Abstract
Purpose
Brazilian anurans are considered the most diverse and species rich around the world. Although in recent years there has been a strong focus on research related to this group of animals, their parasites have not received the same attention. Thus, this study aimed to provide morphological and molecular data on haemogregarines biodiversity infecting Brazilian anurans.
Methods
During 2020, 116 anurans were collected from four Brazilian States and their blood and fragment of organs were screened for haemogregarine parasites.
Results
From the total, seven (6.03%) animals were found infected with species of Hepatozoon and Dactylosoma. Based on the morphological and molecular analysis, four anurans were found infected with Hepatozoon latrensis. The phylogenetic analysis has shown the isolates from this study grouping with the Brazilian anuran Hepatozoon clade, also with gene similarity ranging from 99.70 to 100% to H. latrensis isolates available on GenBank. Furthermore, three specimens (Trachycephalus typhonius, Leptodactylus latrans, and Rhinella diptycha) were infected with the same species of Dactylosoma (100% genetic similarity), with a genetic similarity of 98.56% to Dactylosoma piperis the only other species described in Brazil. In support of the molecular data, different morphological characters were observed in the blood smears as compared to D. piperis, suggesting that the species of Dactylosoma from the present study infecting three different species of Brazilian anurans is an undescribed species.
Conclusion
Thus, this study increases the knowledge of Brazilian anuran blood parasites and demonstrates the importance of using integrative approaches for the diagnosis of haemoparasites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amphibians are considered a diverse group of vertebrates, whose distribution extends from Tropical regions to the Arctic and North temperate zones [1]. Recently, the amphibian populations are declining around the world, due to their susceptibility of environmental pollution and emerging diseases, studies on these animals have increased, however, their parasites have not received the same attention [2, 3].
Anurans are exposed to a great variety of pathogens, and some of their blood parasites include filarial nematodes, haemoflagellates, and apicomplexans [4,5,6]. Intracellular haemogregarines are a diverse group of adeleorinid coccidia (Apicomplexa: Adeleorina) comprising four families: Dactylosomatidae Jakowska and Nigrelli, 1955, Haemogregarinidae Léger, 1911, Hepatozoidae Miller, 1908, and Karyolysidae Labbé, 1894. The genus Hepatozoon includes the most common haemogregarines found in anurans. Several species have been described from Africa, Asia, and Europe [4, 7, 8] and according to Netherlands et al. [9] and Úngari et al. [10] there are 48 recognized species of Hepatozoon infecting anurans globally. In Brazil, four species of Hepatozoon are known, Hepatozoon leptodactyli (Lesage, 1908) Pessoa, 1970 described from a Leptodactylus sp. based solely on morphological data [11], and recently, three additional species described infecting Brazilian anurans, using morphological, morphometric and molecular tools [10].
Other haemogregarines found in anurans belong to the family Dactylosomatidae. These parasites are intracellular haemoparasites that comprises the genera Dactylosoma Labbé, 1894 and Babesiosoma Jakowska and Nigrelli, 1956. In anurans, there are only five recognised species of Dactylosoma [12] with Dactylosoma piperis Úngari, Netherlands, Silva and O´Dwyer, 2020 being currently the only species described from Brazilian anurans [12].
Due to the limited data of anuran haemogregarine parasites from Brazil, this study aimed to bring new insights on haemogregarine parasites diversity on Brazilian anurans, including a characterization of a new species of Dactylosoma using morphological and molecular methods.
Materials and Methods
Anuran Collection
During fieldwork in 2020, 116 anurans were collected from four Brazilian states (Mato Grosso, Mato Grosso do Sul, São Paulo, and Goiás) (Table 1). Specimens were physically restrained and the blood samples were collected by puncture of the cervical paravertebral sinus using sterile and disposable syringes and needles [13]. No ectoparasites were observed on collected animals.
After the blood collection, three thin blood smears were made on glass slides and the remaining blood sample was stored in EDTA tubes and frozen at − 10 ºC for further molecular analysis. All applicable international, national, and institutional guidelines for the ethical handling of animals were followed (SISBIO licence 60,640–1; CEUA-UNESP 1061).
For histological slides, the anurans were euthanized using 50 mg/kg sodium thiopental (Tiopentax®) administered intracerebrally, following the guidelines of Sebben [14] and the Animal Ethics Committee of Veterinary Medicine. The liver, spleen, heart, and kidney were fixed in 4% buffered neutral formalin and stained with hematoxylin–eosin [15].
Morphological Analysis
The blood smears were fixed with absolute methanol and stained with 10% Giemsa Methylene Blue Eosin Merck® diluted in distilled water (pH 7.0 for 50 min), according to Eisen and Schall [15]. For morphological analysis of the blood and tissue parasite stages, digital images were captured and measured using a compound microscope at 1000 × magnification with the Leica software application suite LAS V3.8 (Leica Microsystems). Measurements are in micrometres (µm) comprising the parasite´s length and width, with mean and standard deviation (means ± standard deviation) given. Parasitaemia was calculated per 100 erythrocytes, with ~ 104 erythrocytes examined per blood smear following Cook et al. [16].
Molecular Analysis
DNA was extracted from whole blood samples following the blood protocol of the DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA, USA). Partial 18 S rRNA gene fragments were amplified using two different pairs of primers HepF300/Hep900 (600 bp) and Hemo1/Hemo2 (900 bp) [17, 18]. The PCR amplification reactions for each pair of primers and thermocycler conditions were carried out following Úngari et al. [10].
