Abstract
Environmental induced developmental plasticity occurs in many organisms and it has been suggested to facilitate biological diversification. Here we use ranid frogs to examine whether morphological changes derived from adaptive developmental acceleration in response to pool drying within a species are mirrored by differences among populations and across species. Accelerated development in larval anurans under pool drying conditions is adaptive and often results in allometric changes in limb length and head shape. We examine the association between developmental rate and morphology within population, among populations in divergent environments, and among species inside the Ranidae frog family, combining experimental approaches with phylogenetic comparative analyses. We found that frogs reared under decreasing water conditions that simulated fast pool drying had a faster development rate compared to tadpoles reared on constant water conditions. This faster developmental rate resulted in different juvenile morphologies between the two pool drying conditions. The association between developmental rate and morphology found as a result of plasticity was not mirrored by differences among populations that differed in development, neither was it mirrored among species that differed in development rate. We conclude that morphological differences among populations and species were not driven by variation in developmental time per se. Instead, selective factors, presumably operating on locomotion and prey choice, seem to have had a stronger evolutionary effect on frog morphology than evolutionary divergences in developmental rate in the ranid populations and species studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decade there has been a renewed interest in the role that phenotypic and developmental plasticity might play in generating evolutionary innovation and triggering biological diversification (West-Eberhard 2003, 2005; Pfennig et al. 2010; Moczek et al. 2011). Phenotypic plasticity can favour diversification by permitting populations to colonize new environments while reducing the associated bottlenecks and preserving greater genetic variation (Draghi and Whitlock 2012; Gomez-Mestre and Jovani 2013). Phenotypic plasticity may thus allow populations to rapidly move from one adaptive peak to another (Gomez-Mestre and Jovani 2013). Once established, the traits that were once plastic might become genetically assimilated or accommodated (West-Eberhard 2005), resulting in changes in phenotypic trait values and hence in biological diversification under selection (Pfennig et al. 2010). Genetic assimilation refers to a trait that was originally plastic and that has become under strict genetic regulation, i.e. has become constitutive through canalisation (Waddington 1942; Braendle and Flatt 2006). Genetic accommodation is a broader concept that encompasses genetic assimilation. It refers to the expression of phenotypes resulting from previously cryptic epigenetic or genetic variation, which increases in frequency under selection resulting in divergent reaction norms, whether increased or decreased plasticity (West-Eberhard 2003; Crispo 2007; Fusco and Minelli 2010).
It is unclear how common genetic accommodation may have been in evolution, but we can identify potential cases of this evolutionary process looking for patterns where trait diversity among species or populations exposed to divergent environments mirrors phenotypic plasticity within taxa in response to those same environmental factors (Gomez-Mestre and Buchholz 2006; Wund et al. 2008). This, however, is not a definitive test of genetic accommodation, which would require mechanistic understanding of how phenotypic plasticity affects biological diversification. For example, tests of genetic accommodation would require assessing if different genes are expressed in organisms of the same phenotype, whether it is expressed constitutively or plastically (Snell-Rood et al. 2010; Johansson et al. 2013). Nevertheless, identifying patterns congruent with genetic accommodation is a necessary first step in the study of this process.
Here we use a combination of experimental and phylogenetic comparative approaches to test for associations between developmental rate and morphology in frogs across different levels of organization (within population, among populations, and among species). We quantify plasticity in developmental rate and morphology within species and compare it among populations and species. We hypothesize that the correlation between morphology and development rate seen within a species as a result of phenotypic plasticity, also can be seen across different populations and species that differ in development rate. This would be consistent with evolved differences through genetic accommodation, if not a direct test for it.
Amphibians are excellent study systems for studying the role of phenotypic plasticity in promoting biological diversity because larval amphibians can greatly vary their growth and differentiation rates depending on the conditions experienced. This plasticity allows decoupling of growth and differentiation so that they can have extended growth with little progress in developmental stages under benign conditions, but also accelerated development to evade adverse conditions (Rose 2005). One environmental factor that has strong impact on growth, development, and morphology within frog species is pool drying (Newman 1992). Many amphibian species breed in ephemeral and temporary pools, where pool drying poses a high risk of desiccation to larvae (Newman 1992; Denver et al. 1998). Consequently, having the ability to decouple growth from differentiation and being able to accelerate development when at risk of pool drying is critical for larval amphibians (Wilbur and Colins 1973; Altwegg and Reyer 2003).
It has been suggested that metamorphosis has evolved to unlink distinct functional and morphological stages allowing their independent adaptation to distinct niches: the adaptive decoupling hypothesis (Ebenman 1992; Moran 1994). However, increasing evidence suggests that life stages are not independent (Pechenik 1999). Laboratory and field studies have shown that tadpoles from many taxa have a faster development and usually a lower growth rate under decreasing water levels (Newman 1992; Richter-Boix et al. 2011; Kulkarni et al. 2011). The relative changes in larval growth and developmental rates, result in predictable allometric changes in the morphology of postmetamorphic individuals, particularly on head shape and hind limb length (Richter-Boix et al. 2006; Gomez-Mestre et al. 2010; Tejedo et al. 2010). Such allometric responses are common across amphibian species and result from developmental shifts in response to several environmental factors (Gomez-Mestre et al. 2010; Tejedo et al. 2010). Under high risk of desiccation, developmental acceleration induces shorter hind limbs and wider heads of the emerging juveniles (Richter-Boix et al. 2006; Gomez-Mestre et al. 2010; Tejedo et al. 2010; Johansson and Richter-Boix 2013). If these plastic responses became fixed during population differentiation, it would result in morphological diversification (Fig. 1). Hence, the morphological footprint of evolutionary variation in developmental rate is sometimes detectable among populations or among species with broadly divergent developmental rates (Gomez-Mestre and Buchholz 2006; Johansson and Richter-Boix 2013). Generally, lineages (populations or species) with slower developmental rates typically show relative longer hind limbs and narrower and/or longer heads than those having faster developmental rates (Emerson 1986; Gomez-Mestre and Buchholz 2006; Johansson and Richter-Boix 2013). We can thus test for associations between larval development and postmetamorphic morphology at various taxonomic levels, and determine if variation in developmental rate and morphology across populations and species is congruent with the expectations of accommodation of ancestral plasticity.
