Abstract
Purpose
Babesia canis infection occurs in many locations throughout Europe. However, various studies report different clinicopathological findings in affected dogs. This study was focused on changes in clinical and hematologic parameters in dogs with B. canis infection from eastern Slovakia.
Methods
The study was prospective and included 45 dogs with suspected babesiosis. Babesia canis infection was confirmed by PCR in 34 cases and by blood smear microscopy in 24 (70.6%) of them. Hematology results, clinical examination from these dogs, and possible co-infection with other tick-borne pathogens by PCR were subsequently evaluated.
Results
The major clinical signs found included lethargy (91%), fever (59%), anorexia (59%), pigmenturia (47%) and icterus (18%). Mortality rate was 6%. Thrombocytopenia was the most common hematologic change, observed in 100% of the dogs with B. canis infection. Other frequent findings were lymphopenia (82%) and anemia (68%). No co-infections were detected. Anaplasma phagocytophilum infection was diagnosed by PCR only in one dog, which was not infected with B. canis.
Conclusions
This study showed that B. canis infection in eastern Slovakia should be diagnosed by PCR when there is clinical suspicion of the disease, as almost 30% of the infected sick dogs did not have demonstrable parasites in their blood smear by microscopy. Lymphopenia is a frequent hematologic finding in B. canis infection and observed even more often than anemia. However, in agreement with previous studies, thrombocytopenia remains the most common hematologic finding associated with B. canis infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Babesia canis is a tick-borne pathogen that predominantly affects dogs [1]. It is a protozoal organism mostly found in Europe and transmitted exclusively by the Ixodid tick Dermacentor reticulatus (Fabricius 1794) [1, 2]. Dermacentor reticulatus is found in many areas throughout Europe, including eastern Slovakia [3, 4], where our study was conducted. There are two species of Babesia affecting dogs, which occur in Slovakia. Babesia canis, a large form Babesia species for which Slovakia is considered to be a country with an endemic occurrence [5,6,7], and the small form Babesia gibsoni which occurs rather sporadically in this country [8]. The presence of other large forms of Babesia spp. (Babesia vogeli and Babesia rossi) in Slovakia has not yet been documented.
Typical clinical signs of babesiosis include apathy, anorexia, pale mucous membranes, jaundice, pigmenturia, splenomegaly and fever [2, 9,10,11,12]. A severe form of the disease can lead to multiple organ injury and death [2, 9, 13, 14].
Babesia canis affects the blood system and various changes in hematologic parameters can be observed. Anemia and thrombocytopenia are the most commonly reported [9,10,11,12,13,14,15,16,17,18]. There are several mechanisms, which contribute to the anemia development in babesiosis. It was proposed that the initial decrease of hematocrit is caused by dilution of the blood as a result of hypotension and increased retention of fluid in the blood stream [19]. Piroplasms can damage erythrocytes via several pathways, mainly by direct interaction with the host cell [15, 20, 21]. Immune-mediated hemolytic anemia is another reported cause of red blood cell destruction [9, 11, 21,22,23]. Interestingly, previous studies have shown that parasitemia is not always related to the degree of anemia in B. canis infection [11, 19, 24], nevertheless, the onset of anemia is dependent on the infectious dose [19]. Therefore, dogs with higher infectious dose manifest anemia sooner [19].
Thrombocytopenia is seen more frequently than anemia in dogs with B. canis infection [2, 10, 11, 14, 16, 18, 25] and it is generally accepted as a hallmark of babesiosis [1]. The pathogenesis of thrombocytopenia in babesiosis is not entirely understood and it probably involves immune-mediated platelet destruction, consumption due to coagulopathy and platelet sequestration in the spleen [1, 9, 17, 23]. Nevertheless, the mean platelet volume is usually increased, which indicates a regenerative response of the bone marrow [9]. Interestingly, despite marked thrombocytopenia dogs do not usually manifest clinical signs associated with hemostatic alterations, such as petechia or bleeding [1, 2].