PCR products were subjected to the electrophoresis at 80 V in a 1.5% agarose gel, stained with Gel Red, and observed using an ultraviolet transilluminator. The products of interest were purified by adding 2 µL of ExoSAP-IT® (Affymetrix, Santa Clara, CA, USA) to 5 µL of PCR product according to the manufacturer´s recommendations. Amplicons were then sequenced using PCR primers on a 3500 Genetic Analyser capillary sequencer (Applied Biosystems) and after BigDye Terminator Cycle Sequencing Ready Reaction Kit v.3.1 (Applied Biosystems) according to the manufacturer´s recommendations.
The sequence chromatograms obtained (forward and reverse sequences) were assembled and edited from each primer to obtain a partial 18 S rDNA consensus sequence, and then concatenated [~ 1200 bp] using the Geneious version 7.1.3 [19] (Bomatters, www.geneious.com). Sequences from the haemogregarine group available on GenBank were aligned using Geneious version 7.1.3 [19] with MUSCLE algorithm (Bomatters, www.geneious.com). Adelina dimidiata Schneider, 1875, Adelina grylli Butaeva, 1996, and Klossia helicina Schneider, 1875 were selected as outgroups following Úngari et al. [10]. Phylogenetic relationships were inferred via Bayesian inference (BI) using MrBayes 3.2.2 [20] and Maximum likelihood (ML) analysis using RAxML 7.2.8. [21]. For the BI analysis, the Markov Chain Monte Carlo (MCMC) algorithm was run for 1 million generations, sampling every 100 generations. The first 25% of the trees were discarded as “burn-in”. The Tracer tool was used to assess convergence and the “burn-in” period [22]. For the ML analysis, nodal support was assessed using 1000 rapid bootstrap replicates [23]. The aligned sequences of haemogregarine species from anurans were compared using a pair-wise distance (p-distance) matrix.
Results
From 116 anurans screened (Table 1, Fig. 1), seven (6.03%) which correspond to three genera (Rhinella, Trachycehpalus and Leptodacylus) and three families (Bufonidae, Hylidae and Leptodactylidae), were found infected with species of Dactylosoma and Hepatozoon based on morphological screening [10, 11, 24, 25].
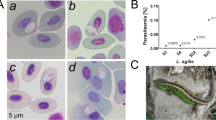
A species of Hepatozoon, latter molecularly identified as Hepatozoon latrensis Úngari, Netherlands, Silva and O´Dwyer, 2021, was identified in four anurans collected at Mato Grosso State, namely two Leptodactylus labyrinthicus, one Leptodactylus sp., and one Rhinella mirandaribeiroi with a parasitaemia of 3.24 and 5.17%, 1.92 and 0.05% respectively. The blood smears were analysed using a light microscope at 100 × magnification, and mature gamonts, immature gamonts, and free gamonts were observed in the peripheral blood of the four positive anurans (Fig. 2). From all the tissue fragments collected, only the spleen and liver were positive for Hepatozoon’s merogony; the histological slides of the four positive anurans revealed micromeronts with micromerozoites and macromeronts with macromerozoites (Fig. 3). The morphometric data are reported in Table 2.
A–E Morphological data on Hepatozoon latrensis in four anurans blood smears from Mato Grosso State, Brazil. A Mature gamont found in one anuran Rhinella mirandaribeiroi (268); B Mature gamont found in one Leptodactylus labyrinthicus; C-D) Free gamonts C and mature gamonts D found in one Leptodactylus sp.; E-Immature (head-arrow) and mature gamonts (arrow) found in one Leptodactylus labyrinthicus. Scale bar: 20 µm
A–F Histological slides containing tissue stage merogony of Hepatozoon latrensis in four anurans from Mato Grosso State, Brazil. A Trizoic cyst found in the liver of the anuran Rhinella mirandaribeiroi; B Macromeronts with macromerozoites found in the liver of Leptodactylus labyrinthicus; C–D Micromeronts with micromerozoites found in the spleen of one Leptodactylus sp.; E–F Macromeront (E) and two micromeronts found in the spleen of one Leptodactylus labyrinthicus. Black arrow (macromeronts). White arrow (micromeronts). Scale bar: 20 µm
Regarding species of Dactylosoma, three anurans were found positive: one Leptodactylus latrans from Mato Grosso do Sul State, one Trachycephalus typhonius from São Paulo State and one Rhinella diptycha from Mato Grosso State with a parasitaemia of 0.09, 0.06 and 0.16%, respectively. Based on the morphological analysis of the peripheral blood stages, it was possible to observe primary merogonic (trophozoites, early-stage meront, meronts, and merozoites) and secondary merogonic (young meront and meronts) developmental stages, of an unidentified species of Datylosoma (Fig. 4). The morphometric data are reported in Table 3.
A–P Morphological data on Dactylosoma amphibia n. sp. infecting Brazilian anurans. A–K Primary merogony: A–C Trophozoites (young trophozoite—arrow); D–E Early-stage meront; F Amoeboid appearance meront G Fan-like shaped primary meront. H Merozoites. I–P Secondary merogony: I–J Young meronts; K–O Mature meronts; K Fan-like shaped meront L Meront with dactylate apperance; P Merozoites. Scale bar: 20 µm
Species Description
Dactylosoma amphibia n. sp. Úngari, Netherlands, Da Silva and O´Dwyer n. sp.
Type-host: Rhinella diptycha [Cope, 1862] (Anura: Bufonidae).
Other hosts: Trachycephalus typhonius [Linnaeus, 1758] (Anura: Hylidae) and Leptodactylus latrans [Steffen, 1815] (Anura: Leptodactylidae).
Type-locality: Mato Grosso State (14°31′10.77″S, 51°41′43.99″W).