Schematics of how developmental plasticity might allow populations and species to adapt along a gradient of pool drying, and the expected associated changes in morphology (compare morphology of frog cartoons). a Plasticity in developmental rate seems to be widespread in amphibians: larvae accelerate metamorphosis when faced with risk of desiccation. Such developmental acceleration, however, results in shorter hind limbs and either shorter or wider heads. b Selection acting on populations adapting to divergent hydroperiods (i.e. to either ephemeral or permanent pools) may drive differences in developmental rate but responsiveness to environmental stimuli may be kept, such that during adaptation, plasticity can be increased or decreased. c As environments stabilise in divergent conditions plasticity can become canalised among speciating lineages, and ultimately result in trait divergence among species. C and D in figures denotes constant and decreasing water levels respectively during development time (larval period)
We use ranid frogs to ask whether developmental acceleration and the associated morphological changes occurring within population are mirrored among populations and species with divergent developmental rate. To answer this question we examined the association between developmental rate and hind limb length and head shape within populations, among populations in divergent environments, and among ranid species, combining experimental approaches with phylogenetic comparative analyses. We predicted that if evolved through genetic accommodation, plastic shifts in developmental rate and morphology within population in response to pond drying would be mirrored among populations occupying ponds of different duration so that populations breeding in more ephemeral ponds would have evolved overall faster developmental rates and shorter hind limbs. We also expected that if genetic accommodation had occurred among species, they would have evolved different larval periods, spurred by variation in the duration of breeding habitats. If evolutionary divergence in larval period among species was large and the causal link between larval developmental rate and post-metamorphic morphology remained unchanged, we would observe a pattern of constitutive differences among species so that faster developing species would also show shorter hind limbs (Gomez-Mestre and Buchholz 2006). Alternatively, if differences in developmental rate among populations did not reflect differences in pond duration, or species differences in developmental rate did not explain morphological variation, the pattern would not be congruent with genetic accommodation. In that case, direct selection on morphology related to its effects on performance aspects such as locomotion or prey capture may be alternative explanations for phenotypic variation.
Materials and Methods
Study Species and System
First, we compared the developmental responsiveness against simulated pool drying among R. temporaria populations adapted to divergent hydroperiod regimes, and tested for differences among sibships within those populations. Second, we compared the relationship between developmental time and morphology among those R. temporaria populations varying in their developmental rate. Third, we compared the relationship between developmental time and morphology in 22 species of ranids.
Laboratory Experiments: Influence of Developmental Rate Plasticity on Morphology
We studied 5 populations of the common frog, R. temporaria, from islands in the Gulf of Bothnia (see Lind and Johansson 2007; Lind et al. (2011) for more details on these island populations). In short, frog populations on these islands have adapted their developmental rate to time metamorphosis to the duration of pools they breed on, such that populations from islands with short-lasting pools have a shorter developmental time than those with permanent water pools (Lind and Johansson 2007). In addition, these island populations are genetically differentiated because they have limited gene flow and the degree of quantitative genetic differentiation (Qst) for life history traits is greater than the degree of neutral genetic differentiation (Fst) (Lind et al. 2011).
The purpose of the laboratory experiment was to estimate the relationship between developmental time and morphology in R. temporaria, within and between island populations. We collected egg clutches from five different island populations in northern Sweden. Estimates on developmental time and choice of island populations were made from data in Lind and Johansson (2007). Because we wanted to compare populations differing in developmental time, we sampled eggs from three islands with permanent pools where frogs typically show long developmental times (Lillhaddingen, Storhaddingen and Petlandsskär), and two islands with temporary pools where frogs show faster (10 % faster on average) development (Åhällan and Ålgrundet). On May 10th 2013, we sampled 10 egg clutches from each island except Ålgrundet, were only 6 clutches were found. The clutches were sampled from 1 to 3 pools depending on the island, but when more than one pool was sampled, pools were always within 10 m of each other. The clutches were in identical developmental stage and were transported to the laboratory at Uppsala University where the experiment was performed in a walk-in climatic room. Each clutch was assumed to represent one female, as female R. temporaria lay only one egg clump per season (Savage 1961) and will hereafter be referred to as a sibship.
Egg clutches were kept at 12 °C (light: dark cycle of 18:6 h) in 3 l plastic containers filled with water until tadpoles had developed to Gosner stage 25 (Gosner 1960). At this stage we started the experiment by individually introducing 6 individuals from each sibship into plastic containers (9.5 cm × 9.5 cm, height 10 cm) filled with 750 mL of reconstituted soft water (RSW; APHA 1985). For three individuals per sibship we simulated pool drying by lowering water level in the containers by 33 % every fourth day, starting at day 5 and continued until day 25, after which the water volume was kept constant at 66 ml. This simulated pool drying conditions allowed us to compare morphology between an induced accelerated developmental time and the constitutive developmental time achieved at the constant water level, i.e. developmental plasticity. Each individual was placed in a randomly chosen place in the constant-temperature room to reduce effects of small temperature differences observed inside the room. The constant water level treatment allowed us to compare development and morphology among islands under a controlled “common garden” condition, reducing environmental variance among populations, and revealing the genetic variance among them. The water was changed every fourth day, and tadpoles were fed chopped spinach: with 0.045 g (±0.005 SD) dry spinach weight during first week, increased to 0.071 ± 0.009 the second week and to 0.097 ± 0.011 from the third week until the end of the experiment. When the forelimbs emerged at Gosner stage 42 water level in the containers was reduced to 100 ml and a wet terrestrial habitat was provided for the metamorphosed froglets until the end of the experiment.