Changes in the white blood cell count can be diverse in babesiosis. Various authors report different findings. Leucopenia [2, 11, 16, 17], neutropenia [2, 11, 16] and lymphopenia [2, 11, 16, 18] have been reported frequently.
Decrease in leucocytes may be caused by their sequestration in the spleen [2], sequestration in the pulmonary vasculature [9], or increased consumption and sepsis [16]. In addition, Rafaj et al. [17] proposed a mechanism based on platelet ability to bind with activated endothelial cells and subsequently react with leucocytes, which causes their so-called “secondary capture”. Developed neutrophil–endothelial interaction can decrease the amount of white blood cells in peripheral blood in the initial days of infection [17]. Such mechanism could contribute to thrombocytopenia as well as leucopenia. This theory was supported by the findings of Schetters et al. [19] who observed the presence of thrombocytopenia in dogs with babesiosis approximately 1 day before leucopenia. The same authors also found that the onset of leucopenia and thrombocytopenia is dependent on the level of infectious dose and dogs infected with a lower infectious dose developed these changes later [19].
Materials and Methods
This study was prospective and involved samples from 45 dogs suspected for canine babesiosis, which visited the Small Animal Clinic at the University of Veterinary Medicine and Pharmacy in Kosice, eastern Slovakia, between 7th May 2020 and 13th May 2021. This group involved 34 males and 11 females. Various breeds were represented in the studied population but crossbreed dogs were the most common group (10 cases). Frequently represented breeds included the Labrador Retriever (3 dogs), German Shepherd (2 dogs), and Irish Setter (2 dogs). Majority of dogs (41 cases) did not travel outside the Slovakia in the last 2 months prior the examination. Therefore, it is assumed that majority of examined dogs was infected in eastern Slovakia.
Dogs with typical clinical signs of babesiosis were examined. Detailed anamnesis, clinical examination and complete blood count (CBC) were obtained and blood smear assessments were performed. Diagnosis of babesiosis was made in the case of positive result of PCR examination. Blood samples were sent to the Laboratory of Molecular Ecology of Vectors (Institute of Parasitology, Slovak Academy of Sciences) for PCR to confirm and identify Babesia spp. By subsequent DNA sequencing, the precise genotyping of Babesia spp. present in the samples was possible. The study included only initial blood samples which were obtained when the dog was admitted for treatment.
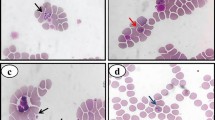
A CBC was obtained by automatic cell counter (ProCyte Dx Hematology Analyzer, ®IDEXX Laboratories, Inc., Hoofddorp, Netherlands). Studied parameters included red blood cell count, hematocrit, hemoglobin concentration, absolute reticulocyte count, total white blood cell count, absolute neutrophil, lymphocyte, monocyte, eosinophil and basophil counts, platelet count, mean platelet volume (MPV) and plateletcrit. Default reference ranges were used to assess the increase or decrease of hematology parameters. Anemia was defined as simultaneous decrease in hematocrit, red blood cell count (RBC) and hemoglobin values below reference ranges [26] and regenerative anemia was diagnosed if the dog had absolute reticulocyte count more than 110,000 reticulocytes per microliter [26]. Blood smears of all hematology samples were made and stained by Hemacolor staining (Merck KGaA, Darmstadt, Germany). Blood smears were assessed under microscope at × 1000 magnification.
DNA from blood samples was extracted using the Thermo Scientific GeneJET Genomic DNA Purification Kit (Vilnius, Lithuania). Subsequently, PCR assays targeting a 411–452 bp long fragment of the 18S rRNA gene of Babesia spp. using primers BJ (5'-GTC TTG TAA TTG GAA TGA TGG-3') and BN (5'-TAG TTT ATG GTT AGG ACT ACG-3') [27] and 334 bp long portion of the A. phagocytophilum msp2/p44 gene using pair of primers ApMSP2F (5'-ATG GAA GGT AGT GTT GGT TAT GGT ATT-3'), ApMSP2R (5'-TTG GTC TTG AAG CGC TCG TA-‘3) according to Massung and Slater [28] were performed.