Other localities: L. latrans: Mato Grosso do Sul State (21°19′53.47″S, 51°56′39.61″W). T. typhonius: São Paulo State (21°50′43.34″S, 48°27′8.95″W).
Site of infection: Peripheral blood.
Prevalence: The total prevalence found was 2.58% (3/116).
Parasitaemia: 0.16%
Other parasitaemia: L. latrans with 0.09% and T. typhonius with 0.06%
Etymology: The epithet “amphibia” refers to the analysed host group (amphibians), since this species has low host specificity parasitizing different species of the amphibian group.
Gene sequence: The 18S rDNA gene sequences obtained were 599 bp (R. diptycha) [OP480618], 593 bp (T. typhonius) [OP480172], and 609 bp (L. latrans) [OP480169].
Type-material: Hapantotype 1 X blood smear from R. diptycha was deposited in the collection of the National Institute of Amazonian Research (INPA), Manaus, Brazil [TO BE ADDED]. Parahapantotype 2 X blood smears: 1 X from T. typhonius and 1X from L. latrans were deposited in the collection of the National Institute of Amazonian Research (INPA), Manaus, Brazil [INPA26].
Note: The authors of the new taxon are different from the authors of this paper; Article 50.1 and Recommendation 50A of the International Code of Zoological Nomenclature [26].
Morphological and Morphometric Analysis
The developmental stages of an unidentified species of Dactylosoma were observed in three hosts, of which when the developmental stage was identified in more than one host, presented similar morphology and morphometry among them. The developmental stages observed were: trophozoites, early-stage meronts, meronts (amoeboid appearance and fan-like shaped meronts), and merozoites from primary merogony. For secondary merogony, early-stage meronts, meronts (fan-like shaped, dactylate appearance, and round-shaped meronts), and merozoites were identified. During primary merogony, meronts producing up to 20 chromatin divisions were observed and during secondary merogony, meronts producing up to six-chromatin divisions were observed (Fig. 4, Table 3). The total number of each developmental stages found in all the positive hosts is included as the letter “n” in the end of each developmental stage description.
Primary Merogony
Trophozoite (Fig. 4A–C): small ovoid to elongated, with both extremities rounded, cytoplasmic vacuoles evidenced and cytoplasm staining whitish, measuring 5.37 ± 0.99 µm long, 2.99 ± 0.53 µm wide, and with area of 13.98 ± 2.46 µm2. Nuclei not clearly defined, sometimes placed at the middle or slightly towards one end of the parasite, non-dense chromatin, and stained dark-purple, measuring 1.94 ± 0.83 µm long, 1.96 ± 0.93 µm wide, and with area of 2.95 ± 1.15 µm2 (n = 13).
Early-stage meront (Fig. 4D–E): Ovoid shape, with large cytoplasmic vacuoles evidenced, occupying half to almost the entire parasite, dislocating towards one end of the cytoplasm and nuclear chromatin of the parasite, with the cytoplasm stained purple-bluish, measuring 6.78 ± 0.58 µm long, 3.95 ± 0.50 µm wide, and with area of 19.90 ± 3.67 µm2. The nuclear chromatin in the division process stained purple, always located close to the parasite’s membrane, measuring 2.40 ± 0.37 µm long, 2.15 ± 0.28 µm wide, and with area of 2.96 ± 0.87 µm2. In some cases, the parasite displaced the cell nuclei towards one side causing hypertrophy of the host cell (n = 10).
Amoeboid shaped meront (Fig. 4F): Large meront with one end tapered and the other rounded, cytoplasm stained bluish with vacuoles always presents, measuring 12.98 ± 1.09 µm long, 9.35 ± 1.39 µm wide, and with area of 76.78 ± 16.16 µm2. Multinucleate with 5–7 nuclei, non-dense nuclear chromatin distributed towards the cytoplasm stained in dark-purple, measuring 2.20 ± 0.40 µm long, 1.05 ± 0.40 µm wide, and with area of 2.90 ± 0.18µm2 (n = 9).
Fan-like shaped meront (Fig. 4G): Large and multinucleate meronts (13 and 20 nuclei), measuring 10.11 ± 0.19 µm long, 7.37 ± 0.13 µm wide, and with area of 58.94 ± 1.21 µm2. In some cases, it is possible to observe small vacuoles in the cytoplasm, which is stained purple-bluish. Ovoid dense nuclear chromatin positioned on one side of meront, forming fan-like shape, measuring 0.99 ± 0.14 µm long, 0.57 ± 0.20 µm wide, and with area of 1.09 ± 0.21 µm2. These shaped meronts always cause dislocation of the nuclei cell towards one side and, in some cases, hypertrophy of the host cell (n = 2).
Merozoite (H): Small, grouped together in the erythrocyte, measuring 3.05 ± 1.13 µm long, 1.72 ± 0.40 µm wide, and with area of 3.71 ± 1.03 µm2. Ovoid small and dense nuclear chromatin stained dark-purple, measuring 1.33 ± 0.33 µm long, 1.69 ± 0.40 µm wide, and with area of 3.35 ± 1.58 µm2 (n = 10).
Secondary Mergony
Early-stage meront (Fig. 4I–J): (I) Round shape with cytoplasm stained whitish, with or without small vacuoles evident, measuring 6.13 µm long, 4.09 µm wide, and with area 17.17 µm2. Multinucleate with four slender nuclei distributed peripherally with chromatin stained dark-purple, measuring 0.41 ± 0.10 µm long, 0.78 ± 0.32 µm wide, and with area of 1.99 ± 0.24 µm2 (n = 1). (J) Elongated with one end tapered and the other rounded, with or without cytoplasmic vacuole, measuring 5.09 µm long, 4.17 µm wide, and with area of 14.91 µm2. Rounded end containing three nuclei, with dense and circular chromatin staining in deep magenta peripherally distributed, the measurements of the three nuclei are 1.15 ± 0.2 µm long, 1.02 ± 0.07 µm wide, and with area of 1.6 ± 0.71 µm2 (n = 1).