The experiment ended when individuals had reached Gosner stage 46, i.e. the tailed resorbed, since at this stage metamorphosis is completed and larval period is over. At this stage froglets were anesthetised with immersion in an aqueous solution of MS222 and ventrally photographed from a standardized distance together with a scale. We estimated morphological traits from these photographs using the software ImageJ (Abramoff et al. 2004). The morphological traits measured were: snout-vent length (SVL), head width (HW), head length (HL) femur length (FeL), fibio-tibula length (TL) and foot length (FoL) (see Richter-Boix et al. 2006 for details). SVL was used as an overall measure of body size; other metamorphic traits were size-corrected. For size-corrected traits we first regressed each trait on SVL and retrieved the trait residuals, which reflected whether individual traits were relatively short or long for their size. Our experimental design does not allow us to take maternal effects of traits into account. However, maternal genetic variance in life history traits are low in R. temporaria (Laugen et al. 2005), and Lind and Johansson (2007) showed that it was only 5 % in the Storhaddingen population, one of the populations used in the current study.
Morphological Comparisons Among Species
For this comparative study we included 22 species of ranid frogs belonging to five different genera. Ranidae is one of the most widespread frog families in the world, ranging across all continents except Antarctica, and in a wide range of habitats encompassing wetlands in deserts, tundra, boreal- and deciduous forests, and breeding in marshes, streams, pools and lakes (Frost et al. 2006).
The species included are found across Europe, North America, Asia, and Africa, where they occupy a wide array of aquatic habitats, from temporary pools and ditches, to lakes and streams (Table 1). Some of the species included are rather habitat specialists (e.g. stream dwelling Rana pyrenaica or vernal pool specialist Rana sylvatica) whereas other species exploit diverse habitats (e.g. Rana temporaria, Rana sphenocephala). Therefore, morphology in these species is likely to have evolved under selection driven by various environmental factors including predation, diet, temperature, competition, or water current, in addition to the influence that developmental rate may or may not have exerted over it. To conduct explicit tests regarding the possible association of habitat preference and morphology, we coded each species as belonging to either one of three categories: typical of lentic systems (pool, lakes, swamps, flooded plains), restricted to lotic systems (streams and rivers), or found in both.
Morphological measurements were obtained from specimens deposited at the Museo Nacional de Ciencias Naturales (Madrid, Spain) and at Estación Biológica de Doñana (Seville, Spain). Using callipers, we took the same morphological measurements from ethanol-preserved specimens as those taken for experimental individuals. The number of individuals studied for each species was determined by specimen availability in the collections visited, and varied between 1 and 10 (Table 2), with an average of two populations included per species. Only adults were measured for the study, the majority of which were males, as they tend to be overrepresented in museum collections given the biased sex ratios observed in the field during reproduction for these species. The original measurements, together with museum identifiers are given in supplementary Table S1. Duration of the larval period for each species was obtained from the literature and the public database AmphibiaWeb (http://amphibiaweb.org, accessed March 2014). This search resulted in a range of values for duration of larval period for each species, encompassing natural observations from field guides to controlled laboratory experiments. For each species we used the range median for phylogenetic analyses.
Statistical Analyses
Laboratory Experiments: Influence of Developmental Rate Plasticity on Morphology
To test if average pool duration in each island affected developmental time, we ran a general linear model using island category as a factor with two levels (permanent or temporary) and population as random factor nested within island category. Estimates of average pool duration were taken from Lind and Johansson (2007). We used a one tailed hypothesis for this test since we predicted that developmental time should be shorter on islands with temporary pools. Because island distribution across the hydroperiod did not show a perfect segregation between well-defined transitions (the island Åhällan did not behave as a temporary pool island with regard to developmental time as in our past studies: larval developmental time was almost as long for this island as it was for the permanent islands) we also analysed if developmental plasticity and morphology differed among islands considering pool duration as a continuum. For this we fitted a model using treatment and island as fixed effects (since island choice was made after their average pond duration), and developmental time and morphology as dependent variables. Finally, to examine the relation between development time and morphology among islands, we also run a linear regression of morphological traits against developmental time in individuals subjected to constant water using each island as a replicate. Given the multiple test performed on morphological traits, we applied the Benjamini and Hochberg (BD) false discovery rate (Benjamini and Hochenberg 1995) with the library sgof (Carvajal-Rodríguez et al. 2009) in R. For all other analyses we used the nlme library (Pinheiro et al. 2008) in R (version 2010.1, Core Development Team) using the corCompSymm argument in the gls function.