Blood samples were also tested for the presence of other vector-borne pathogens, such as portion of the P18 gene of B. gibsoni with primers d3 (3'-TCC GTT CCC ACA ACA CCA GC-3') and d4 (5'-CGA ATG AGG ATG ATG AGG AGG A-5'), conventional PCR assay which amplifies a 203-bp fragment of the cytochrome c oxidase subunit 1 (COI) gene of D. immitis (with primers DICOI-F1 5'-AGT GTA GAG GGT CAG CCT GAG TTA and DICOI-R1 5'-ACA GGC ACT GAC AAT ACC AAT-3') and a 209-bp portion of D. repens COI gene (with pair of primers DRCOI-F1 5'-AGT GTT GAT GGT CAA CCT GAA TTA-3' and DRCOI-R1 5'-GCC AAA ACA GGA ACA GAT AAA ACT-3') according to Rishniw et al. [29] to screen for possible coinfections.
The results of PCR assays were visualized after gel electrophoresis under UV light, using a UV transilluminator. Positive PCR products were purified using the Nucleospin Extract II kit (Macherey Nagel, Düren, Germany) and sent for sequencing in both directions to the Institute of Immunology, University of Veterinary Medicine and Pharmacy in Košice, Slovakia, using the same primers as used for the PCRs.
The obtained nucleotide sequences were manually edited for removal of misread nucleotides using the MEGA X software [30]. To confirm the identity of each sequence, the Basic Local Alignment Search Tool (BLAST) algorithm was used [31]. Sequences obtained in this study were compared with GenBank entries. Nucleotide sequences of the B. canis 18S rRNA gene fragment and a portion of the A. phagocytophilum msp2 gene obtained in this study were deposited in the GenBank database and are available under the following Accession numbers: OK446632–OK446651 for the B. canis fragments and OK523377 for the A. phagocytophilum nucleotide sequence.
Results
Altogether 45 blood samples from 45 dogs were tested for the presence of tick-borne pathogens by PCR. DNA of Babesia spp. was amplified from 34 dogs during the initial molecular screening, representing a prevalence of 75.6% among the dogs suspected for babesiosis in this study. From 34 positive samples, 24 (70.6%) were positive by blood smear and PCR, while 10 (29.4%) samples were diagnosed only by PCR. Blood smears in these dogs were negative.
Genotyping of PCR-positive samples by further sequencing confirmed the presence of B. canis in all positive samples. Nucleotide sequences with 100% coverage with homologous sequences from GenBank database were 97.95–100% identical with several B. canis isolates including those previously amplified from dogs in Slovakia (MK508870, MK508874), the Mesh 4 isolate of B. canis from An Iranian dog (MN173223), BCCRO10 isolate from a Croatian dog (MK089785), and others.
No co-infection with other parasite pathogens has been observed by PCR in any of the samples. However, one sample which was negative for B. canis tested positive for A. phagocytophilum DNA.
The obtained nucleotide sequence was deposited in GenBank (Acc. No. OK523377). BLAST analysis confirmed that this was 100% identical with several A. phagocytophilum isolates previously detected in an I. ricinus tick removed from a red fox in Slovakia (MG334167), a questing I. ricinus from Poland (MK802161), and ticks removed from red deer (MK625083) and roe deer (MK625087) in Poland, and from the blood of a Polish red deer (MK625084).
In the group of the dogs with B. canis infection, the main complaint of the owners was lethargy that was described in 31 dogs (91%). Other clinical signs included fever (20 dogs; 59%), anorexia (20 dogs; 59%), pigmenturia (16 dogs; 47%) and icterus (6 dogs; 18%). Two dogs (6%) developed acute renal disease and other two dogs (6%) died despite the administered treatment. Therefore, mortality rate in presented study was 6% (2 dogs).