Fan-like shaped meront (Fig. 4K): Multinucleate meront with a tapered side and the other rounded with 6 nuclei, forming fan-like shape; in some cases, it is possible to observe small cytoplasmic vacuoles, the cytoplasm stained bluish, measuring 7.01 ± 0.22 µm long, 3.99 ± 0.14 µm wide, and with area of 16.98 ± 0.52 µm2. Ovoid dense chromatin positioned on one side of meront stained dark-purple, measuring 1.15 ± 0.24 µm long, 1.02 ± 0.07 µm wide, and with area of 1.59 ± 0.71 µm2 (n = 2).
Dactylate shaped meront (Fig. 4L): Round shape (hand-like appearance) with transparent cytoplasm with or without small cytoplasmic vacuoles evidenced, measuring 6.24 µm long, 4.19 µm wide, and with area of 17.52 µm2. Multinucleate with five nuclei located peripherally with dense chromatin staining in deep magenta, with the five nuclei measuring 0.56 ± 0.11 µm long, 0.89 ± 0.31 µm wide, and with area of 1.91 ± 0.32 µm2 (n = 1).
Mature meronts (Fig. 4M–O): Ovoid multinucleated (up to six nuclei) meronts, in some cases with one end more tapered than the other, intra or extracellular, with or without cytoplasm vacuoles; measuring 10.0 ± 0.65 µm long, 6.13 ± 1.06 µm wide, and with area of 42.64 ± 5.30 µm2. Rounded to irregular shaped nuclei stained purplish, measuring 1.78 ± 0.45 µm long, 1.32 ± 0.52 µm wide, and with area of 2.44 ± 1.20 µm2 (n = 3).
Merozoites (Fig. 4P): Small and located within the erythrocyte, with one end more tapered, measuring 5.33 ± 0.14 µm long, 4.39 ± 0.44 µm wide, and with area of 17.24 ± 0.11 µm2. Rounded and dense nuclear chromatin stained in dark-purple, measuring 2.02 ± 0.35 µm long, 1.89 ± 0.43 µm wide, and with area of 4.85 ± 1.36 µm2 (n = 3).
Remarks
Regarding morphological and morphometric data, the seven species currently recognized have their peculiarities, which proved the one described here to be a new species. Of these seven, two were described infecting fish hosts, Dactylosoma salvelini Fantham, Porter and Richardson, 1942 from Canada and Dactylosoma iethrinorum Saunders, 1960 from Egypt. The other five were described from anuran hosts: Dactylosoma sylvatica Fantham, Porter and Richardson, 1942 infecting Lithobates sylvatica LeConte, 1825 from Canada; Dactylosoma taiwanensis Manwell, 1964 infecting Fejervarya limnocharis Gravenhorst, 1829 from Taiwan; Dactylosoma ranarum Kruse, 1890 (syn. D. splendes) infecting Pelophylax kl. esculentus Linnaeus, 1758; Dactylosoma kermiti Netherlands, Cook, Du Preez, Vanhove, Brendonck and Smit, 2020 infecting Ptychadena anchietae Bocage, 1868; Sclerophrys gutturalis Power, 1927 from South Africa; and Dactylosoma piperis Úngari, Netherlands, Silva and O´Dwyer 2020 infecting Leptodactylus labyrinthicus Spix, 1824 from Brazil (Table 4).
Dactylosoma amphibia n. sp. has unique characteristics that distinguish it from the seven previously described and recognized species. The first merogony is characterized by small trophozoites with cytoplasmic vacuoles, early-stage meronts with cytoplasmic vacuoles occupying almost the entire parasite, and mature meronts containing variable shapes and sizes with up to 20 nuclei per meront. Secondary merogony contained of mature meronts with up to six nuclei and free, small, and elongated intraerythrocytic merozoites. In both merogonic cycles, variable meront shape were observed, such as the fan-like, dactylate, amoeboid, and rounded shape.
According to literature, primary merogony is characterized by producing up to 16 merozoites forming a large multinucleate meront in hand-like or rosette-like shape [27]. From this study, up to 20 chromatin divisions (nuclei) in a fan-like shape and amoeboid appearance were observed. However, the second merogony is represented by up to eight merozoites [27] and in this study up to six-chromatin division were observed.
Netherlands et al. [12] reported secondary merogony of D. kermiti with meronts producing up to six nuclei (chromatin division), similar to the present study. However, the trophozoite morphology reported in D. kermiti can be distinguished from Dactylosoma amphibia n. sp. with the nuclei not evident in D. kermiti, while in this study it was possible to identify in some cases placed at the middle or slightly towards one end of the parasite, non-dense chromatin and stained dark-purple. Moreover, D. kermiti primary merogony was characterized by up to 14 nuclei formed differing from this study.
Regarding D. ranarum, the first species in Dactylosomatidae, it has some similarities with Dactylosoma amphibia n. sp.; the secondary merogony of both species reported a nucleus formation stage with up to six-chromatin divisions. However, the morphometric values of D. ranarum are distinct in comparison with Dactylosoma amphibia n. sp.