Morphological Comparisons Among Species
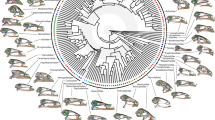
To determine if developmental time of species affects their morphology we performed a comparative phylogenetic analysis. We obtained a time-calibrated phylogeny of 22 ranid species (Fig. 2) for which we could collect matching data on six morphological traits and on duration of the larval period, by trimming down the large-scale phylogeny of Pyron and Wiens (2013). All morphological traits were log-transformed prior to analyses. Given that all morphological variables were susceptible of measurement error, we obtained size-corrected length estimates for femur length, fibio-tibula length, foot length, total hindlimb, head length and head width, extracting the residuals of phylogenetic reduced major axis regressions (RMA) between each of these variables and snout-to-vent length. These models were fit using phyl. RMA from the phytools package (Revell 2010). We then used the resulting residuals as size-corrected variables in subsequent analyses.
Time-calibrated phylogeny indicating the relationships among the frog species included in our comparative analyses. The tree was extracted from Pyron and Wiens (2013), trimming down from a large-scale phylogeny including 2871 amphibian species
We estimated the phylogenetic signal for each of the morphological traits and for larval period calculating Blomberg’s K and Pagel’s λ using the phylosig function from the phytools package (Revell 2010). We then tested alternative models of evolution of developmental rate and morphological traits. We used fitContinuous in the ape package to compare three models of trait evolution: a Brownian Motion (BM) model where trait variation is fully explained by phylogenetic distances; an Ornstein–Uhlenbeck model (OU), where traits were modelled to have varied under stabilizing selection around a single optimum); and an estimated λ model where the level of phylogenetic signal is estimated. We compared the goodness of fit of these models by means of their corrected Akaike Information Criterion parameter (AICc). Differences in AICc between competing models were considered negligible if <3, moderately strong between 4 and 7, and very strong if >10 (Anderson and Burnhamn 2002). We then performed analyses using the best-fitting model. We tested for phylogenetic associations between duration of the larval period and body size, and between larval period and size-corrected morphological traits through phylogenetic generalized linear models (PGLS) using the pgls function from the caper package (Orme et al. 2012) using the complete data set. PGLS analyses were conducted allowing estimation of the λ parameter through maximum likelihood. Finally, in contrast to similar studies on other amphibian groups, ranid species differ broadly in habitat use. As an alternative hypothesis of morphological evolution associated to accommodated differences in larval period among species, we also tested if habitat type (lentic, lotic, or both) explained morphological differences among species using phylogenetic analyses of variance using the phylANOVA function from the phytools package (Revell 2010). This analysis thus compares morphological differences among species across habitats, using species as data points and habitat as factor.
We are currently lacking experimental determination of developmental plasticity for most of the ranid species included in this analysis. However, the duration of the larval period is highly plastic across even distantly related species (Gomez-Mestre and Buchholz 2006; Gomez-Mestre et al. 2010; Richter-Boix et al. 2011), and therefore it is safe to assume that plasticity is ancestral to most, if not all, anurans and could have the potential to affect morphological diversification.
Results
Laboratory Experiments
Populations from temporary islands (Åhällan and Ålgrundet), tended to have shorter developmental times (Fig. 3), and a generalized linear model with island category (permanent and temporary) as fixed factor suggested a trend for a faster development in islands with temporary pools (F1, 3, 119 = 3.69, P = 0.07). A mixed model ANOVA using island (population) as fixed factor rather than island category, showed that developmental time differed among populations and that our water level treatment affected developmental time significantly so that tadpoles under drying water conditions significantly shortened their time to metamorphosis (Table 3; Fig. 3).
Larval development time for individuals from island population raised under constant and decreasing water level. Dots are islands with permanent pools and triangles islands with temporary pools: Lillhadingen (LH), Petlandsskär (PE), Stor Haddingen (SH), Ålgrundet (AG) and Åhällan AH). Filled and open symbols denote constant and decreasing water level conditions and dotted lines are drawn to connect these two conditions for each island and the slope of the lines represents the amount of phenotypic plasticity. Error bars denote S.E
There was a trend for a significant differences in body length among populations, and the island Ålgrundet, which had temporary pools, showed the shortest body length, being 5 % shorter than those of the permanent pool island Lill Haddingen which had the longest body length (Table 3; Fig. 4). Tadpoles raised under simulated drying pool conditions showed a significantly shorter body length (Table 3), being on average 9 % shorter. There was no significant interaction between populations and pool drying.
Snouth vent length, relative head width, relative head length, relative femur length, relative, fibio-tiula length, and relative foot length for individuals from island population raised under constant and decreasing water level. The relative lengths and widths are residuals, see text. Dots are islands with permanent pools and triangles islands with temporary pools: Lillhadingen (LH), Petlandsskär (PE), Stor Haddingen (SH), Ålgrundet (AG) and Åhällan AH). Filled and open symbols denote constant and decreasing water level conditions and dotted lines are drawn to connect these two conditions for each island and the slope of the lines represents the amount of phenotypic plasticity. Error bars denote S.E
Relative head length differed significantly between islands with the Lill Haddingen population having the shortest relative head width and being 9 % shorter than that of the temporary pool island Ålgrundet, which had the longest head width (Table 3; Fig. 4). Treatment had significant effect on head length with simulated pool drying condition causing longer head lengths in two populations and shorter in three populations. However, the interaction effect between the two factors was not significant.
Relative head width differed significantly among islands and the island Petlandsskär having permanent pools, had the narrowest head, with a 6 % shorter head width than that of the temporary pool island Ålgrundet which had the longest (Table 3; Fig. 4). There was no significant effect related to treatment and no significant interaction between populations and treatment.
Relative femur length and relative fibio-tibula length showed no significant difference among islands (Table 3; Fig. 4). In contrast, simulated pool drying conditions caused a significant treatment effect in those traits, with shorter relative femur (8 %) and relative fibio-tibula (8 %) under decreasing water conditions. There was no significant island x treatment interaction effect.