Fever was a common clinical finding. 14 dogs had a moderate fever (39.3–40.0 °C) and 6 others had a higher fever (40.1–41.0 °C). No dog had body temperature above 41.0 °C.
Twenty-nine dogs were males (85%) and five were females (15%). Data were statistically evaluated by the GraphPad Prism software using the Fisher´s exact test and showed that there was a statistically significant difference between sexes (p ˂0.05).
The youngest dog was 4 months of age and the oldest was 14 years old. The average age of infected dogs was 5.4 years. The most commonly infected breed was crossbreed (10 cases). Other represented dog breed categories were herding group (7 cases), sporting group (7 cases), working group (5 cases), toy group (2 cases), terrier group (2) and hound group (1).
Thrombocytopenia was the most frequent hematologic abnormality (Table 1). It was present in all 34 dogs diagnosed with B. canis infection. The plateletcrit was similarly decreased in all 34 dogs, and the MPV was increased in 33 dogs (97%). Thirty-one dogs (91%) had a platelet count of less than 50 × 109/l.
Another very common finding was lymphopenia (28 dogs; 82%) which were observed in more infected dogs than anemia (25 dogs; 74%) (Table 1). Leucopenia (19 dogs; 56%), neutropenia (12 dogs; 35%) and monocytosis (12 dogs; 35%) were common findings as well.
The majority of anemic dogs (12 dogs; 35%) had mild anemia [26] with hematocrit between 0.3 l/l and 0.37 l/l, while 6 dogs (18%) had hematocrit levels between 0.20 l/l and 0.29 l/l, classified as moderate anemia [26]. Two dogs (6%) had hematocrit between 0.2 l/l and 0.14 l/l and three dogs (9%) suffered from very severe anemia with hematocrit levels below 0.13 l/l [26].
There were two dogs with babesiosis with increased reticulocyte counts. One had mild RBC regeneration, while the other had marked regeneration. One dog had an increased MCV (mean corpuscular volume) and another dog had decreased MCHC (mean corpuscular hemoglobin concentration); however, these two dogs did not have increased reticulocyte counts.
Discussion
Canine babesiosis is a common disease in Slovakia which can have serious consequences [7]. Its nonspecific clinical signs make the establishment of its diagnosis difficult. This study aimed to describe the most common clinical and hematologic finding in dogs infected with B. canis.
Blood smear evaluation detected B. canis in 24 dogs, while 10 dogs that were positive by PCR had negative blood smear. Therefore, 70.6% of the dogs could be diagnosed by blood smear, while 29.4% needed PCR to establish the diagnosis. This finding highlights need of molecular testing in dogs with typical clinical findings of babesiosis even if they had a negative blood smear evaluation.
Lymphopenia was reported in 82% of dogs, which was more frequent than anemia. Older literature reports lymphopenia as a common finding in canine babesiosis, but not as frequent as anemia [11]. Recently, a study by Thongsahuan et al. [18] conducted in Thailand on privately owned dogs reported similar findings. However, this study did not include molecular testing as part of the diagnostics to confirm the identity of the infecting Babesia species found. A possible reason for lymphopenia could be as a response to acute infection or corticosteroid influence due to stress. Steroid treatment was not administered to any dog; therefore, this medication could not influence hematology results in this study.
Our study also showed substantial monocytosis in 35% of cases. This finding is similar to recent findings in the study by Thongsahuan et al. [18], who report 26.7% monocytosis in dogs with babesiosis. Since a common cause of monocytosis is the influence of endogenous corticosteroids [32], these findings might be connected with stress when Babesia infection is presented. Another possible cause of monocytosis could be a cellular immune response to the infection.