Comparing the new species with D. taiwanensis and D. sylvatica different morphometric values were observed. Dactylosoma taiwanensis also presented square-shaped meronts and second merogony up to eight chromatin divisions, differing from this study. Moreover, only a single, most probably the secondary merogonic cycle of D. sylvatica is reported in the literature, with meronts only producing up to eight merozoites, differing from this study.
Regarding the only Brazilian species described so far, D. piperis, there are some differences observed in comparison with the species from this study. The morphometric values from both species, the number of chromatin divisions from the two merogonies, and the presence in this study of amoeboid appearance meronts from the first merogony.
Molecular and Phylogenetic Analysis:
From the seven positive specimens, four with Hepatozoon and three with Dactylosoma, seven newly generated sequences were obtained, with the species of Dactylosoma generating amplicons of 599 bp (OP480168), 609 bp (OP480169), and 593 bp (OP480172); and the species of Hepatozoon generating four concatenated sequence amplicons of 1.319 bp (OP477334); 1.365 bp (OP477337), 1359 (OP477331), and 1.391 (OP477333).
In the phylogenetic analysis based on the alignment of the SSU rRNA sequences available on GenBank, both Maximum likelihood (ML) and Bayesian inference (BI) methods reported the same topologies with supported nodes and values in the clades (Fig. 5). The trees were generated with isolates from adeleorinid parasites (Haemogregarinidae, Hepatozoidae, Karyolysidae, and Dactylosomatidae) with Klossia helicina (HQ224955), Adelina dimidiata (DQ096835), and Adelina grylli (DQ096836) as outgroups. From the trees, the Dactylosomatidae clade forms a sister group to the large well-supported clade comprising isolates of Haemogregarinidae, Karyolysidae, and Hepatozoidae clades. The Dactylosomatidae clade comprises only Dactylosoma species, of which the isolates from this study grouped close to D. piperis (MW264134), but in a separate branch. Regarding the large well-supported clade, the monophyletic Haemogregarinidae clade was located as a sister group of a larger-clade comprising both Karyolysidae and Hepatozoidae polyphyletic groups. Species of Hepatozoon isolated from large mammals formed a sister group to the Karyolysidae clade comprising species of Karyolysus. Within the reptile and anuran Hepatozoon clade, the isolates from the present study grouped with isolates from Brazil, H. formosus Úngari, Netherlands, Da Silva and O´Dwyer, 2021 (MW584356), H. longinucleus Úngari, Netherlands, Da Silva and O´Dwyer, 2021 (MW584361), and H. latrensis. (MW584362/ MW584365). The isolates from Brazilian anurans formed a sister group to a clade comprising species of Hepatozoon from Brazilian snakes, Hepatozoon musa Borges-Nojosa, Borges-Leite, Maia, Zanchi-Silva, Braga and Harris, 2017 (MF497767), Hepatozoon sp. (MF497768), and Hepatozoon cuestensis O’Dwyer, Moço, Kdos, Spenassatto, da Silva and Ribolla, 2013 (MF497077).
Consensus phylogram of haemogregarines based on 18S rDNA sequences (486 nt). The topology trees were inferred by Bayesian (BI) and Maximum Likelihood (ML) methods (represented by ML tree). The scale bar represents 0.01 nucleotide substitutions per site. The isolates Adelina dimidiata (DQ096835), Adelina grylli (DQ096836) and Klossia helicina (HQ224955) were used as an out-group
The Hepatozoon isolates from the current study, were identified as H. latrensis, with similarity varying beetween 99.70–100% with H. latrensis (MW584365) from GenBank, with an intragenetic pair-wise divergence of 0.001 observed in the isolate OP477334 (Table 5).
Discussion
In the present study anurans of Bufonidae, Hylidae, and Leptodactylidae were screened for intracellular blood parasites, and have been found infected with Hepatozoon latrensis and Dactylosoma amphibia n. sp. Haemogregarines are considered to have a cosmopolitan distribution and have been found in all continents, except the Arctic and Antarctic regions. Some interesting questions still need to be answered regarding the evolution and dispersal of these parasites. How have they adapted to so many different host species distributed across so many varying environmental conditions and habitats? Brazilian anurans are considered a rather diverse group and more studies are required to understand their haemogregarine parasites. The present study has provided new information, helping to set a base for future work on the biodiversity of haemogregarine parasites from Brazilian anurans. Build on this knowledge will aid in answering questions on the biodiversity, evolution and dispersal of these parasites. Moreover, understanding the parasitic biodiversity and its occurrence in anurans may lead to a better comprehension of the host species condition, adaptation to the surrounding environment, and how they are affected by these parasites.
In Brazil, to date, only a handful of studies on haemogregarine parasites from anurans have been published (10, 24, 33–35). Côelho et al. [33] reported the occurrence of Dactylosoma sp. infecting four Rhinella major [Muller and Hellmich, 1936] with a prevalence of 5.5%, and Rhinella marina [Linnaeus, 1758] (the only one screened). Úngari et al. [24] reported on species of Dactylosoma, with a description of a new species, D. piperis, infecting one L. labyrinthicus. With regards to species of Hepatozoon, from the 145 frogs screened by Leal et al. [35], 18 (26.47%) Leptodactylus chaquensis Cei, 1950 and 24 (31.17%) Leptodactylus podicipinus Cope, 1862 were found parasitized. Another study from Ferreira et al. [34] analysed 36 anurans, and two (5.5%), one L. latrans and one R. diptycha, were infected with Hepatozoon sp. In addition, Úngari et al. [10] screened 66 anurans and reported a prevalence of 2.64% (4 host specimens) positive for species of Hepatozoon, two L. latrans and two L. labyrinthicus. As shown above, there have only been a few studied on haemogregarine parasites from Brazilian anurans. Furthermore, is it clear from the current data that the preevelence of haemogregarines infecting anurans from Brazil is surprisingly low. The prevalence found in the present study is 6.03%, supporting the results from similar studies conducted in Brazil [10, 33].