Relative foot length differed significantly among islands and the permanent pool island Lill Haddingen had the longest foot length, being 23 % longer than that of the temporary pool island Ålgrundet, (Table 3; Fig. 4). Treatment had a significant effect on foot length; being 10 % shorter under decreasing water conditions. No significant interaction between populations and treatment was found. After adjusting p-values for multiple comparisons the effect of treatment was non-significant.
The linear regression between morphological traits and development using island as replicate showed no significant relationship for any of the five morphological traits (relative head length: P = 0.165, r2 = 0.53; relative head width: P = 0.06, r2 = 0.74; relative femur length: P = 0.48, r2 = 0.17; relative fibio-tibula length: P = 0.76, r2 = 0.04; relative foot length: P = 0.87, r2 = 0.02).
Overall, all populations accelerated development in response to reduced water levels but did so to a similar extent across all populations. Populations differed in their mean duration of the larval period. Developmental acceleration resulted in changes in morphology within population, but environmentally induced morphological variation was not mirrored by differences among populations.
Comparison Among Species
We detected substantial phylogenetic signal for most of the morphological traits measured, but not for larval period, body length or head width (Table 4). Estimated lambda and OU models showed a better fit than Brownian models for the evolution of morphological traits in all cases, with differences in AIC in favour of the lambda and OU models being low for foot and head length, very strong for femur and overall hindlimb length and head width, and moderate for the rest. Since goodness of fit was nearly identical for OU and estimated lambda models with very slight improvement towards lambda-estimated models, we opted for these latter for further phylogenetic least squares tests of trait associations. However, we found no significant relationships between any of the size-corrected morphological traits and developmental time (all P > 0.20). Phylogenetic ANOVAs, however, indicated that head length (F = 9.545, P = 0.002) and foot length (F = 4.697, P = 0.046) varied significantly across habitat types with fibio-tibula showing the same trend, albeit with a marginally non-significant result (F = 3.976, P = 0.064), (Fig. 5). Species specialised in lotic environments tended to have a more elongated shape, with longer fibiotibulas, longer feet, and greater head length. All other morphological traits failed to show any variation among habitat types.
Relationships among morphology, larval period, and habitat type in 22 ranid species studied. a Phylogenetic general least square regressions between larval period and size-adjusted morphological traits. The plotted values from morphological traits are residuals from phylogenetic RMA regressions between each original trait and snout-to-vent length. Dots and triangles denote lenthic and lotic species, respectively, while plus signs denote species occupying both habitats. We found no evidence for an effect of developmental rate on among-species morphology. b Phylogenetic least square regressions between morphological traits and snout-to-vent length. c Boxplots showing differences among species grouped as being specialists of lentic environments, lotic environments, or occupying both types of habitats. Species from lotic environments tended to have a more elongated shape, with longer fibiotibulas, longer feet, and greater head length
Discussion
We found that our island populations responded to simulated pool drying by speeding up their development (Fig. 3). Such acceleration in development is adaptive because it allows tadpoles to metamorphose before the pools dry up. This adaptive response has been shown previously in this species (Laurila and Kujasalo 1999; Lind and Johansson 2007), and other amphibians (reviewed in Richter-Boix et al. 2011). The accelerated development resulted in shorter limb length for the three limb traits. In contrast, head length and width showed no consistent pattern of variation among populations. The morphological changes we found for limb length is typical for frogs subjected to simulated pool drying conditions (Richter-Boix et al. 2006; Johansson and Richter-Boix 2013). In contrast head width does not show a consistent pattern across study species because wider as well as narrower responses to pool drying have been found (Tejedo et al. 2010).
Mirrored Pattern Within and Among Populations
We predicted that, if evolved through genetic accommodation, the morphological changes caused by developmental acceleration in response to simulated pool drying would be mirrored across populations locally adapted to divergent hydroperiods. Specifically, we predicted that populations adapted to longer lasting ponds, and hence with longer developmental times, typically would have relatively longer limbs and narrower and/or longer heads than those having faster developmental rates. We found that islands differed significantly in developmental time and there was a trend for a significant shorter developmental time in islands with temporary pools as has been shown in previous studies of these islands (Lind and Johansson 2007). Island populations also showed differences in morphology, but we found no association between morphology and developmental rate among populations. Therefore, although the observed among-islands differentiation in developmental rate is congruent with genetic accommodation of ancestral developmental plasticity, such accommodation of larval period does not seem to have caused a concomitant accommodation of morphological plastic changes. For example, the island Ålgrundet, which has temporary pools and showed the fastest development under constant water conditions, did not show a consistent shorter limb length for any of the three limb traits measured compared to the other populations. Neither did this population show a shorter head length, as often induced by pool drying. Similarly, the permanent pool island Petlandsskär, which had a long developmental time, showed a short relative foot length compared to the other permanent islands. Thus, we found morphological differentiation among islands, but it was seemingly unrelated to differences in developmental rate.
The lack of mirrored pattern between plastic responses within population and variation among populations is unlikely to be due to a low number of islands being considered. In 2010 we performed a similar experiment using three other islands from the same archipelago that also differ in pool permanence. That study also showed that the plastic responses to simulated pool drying within islands were not mirrored across island with regard to morphology, although the developmental response was mirrored (see Figure S1 in supplementary material). Altogether, these results suggest that larval life-history traits and frog morphological traits are evolutionarily dissociated and with capacity to respond independently.
