (1965). An analysis of variance test for Normality (complete samples). Biometrika, 52, 591–611.
Shelomi, M. (2012). Where are we now? Bergmann’s rule sensu lato in insects. American Naturalist, 180, 511–519.
Shine, R. (1989). Ecological Causes for the evolution of sexual dimorphism: A review of the evidence. The Quarterly Review of Biology, 64, 419–461.
Sidlauskas, B. L., Mol, J. H., & Vari, R. P. (2011). Dealing with allometry in linear and geometricmorphometrics: A taxonomic case study in the Leporinus cylindriformis group (Characiformes: Anostomidae) with description of a new species from Suriname. Zoological Journal of the Linnean Society, 162, 103–130.
Smith, R. J. (1999). Statistics of sexual size dimorphism. Journal of Human Evolution, 36, 423–459.
Smith, R. J. (2009). Use and misuse of the reduced major axis for line-fitting. American Journal of Physical Anthropology, 140, 476–486.
Sokal, R. R., & Rohlf, F. J. (1985). Biometry. New York: W.H. Freeman and Company.
Somero, G. N. (2002). Thermal physiology and vertical zonation of intertidal animals: Optima, limits and cost of living. Integrative and Comparative Biology, 42, 780–789.
Stillwell, R. C., & Fox, C. W. (2007). Environmental effects on sexual size dimorphism of a seed-feeding beetle. Oecologia, 153, 273–280.
Stramma, L., & England, M. (1999). On the water masses and mean circulation of the South Atlantic Ocean. Journal of Geophysical Research Washington, 104, 863–883.
Stuart-Fox, D. M., & Ord, T. J. (2004). Sexual selection, natural selection and the evolution of dimorphic coloration and ornamentation in agamid lizards. Proceedings of the Royal Society of London. Series B, Biological Sciences, 271, 2249–2255.
Tavolga, W. N. (1950). Development of the gobiid fish, Bathygobius soporator. Journal of Morphology, 87, 467–492.
Thacker, C. E. (2003). Molecular phylogeny of the gobioid fishes (Teleostei: Perciformes: Gobioidei). Molecular Phylogenetics and Evolution, 26, 354–368.
Tsuboi, M., Gonzalez-Voyer, A., Hoglund, J., & Kolm, N. (2012). Ecology and mating competition influence sexual dimorphism in Tanganyikan cichlids. Evolutionary Ecology, 26, 171–185.
Walsh, M. R., & Reznick, D. N. (2009). Phenotypic diversification across an environmental gradient: A role for predators and resource availability on the evolution of life histories. Evolution, 63, 3201–3213.
Warton, D. I., Duursma, R. A., Falster, D. S., & Taskinen, S. (2012). Smatr 3—an R package for estimation and inference about allometric lines. Methods in Ecology and Evolution, 3, 257–259.
Warton, D. I., Wright, I. J., Falster, D. S., & Westoby, M. (2006). Bivariate line-fitting methods for allometry. Biological Reviews, 81, 259–291.
Webb, T. J., & Freckleton, R. P. (2007). Only half right: Species with female-biased sexual size dimorphism consistently break Rensch’s Rule. PLoS ONE, 2(9), e897. doi:10.1371/journal.pone.0000897.
Wong, B. B. M., & Candolin, U. (2005). How is female mate choice affected by male competition? Biological Reviews of the Cambridge Philosophical Society, 80, 559–571.
Zar, J. H. (2010). Biostatistical Analysis. Upper Saddle River, NJ: Pearson Prentice-Hall.
Acknowledgements
We are grateful to the Coordination for the Improvement of Higher Education Teaching Personnel (CAPES) and the National Council for Scientific and Technological Development (CNPq) (Project No. 556793/2009-9) for their financial support, and to IBAMA (Process No. 19135/1) and José Garcia Júnior for taxonomic identification of species.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Lima-Filho, P.A., Bidau, C.J., Alencar, C.E.R.D. et al. Latitudinal Influence on the Sexual Dimorphism of the Marine Fish Bathygobius soporator (Gobiidae: Teleostei). Evol Biol 44, 374–385 (2017). https://doi.org/10.1007/s11692-017-9416-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-017-9416-9