Four anurans were infected with H. latrensis in the present study. Úngari et al. [10] reported this parasite infecting two L. latrans from Mato Grosso State. Anurans of Leptodactylidae are known to harbour a great number of haemoparasites globally (30–32). Moreover, studies on haemogregarine parasites and different genera of anurans have shown more infection around anurans of the Leptodactylidae [10, 32]. According to Netherlands et al. [36], semi-aquatic and aquatic anurans normally show higher prevalence compared to terrestrial anurans, suggesting that the vectors may live around aquatic habitats, such as leeches. Besides, in Brazil, the first species of Hepatozoon described from an anuran host was Hepatozoon leptodactyli [Lesage, 1908] Pessoa, 1970 infecting Leptodactylus sp. based solely on morphological data [11]. Costa et al. [11] screened 90 L. latrans [Steffen, 1815] and Leptodactylus pentadactylus [Laurent, 1768] reporting 17 infected with H. leptodactyli. Furthermore, the leech Haememteria lutzi Pinto, 1920 was considered as the possible vector of H. leptodactyli [11]. Although the hosts from the present study were collected from the same State where H. latrensis was described [10], the anuran hosts were different, R. mirandaribeiroi, L. labyrinthicus, and Leptodactylus sp. Anurans from the Bufonidae include nocturnal and terrestrial anurans, while in the Leptodactylidae, the species were semi-aquatic and have nocturnal behaviour [11]. Leeches as possible vectors could be related to anurans of the Leptodactylidae, but are less likely for anurans from the Bufonidae, since bufonids spend time in the aquatic environment only in the breeding season, so it is more likely that possible vectors could be mosquitoes, sandflies, and other dipteran vectors [11]. Furthermore, it is important to highlight the generalist specificity of H. latrensis, infecting anurans from different families of Brazilian amphibians. This species has been proving how well adapted it is, infecting hosts with different ecological habits and behaviour.
To date, the complete cycle of Dactylosoma has not been clarified. However, Barta [43] experimentally infected Desserobdella picta (Verrill, 1872), the natural vector of B. stableri, with D. ranarum using frogs captured on the French island of Corsica. Although there was no development of zygote formation or gametes, it was observed D. ranarum sporogonic formation in the intestinal epithelium of the experimentally infected leech host (Barta, 1991). Moreover, Nöller [44] was the first to attempt transmission of D. ranarum using the leech Hemiclepsis margmata (Müller, 1774), but failed despite repeated attempts. In another study, Boulard et al. [45] tested Culicoides nubeculosus (Meigen, 1830) mosquitoes as a potential vector of D. ranarum, but the experiments were inconclusive. Therefore, studies aimed at possible vectors of Dactylosoma were developed experimentally. Future studies should be done emphasizing life cycles, with possible vectors captured in their environment, enriching the poor knowledge about Dactylosoma life cycles.
Regarding the morphological data, H. latrensis from this study presented some new developmental stages in blood smears, with free gamonts and microgamonts reported for the first time. Moreover, through molecular analysis, it was possible to identify the haemoparasites species found in this study, and also aid in the description of an undescribed species. Thus, the usefulness of molecular analysis as a diagnostic tool is shown in this study. Moreover, although the molecular tool provides inferences about species description, only by integrating the techniques of morphology, morphometry and molecular analysis, we can have advances in species description and how these parasites behave in different hosts.
These biodiversity of parasites infecting anurans only shows how important it is study this group of animals, of which is experiencing large-scale declines in species diversity. Over the last years, a third of the estimated amphibian species have declined, according to the IUCN Global Amphibian Assessment [28]. Nowadays, 8,425 anuran species have been described around the world, with a notable number of 1,146 Brazilian species [29]. Therefore, since anurans are known to harbour a great number of haemoparasites, with haemogregarines being the most commonly reported [30,31,32], this study has its relevancy for science, increasing the knowledge of parasites biodiversity.
Conclusion
The number of studies focussing on amphibian has been increasing in recent years, due to the possible extinction of this group [1]. However, in Brazil, the richest country in amphibian diversity, studies on parasites have not received the same attention, even with researches showing that parasites directly influence their quality of life, behaviour, reproduction, fecundity, fitness, and reproduction [32, 37, 38].
Thus, the first step for a better comprehension of host-parasite relation, potential pathogenicity, and their consequences for the anuran hosts is the correct identification of these parasites. Based on the information reported above, our study contributes to reducing the lack of knowledge on haemogregarine biodiversity infecting Brazilian anurans from the Midwest and Southeast regions, and includes the description of a new species of Dactylosoma, Dactylosoma amphibia n. sp., through morphological, morphometric and molecular approaches.