Among Species
We also predicted that the morphological changes caused by phenotypic plasticity responses to simulated pool drying should be mirrored across species such that species with longer development typically should have longer limbs and narrower and/or longer heads than those having faster developmental rates. Gomez-Mestre and Buchholz (2006) observed that plastic responses in developmental rate within spadefoot toad species were mirrored in among species differences. In a study comparing within and among populations of Eurasian spadefoot toads Johansson and Richter-Boix (2013) found that morphological changes induced by decreasing water level was mirrored among populations of Pelodytes punctatus that differed in breeding pool hydroperiod and developmental rate. In the first study developmental acceleration was induced by temperature and in the latter by pool drying, but both environmental stimuli would enhance differentiation over growth, triggering an early metamorphosis and were thus expected to result in the same morphological consequences (Gomez-Mestre et al. 2010). Interestingly, both studies (Gomez-Mestre and Buchholz 2006; Johansson and Richter-Boix 2013) were conducted on pelobatoids frogs, which included scaphiopodids (North American spadefoot toads), pelobatids (Eurasian spadefoot toads) and pelodytids (Eurasian parsley frogs) (Frost et al. 2006; Pyron and Wiens 2011), whereas our study was performed on ranids. One potential explanation for the absence of support in our study could be that ranid frogs do not experience as extreme variation in pool duration among species as pelobatid frogs do. Consequently, larval period does not vary among the ranid species included here to the extent that it varies among spadefoot toads and parsley frogs (Buchholz and Hayes 2002; Zeng et al. 2014). In consequence, a very large sample size of ranids would be needed to find the mirrored pattern found in pelobatoid frogs. An alternative explanation for absence of a mirrored pattern is that selection on juvenile and adult morphology could be masking the relationship between larval period and morphology, perhaps through compensatory growth in the post-metamorphic stage. Relative shorter limbs are a consequence of growing and developing under stressful conditions like pool drying, but under certain environmental conditions it might be adaptive in the adult stage, or simply evolve neutrally.
Limb length in frogs is related to mode of locomotion (Emerson 1978) and climate conditions (Vidal-García et al. 2014). Shorter legs are common in species that walk or in burrowing species (Vidal-García et al. 2014), whereas long limbs are common in species inhabiting very wet environments, to enhance swimming ability (James and Wilson 2008), or in riparian and stream breeders to resist high flow rates (Lewis and Rohweder 2005; Hoskin 2010). Though we did not find a relationship between development time and morpohology in our comparative analyses, we found an association between morphology and habitat type, despite the low number of species in our analysis. Species specialised in lotic systems tended to have longer feet and fibio-tibula, and greater head lengths than species typical of lentic systems or than generalist species that occupy both lentic and slow-flowing lotic systems. Therefore, in at least the ranid species sampled, selective factors, presumably operating on locomotion and prey choice, seem to have had a stronger evolutionary effect on frog morphology than evolutionary divergences in developmental rate. Under this scenario even if species breed and develop in temporary pools and faster development is selected for, the juvenile morphology will not be genetically accommodated if there is strong selection for an alternative phenotype (Pigliucci 2001; Braendle and Flatt 2006). That is, a mirrored pattern in plasticity among populations or species would only be expected when the plastic morphological trait is adaptive, or neutral and linked to an adaptive trait (West-Eberhard 2003; Gomez-Mestre and Buchholz 2006).
Summary
We found no strong link between morphology and development within, among and population or among species of ranid frogs, and thus no support for genetic accommodation in the organism group studied. The absence of a strong pattern partly supports the adaptive decoupling hypothesis (Hanken 1992; Moran 1994) that predicts that larval and adult traits should be independent of each other, and that metamorphosis has evolved to unlink distinct stages and allows their independent adaptation to distinct niches (Moran 1994). However, such decoupling is far from complete and organisms cannot escape carry-over effects of the growing conditions experienced, as shown here in the form of morphological changes due to developmental acceleration induced by pond drying. Future studies about phenotypic integration across stages and independence between developmental modules will help us understand internal constraints in morphology, and evolution and diversification of organisms with complex life cycles, and reveal how and why patterns differ among organism groups, and whether these differences are due to adaptation or phylogenetic constraints.
References
Abramoff, M. D., Magelhaes, P. J., & Ram, S. J. (2004). Image Processing with ImageJ. Biophotonics International, 11, 36–42.
APHA. (1985). Standard methods for the examination of water and wastewater (16th ed.). Washington, DC: American Public Health Association.
Altwegg, R., & Reyer, H. U. (2003). Patterns of natural selection on size at metamorphosis in water frogs. Evolution, 57, 872–882.
Anderson, D. R., & Burnhamn, K. P. (2002). Avoiding pitfalls when using information-theoretic methods. The Journal of Wildlife Management, 66, 912–918.
Baskett, M. L., Weitz, J. S., & Levin, S. A. (2007). The evolution of dispersal in reserve networks. The American Naturalist, 170, 59–78.
Benjamini, Y., & Hochenberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, 57, 289–300.
Blem, C. R., Steiner, J. W., & Miller, M. A. (1978). Comparison of jumping abilities of the cricket frogs Acris gryllus and Acris crepitans. Herpetologica, 34, 288–291.
Braendle, C., & Flatt, T. (2006). A role for genetic accommodation in evolution? BioEssays, 28, 868–873.
Buchholz, D. R., & Hayes, T. B. (2002). Evolutionary patterns of diversity in spadefoot toad metamorphosis (Anura: Pelobatidae). Copeia, 2002, 180–189.
Carvajal-Rodríguez, A., de Uña-Álvarez, J., & Rolán-Álvarez, E. (2009). A new multitest correction (SGoF) that increases its statistical power when increasing the number of tests. BMC Bioinformatics, 10, 1–14.