References
Wake D, Vredenburg VT (2008) Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA 105:11466–11473. https://doi.org/10.1073/pnas.0801921105
Isaak-Delgado AB, López-Díaz O, Romero-Callejas E, Martínez-Hernández F, Muñoz-Garcia CI, Villalobos G, Randón-Franco E (2020) Morphological and molecular characteristics of hemoparasites in vaillant’s frogs (Lithobates vaillanti). Parasitol Res 119:1891–1901. https://doi.org/10.1007/s00436-020-06689-1
Stuart S, Chanson J, Cox N, Young B (2006) El estado global de los anfíbios. In: Ângulo A, Rueda J, Rodríguez J, La Marca E (eds) Técnicas de inventario y monitoreo para los anfibios de la región tropical andina. Conservación internacional serie manuales para la conservación, vol 2. Panamericana Formas e Impresos S A, Bogotá, pp 19–34
Netherlands EC, Cook CA, Smit NJ (2014) Hepatozoon species [Adeleorina: Hepatozoidae] of African bufonids, with morphological description and molecular diagnosis of Hepatozoon ixoxo sp. nov. parasiting three Amietophrynus species (Anura: Bufonid). Parasit Vectors 7:552–563. https://doi.org/10.1186/s13071-014-0552-0
Barta JR, Ogedengbe JD, Martin DS, Smith TG (2012) Phylogenetic position of the Adeleorinid Coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rDNA sequences. J Eukaryot Microbiol 59:171–180. https://doi.org/10.1111/j.1550-7408.2011.00607.x
Davies A, Johnston M (2000) The biology of some intraerythrocytic parasites of fishes, Amphibia and reptiles. Adv Parasitol 45:1–107. https://doi.org/10.1016/S0065-308X(00)45003-7
Netherlands EC, Cook CA, Smit NJ, Du Preez LH (2014) Redescription and molecular diagnosis of Hepatozoon theileri (Laveran, 1905) (Apicomplexa: Adeleorina: Hepatozoidae) infecting Amietia quecketti (Anura: Pyxicephalidae). Folia Parasitol 61:239–300. https://doi.org/10.14411/fp.2014.046
Levine ND (1988) The protozoan phylum apicomplexan, vol 2. CRC Press, Boca Raton
Netherlands EC, Cook CA, Du Preez LH (2018) Monophyly of the species of Hepatozoon (Adeleorina: Hepatozoidae) parasiting (African) anurans, with the description of three new species from hyperoliid frogs in South Africa. Parasitol 145:1039–1050. https://doi.org/10.1017/S003118201700213X
Úngari LP, Netherlands EC, Santos ALQ, Alcantara EP, Emmerich E, Silva RJ, O´Dwyer LH (2021) New insights on the diversity of Brazilian anuran blood parasites: with the description of three new species of Hepatozoon (Apicomplexa:Hepatozoidae) from Leptodactylidae anurans. Int J Parasitol 14:190–201. https://doi.org/10.1016/j.ijppaw.2021.02.009
Costa SCG, Pessoa SB, Pereira NM, Colombo T (1973) The life history of Hepatozoon leptodactyli (Lesage, 19080) Pessoa, 1970: a parasite of the common laboratory animal – the frog of the genus Leptodactylus. Mem Inst Oswaldo Cruz 71:1–18. https://doi.org/10.1590/S0074-02761973000100001
Netherlands EC, Cook CA, Du Preez LH, Vanhove MPM, Bren- donck L, Smit NJ, (2020) An overview of the Dactylosomatidae (Apicomplexa: Adeleorina: Dactylosomatidae), with the description of Dactylosoma kermiti n. sp. parasitising Ptychadena anchietae and Sclerophrys gutturalis from South Africa. Int J Parasitol 11:246–260. https://doi.org/10.1016/j.ijppaw.2019.12.006
Zippel KC, Lillywhite HB, Mladinich CR (2001) New vascular system in reptiles: anatomy and postural hemodynamics of the vertebral venous plexus in snakes. J Morphol 250:173–184. https://doi.org/10.1002/jmor.1063
Sebben A (2007) Microdissecção fisiológica a fresco: uma nova visão sobre a anatomia de anfíbios e répteis. In: Nascimento LB, Oliveira ME (eds) Herpetologia no Brasil, vol 2. Sociedade Brasileira de Herpetologia, Belo Horizonte, pp 311–325
Eisen RJ, Schall JJ (2000) Life history of malaria parasite (Plasmodium mexicanum): independent traits and basis for variation. Proc R Soc B: Biol Sci 267:793–799. https://doi.org/10.1098/rspb.2000.1073
Cook CA, Smit NJ, Davies AJ (2009) A redescription of Haemogregarina fitzsimonsi Dias, 1953 and some comments on Haemogregarina parvula Dias, 1953 (Adeleorina: Haemogregarinidae) from Southern African tortoises (Cyptodira: Testudinidae) with new host data and distribution records. Folia Parasitol 56:173–179. https://doi.org/10.14411/fp.2009.021
Ujvari B, Madsen T, Olsson M (2004) High prevalence of Hepatozoon spp. (Apicomplexa, Hepatozoidae) infection in water pythons (Liasis fuscus) from Tropical Australia. J Parasitol 90:670–672. https://doi.org/10.1645/GE-204R
Perkins SL, Keller AK (2001) Phylogeny of nuclear small subunit rRNA genes of hemogregarines amplified with specific oligonucleotídeos. J Parasitol 87:870–876. https://doi.org/10.1645/0022-3395(2001)087[0870:PONSSR]2.0.CO;2
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. https://doi.org/10.1093/bioinformatics/btg180
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:312–1313. https://doi.org/10.1093/bioinformatics/btu033
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67:901–904. https://doi.org/10.1093/sysbio/syy032
Rambaut A (2012) FigTree v1.4.2. Available at: http://tree.bio.ed.ac.uk/software/figtree/ (Accessed Mar 2022).
Úngari LP, Netherlands EC, Santos ALQ, Alcantara EP, Emmerich E, Silva RJ, O´Dwyer LH (2020) A new species, Dactylosoma piperis n. sp. (Apicomplexa, Dactylosomatidae), from the pepper frog Leptodactylus labyrinthicus (Anura, Leptodactylidae) from Mato Grosso State, Brazil. Parasite 27:73. https://doi.org/10.1051/parasite/2020070
O´Dwyer LH, Moço TC, Paduan KS, Spenassatto C, Silva RJ, Ribolla PE (2013) Description of three new species of Hepatozoon (Apicomplexa, Hepatozoidae) from Rattlesnakes (Crotalus durissus terrificus) based on molecular, morphometric and morphologic characters. Exp Parasitol 135:200–207. https://doi.org/10.1016/j.exppara.2013.06.019
ICZN, International Code of Zoological Nomenclature (1999) The International Trust for Zoological Nomenclature: London. Available at: http://www.nhm.ac.uk/hosted-sites/iczn/code/ (Accessed July 2021).
Barta JR, Boulard Y, Desser SS (1987) Ultrastructural observations on secundary merogony and gametogony of Dactylosoma ranarum Labbé, 1894. J Parasitol 73:1019–1029. https://doi.org/10.2307/3282527
Frost DR (2019) Amphibian species of the world. American Museum of Natural History, New York
Frost DR (2022) Amphibian species of the world: an online reference. American Museum of Natural History, New York
Telford SR Jr (2009) Hemoparasite of the reptilian: color atlas and text. CRC Press, Boca Raton
Smith TG (1996) The genus Hepatozoon (Apicomplexa: Adeleorina). J Parasitol 82:565–585. https://doi.org/10.2307/3283781
Barta JR, Desser SS (1984) Blood parasites of amphibians from Algonquin Park, Ontario. J Wildl Dis 20:180–189. https://doi.org/10.7589/0090-3558-20.3.180
Côelho TA, De Souza DC, Oliveira EC, Correa LL, Viana LA, Kawashita-Robeiro RC (2021) Haemogregarine of Genus Dactylosoma (Adeleorina: Dactylosomatidae) in species of Rhinella (Anura: Bufonidae) from the Brazilian Amazon. Acta Parasitol 66:1574–1580. https://doi.org/10.1007/s11686-021-00399-z
Ferreira DAR, Perles L, Machado RZ, Prado CPA, André MR (2020) Molecular detection of Apicomplexan hemoparasites in anurans from Brazil. Parasitol Res 119:3469–3479. https://doi.org/10.1007/s00436-020-06835-9
Leal DDM, Dreyer CS, Da Silva RJ, Ribolla PEM, Paduan KS, Blanchi I, O´Dwyer LH, (2015) Characterization of Hepatozoon spp. in Leptodactylus chaquensis and Leptodactylus podicipinus from two regions of the Pantanal, state of Mato Grosso do Sul, Brazil. Parasitol Res 114:1541–1549. https://doi.org/10.1007/s00436-015-4338-x
Netherlands EC, Cook CA, Du Kruger DJD, Du Preez LH, Smit NJ (2015) Biodiversity of frog haemoparasites from sub-tropical northern KwaZulu-Natal. South Africa, IJP:PAW 4:135–141. https://doi.org/10.1016/j.ijppaw.2015.01.003
Segalla M, Berneck B, Canedo C, Caramaschi U, Cruz CAG, Garcia PCA, Grant T, Haddad CFB, Lourenço AC, Mangia S, Mott T, Nascimento L, Toledo LF, Werneck F, Langone LA (2021) List of Brazilian amphibians. Herpetol Bras 10:121–216. https://doi.org/10.5281/zenodo.4716176
Lainson R, Paperna I, Naiff RD (2003) Developmental of Hepatozoon caimani (Carini, 1909) Pessoa, de Biasi and Souza, 1972 in the caiman Caiman c. crocodilus, the frog Rana catesbianae and the mosquito Culex fatigans. Mem Inst Oswaldo Cruz 98:103–113
Fantham HB, Porter A, Richardson LR (1942) Some haematozoa observed in vertebrates in Eastern Canada. Parasitology 34:199–226. https://doi.org/10.1017/S0031182000016176
Saunders DC (1960) A survey of the blood parasites in the fishes of the Red Sea. Trans Am Micros Soc 79:239–252. https://doi.org/10.2307/3223730
Manwell RD (1964) The genus Dactylosoma. J Protozool 11:526–530. https://doi.org/10.1111/j.1550-7408.1964.tb01792.x
Kruse W (1890) Archiv fur pathologische. Anatomie Physiol fur klin Med 120:173–183
Barta JR (1991) The dactylosomatidae. Adv Parasitol 30:1–37
Nöller W (1913) Die Blutprotozoen des wasserfrosches und ihre übertragung. Archiv fur Protistenkde 31:169–240
Boulard Y, Vivier E, Landau I (1982) Ultrastucture de Dactylosoma ranarum (Kruse, 1890); affinites avec les coccidies; revision du statut taxonomique des dactylosomides. Protistologica 18:103–112
Acknowledgements
We thank the team of the Laboratory for Teaching and Research in Wild Animals (LAPAS) and Non-governmental organization for the preservation of wild animals in Brazil (ONG PAS). Financial assistance: R.J.S. is supported by FAPESP (2016/50377-1); CNPq (309125/2017-0); CNPq-PROTAX (440496/2015-2). L.P.U is supported by FAPESP (2018/00754-9; 2018/09623-4). L.H.O is supported by FAPESP (2018/09623-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed (IBAMA licence 60640–1; CEUA-UNESP 1061).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Úngari, L.P., Netherlands, E.C., Santos, A.L.Q. et al. Diversity of Haemogregarine Parasites Infecting Brazilian Anurans, with a Description of New Species of Dactylosoma (Apicomplexa: Adeleorina: Dactylosomatidae). Acta Parasit. 67, 1740–1755 (2022). https://doi.org/10.1007/s11686-022-00624-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00624-3