Crispo, E. (2007). The Baldwin effect and genetic assimilation: Revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evolution, 61, 2469–2479.
Denver, R. J., Mirhadi, N., & Phillips, M. (1998). Adaptive plasticity in amphibian metamorphosis: Response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology, 79, 1859–1872.
Draghi, J. A., & Whitlock, M. C. (2012). Phenotypic plasticity facilitates mutational variance, genetic variance, and evolvability along the major axis of environmental variation. Evolution, 66, 2891–2902.
Ebenman, B. (1992). Evolution in organisms that change their niches during the life-cycle. American Naturalist, 139, 990–1021.
Emerson, S. B. (1978). Allometry and jumping in frogs: Helping the twain to meet. Evolution, 32, 551–564.
Emerson, S. B. (1986). Heterochrony and frogs: The relationship of a life history trait to morphological form. American Naturalist, 127, 167–183.
Frost, D. R., Grant, T., Faivovich, J., Bain, R. H., Haas, A., et al. (2006). The amphibian tree of life. Bulletin of the American Museum of Natural History, 297, 1–370.
Fusco, G., & Minelli, A. (2010). Phenotypic plasticity in development and evolution: Facts and concepts. Philosophical Transactions of the Royal Society B: Biological Sciences, 365, 547–556.
Gomez-Mestre, I., & Buchholz, D. R. (2006). Developmetal plasticity mirrors differences among taxa in spadefoot toads linking plasticity and diversity. Proceedings of the National Academy of Sciences USA, 103, 19021–19026.
Gomez-Mestre, I., & Jovani, R. (2013). A heuristic model on the role of plasticity in adaptive evolution: Plasticity increases adaptation, population viability and genetic variation. Proceedings of the Royal Society of London B, 280, 1869.
Gomez-Mestre, I., Saccoccio, V. L., Iijima, T., Collins, E. M., Rosenthal, G. G., & Warkentin, K. M. (2010). The shape of things to come: Linking developmental plasticity to post-metamorphic morphology in anurans. Journal of Evolutionary Biology, 23, 1364–1373.
Gosner, K. L. (1960). A simple table for staging anuran embryos and larvae with notes on identification. Herpetologica, 16, 183–190.
Hanken, J. (1992). Life history and morphological evolution. Journal of Evolutionary Biology, 5, 549–557.
Hoskin, C. (2010). Breeding behaviour of the Barred frog Mixophyes coggeri. Memoirs of the Queensland Museum—Nature, 55, 1–7.
James, R., & Wilson, R. (2008). Explosive jumping: Extreme morphological and physiological specializations of Australian rocket frogs (Litoria nasuta). Physiological and Biochemical Zoology, 81, 176–185.
Johansson, F., Lind, M. I., & Lederer, B. (2010). Trait performance correlations across life stages under environmental stress conditions in the common frog, Rana temporaria. PLoS One, 5(7), e11680.
Johansson, F., & Richter-Boix, A. (2013). Within-population developmental and morphological plasticity is mirrored in between- population differences: Linking plasticity and diversity. Evolutionary Biology, 40, 494–503.
Johansson, F., Veldhoen, N., Lind, M. I., & Helbing, C. (2013). Phenotypic plasticity in the hepatic transcriptome of the European common frog (Rana temporaria): The interplay between environmental induction and geographic lineage on developmental response. Molecular Ecology, 22, 5608–5623.
Kulkarni, S., Gomez-Mestre, I., Moskalik, C., Storz, B., & Buchholz, D. (2011). Evolutionary reduction of developmental plasticity in desert spadefoot toads. Journal of Evolutionary Biology, 24, 2445–2455.
Laugen, A. T., Kruuk, L. E., Laurila, A., Räsänen, K., Stone, J., & Merilä, J. (2005). Quantitative genetics of larval life-history traits in Rana temporaria in different environmental conditions. Genetical Research, 86, 161–170.
Laurila, A., & Kujasalo, J. (1999). Habitat duration, predation risk and phenotypic plasticity in common frog tadpoles. Journal of Animal Ecology, 68, 1123–1132.
Lewis, B., & Rohweder, D. (2005). Distribution, habitat, and conservation status of the Giant Barred Frog, Mixophyes iteratus in the Bungawalbin catchment, northeastern New South Wales. Pacific Conservation Biology, 11, 189–197.
Lind, M. I., Ingvarsson, P. K., Johansson, H., Hall, D., & Johansson, F. (2011). Gene flow and selection on phenotypic plasticity in an island system. Evolution, 65, 684–697.
Lind, M., & Johansson, F. (2007). The degree of adaptive phenotypic plasticity is correlated with spatial environmental heterogeneity experienced by island populations of Rana temporaria. Journal of Evolutionary Biology, 20, 1288–1297.
Minelli, A., Brena, C., Deflorian, G., Maruzzo, D., & Fusco, G. (2006). From embryo to adult—beyond the conventional periodization of arthropod development. Development genes and evolution, 216, 373–383.
Moczek, A. P., Sultan, S., Foster, S., Ledon-Rettig, C., Dworkin, I., Nijhout, H. F., et al. (2011). The role of developmental plasticity in evolutionary innovation. Proceedings of the Royal Society of London B B, 278, 2705–2713.
Moran, N. A. (1994). Adaptation and constraint in the complex life cycles of animals. Annual Review of Ecology and Systematics, 25, 73–600.
Newman, R. A. (1992). Adaptive plasticity in amphibian metamorphosis. BioScience, 42, 671–678.
Orme, D., Freckleton, R., Thomas, G., Petzoldt, T., Fritz, S., Isaac, N., et al. (2012). Caper: Comparative analyses of phylogeneticsand evolution in R. version 0.5. http://CRAN.R-project.org/package=caper.
Pechenik, J. A. (1999). On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Marine Ecology Progress Series, 177(1), 269–297.
Pechenik, J. A. (2006). Larval experience and latent effects—metamorphosis in not a new beginning. Integrative and Comparative Biology, 46, 323–333.
Pfennig, D., Wund, M. A., Snell-Rood, E. C., Cruickshank, T., Schlichting, C. D., & Moczek, A. P. (2010). Phenotypic plasticity’s impacts on diversification and speciation. Trends in Ecology and Evolution, 25, 459–467.
Pigliucci, M. (2001). Phenotypic plasticity: Beyond nature and nurture. Baltimore and London: The John Hopkiss University Press.328 pp.
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R. C. team. (2008). Nlme: Linear and nonlinear mixed effects models. R package version 3.1-90.
Pyron, R. A., & Wiens, J. J. (2011). A large-scale phylogeny of Amphibia including over 2,800 species, and a revised classification of extant frogs, salamanders, and caecilians. Molecular Phylogenetics and Evolution, 61, 543–583.
Pyron, R. A., & Wiens, J. J. (2013). Large-scale phylogenetic analyses reveal the causes of high tropical amphibian diversity. Proceedings of the Royal Society of London B: Biological Sciences, 280(1770), 20131622.
Revell, L. J. (2010). phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223.
Richter-Boix, A., Llorente, G. A., & Montori, A. (2006). Effects of phenotypic plasticity on post-metamorphic traits during pre-metamorphic stages in the anuran Pelodytes punctatus. Evolutionary Ecology Research, 8, 309–320.
Richter-Boix, A., Tejedo, M., & Rezende, E. L. (2011). Evolution and plasticity of anuran larval development in response to desiccation. A comparative analysis. A comparative analysis. Ecol Evol., 1, 15–25.
Rose, C. S. (2005). Integrating ecology and developmental biology to explain the timing of frog metamorphosis. Trends in Ecology and Evolution, 20, 129–135.
Savage, R. M. (1961). The ecology and life history of the common frog. London: Sir Isac Pitman and Sons.
Snell-Rood, E. C., Van Dyken, J. D., Cruickshank, T., Wade, M. J., & Moczek, A. P. (2010). Toward a population genetic framework of developmental evolution: The costs, limits, and consequences of phenotypic plasticity. BioEssays, 32, 71–81.
Stoks, R., & Córdoba-Aguilar, A. (2012). Evolutionary ecology of odonata: A complex life cycle perspective. Annual Review of Entomology, 57, 249–265.
Suzuki, Y., & Nijhout, H. F. (2006). Evolution of polyphenism by genetic accommodation. Science, 311, 650–652.
Tejedo, M., Marangoni, F., Pertoldi, C., Richter-Boix, A., Laurila, A., Orizaola, G., et al. (2010). Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Climate Research, 43, 31–39.
Tejedo, M., & Reques, R. (1994). Plasticity in metamorphic traits of natterjack tadpoles: The interactive effects of density and pond duration. Oikos, 71, 295–304.
Vidal-García, M., Byrne, P. G., Roberts, J. D., & Keogh, J. S. (2014). The role of phylogeny and ecology in shaping morphology in 21 genera and 127 species of Australo-Papuan myobatrachid frogs. Journal of evolutionary biology, 27, 181–192.
Waddington, C. H. (1942). Canalization of development and the inheritance of acquired characters. Nature, 150, 563–565.
Waddington, C. H. (1952). Selection of the genetic basis for an acquired character. Nature, 169, 278.
Watkins, T. B. (2001). A quantitative genetic test of adaptive decoupling across metamorphosis for locomotor and life-history traits in the pacific tree frog, Hyla regilla. Evolution, 55, 1668–1677.
West-Eberhard, M. J. (2003). Developmental plasticity and evolution (p. 794). New York: Oxford University Press.
West-Eberhard, M. J. (2005). Developmental plasticity and the origin of species differences. Proceedings of the National Academy of Sciences USA, 102, 6543–6549.
Wilbur, H. M., & Colins, J. P. (1973). Ecological aspects of amphibian metamorphosis. Science, 182, 1305–1314.
Wilson, A. D. M., & Krause, J. (2012). Personality and metamorphosis: Is behavioral variation consistent across ontogenetic niche shifts? Behavioral Ecology, 23, 1316–1323.
Wund, M. A., Baker, J. A., Clancy, B., Golub, J., & Fosterk, S. A. (2008). A test of the “Flexible stem” model of evolution: Ancestral plasticity, genetic accommodation, and morphological divergence in the threespine stickleback radiation. The American Naturalist, 172, 449–462.
Zeng, C., Gomez-Mestre, I., & Wiens, J. J. (2014). Evolution of rapid development in spadefoot toads is unrelated to arid environments. PLoS One, 9, e96673.
Acknowledgments
We thank J.J. Wiens for assistance in deriving the phylogeny used in this article from a previously published large-scale phylogeny. We also tank J. Cabot and M. Calvo for granting access to the herpetological collections at Estación Biológica de Doñana and Museo Nacional de Ciencias Naturales, respectively. FJ was supported by The Swedish Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Johansson, F., Richter-Boix, A. & Gomez-Mestre, I. Morphological Consequences of Developmental Plasticity in Rana temporaria are not Accommodated into Among-Population or Among-Species Variation. Evol Biol 43, 242–256 (2016). https://doi.org/10.1007/s11692-015-9363-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-015-9363-2