Thrombocytopenia was the most common hematologic finding observed in all dogs with B. canis infection. Thirty one dogs (91%) had platelet counts of less than 50 × 109/l, under which haemorrhage caused by thrombocytopenia may occur [33]. However, external signs of haemorrhage, such as presence of petechial skin lesions, epistaxis or melena, was not reported in any dog in the studied population. These findings are consistent with information from similar studies [2, 10, 11, 14, 16, 18, 25]. Possible explanation of this finding offers study by Rafaj et al., [34], which reported decreased antithrombin III levels and increased production of thrombin–antithrombin complexes in dogs with B. canis infection, which indicated hypercoagulable state [34]. Therefore, signs of haemorrhage probably did not occur due to hypercoagulable state of the dogs.
The clinical signs of the disease observed in the study were in accordance with other studies [11]. The study also showed statistically significant difference between both sexes. Majority (85%) of the infected dogs were males, which is commonly observed in naturally occurring cases of canine babesiosis [11, 13, 23]. This phenomenon could be connected with the increased use of male dogs in outdoor activities, such as hunting. This is in accordance with the fact that the most commonly affected breed groups were herding (7 cases) and sporting (7 cases) group with terriers (2 cases) and hound (1 case) dogs also represented.
Our study’s main limitation was the relatively small size of studied dog population. Despite this, there were clearly hematologic and clinical abnormalities which were found associated with B. canis infection, such as thrombocytopenia, lymphopenia, anemia, lethargy and pigmenturia, which should raise the index of suspicion for babesiosis for clinicians in areas, where B. canis infection is present, and in addition infection was more frequent in male dogs.
Conclusions
Our study confirmed that B. canis is the main large Babesia species infecting dogs in eastern Slovakia. Infection was associated with several hematologic and clinical changes but not necessarily with anemia and in particular regenerative anemia. And finally, diagnosis if babesiosis caused by B. canis should be performed by specific PCR and not rely only on blood smear evaluation.
Change history
31 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11686-022-00619-0
References
Schoeman JP (2009) Canine babesiosis. Onderstepoort J Vet Res 76:59–66. https://doi.org/10.4102/ojvr.v76i1.66
Mathe A, Vörös K, Németh T, Biksi I, Hetyey C, Manczur F, Tekes L (2006) Clinicopathological changes and effect of imidocarb therapy in dogs experimentally infected with Babesia canis. Acta Vet Hung 54:19–33. https://doi.org/10.1556/avet.54.2006.1.3
Bullová E, Lukáň M, Stanko M, Peťko B (2009) Spatial distribution of Dermacentor reticulatus tick in Slovakia in the beginning of the 21st century. Vet Parasitol 165:357–360. https://doi.org/10.1016/j.vetpar.2009.07.023
Rubel F, Brugger K, Pfeffer M, Chitimia-Dobler L, Didyk Y, Leverenz S, Dautel H, Kahl O (2016) Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis 7:224–233. https://doi.org/10.1016/j.ttbdis.2015.10.015
Majlathová V, Majláth I, Víchová B, Guľová I, Derdáková M, Sesztáková E, Peťko B (2011) Polymerase chain reaction confirmation of Babesia canis canis and Anaplasma phagocytophilum in dogs suspected of babesiosis in Slovakia. Vector Borne Zoonotic Dis 11:1447–1451. https://doi.org/10.1089/vbz.2010.0276
Kubelová M, Tkadlec E, Bednář M, Roubalová E, Široký P (2001) West-to-east differences of Babesia canis canis prevalence in Dermacentor reticulatus ticks in Slovakia. Vet Parasitol 180:191–196. https://doi.org/10.1016/j.vetpar.2011.03.033
Vatoliková I, Dekány D, Matušková H, Miklošovičová B, Macenauer Z, Szaboóvá A, Šimek J, Hanzlíček D (2019) Babezióza psov na západnom Slovensku: retrospektívna klinická štúdia z rokov 2014–2018. Veterinářství 69:144–150
Víchová B, Horská M, Blaňarová L, Švihran M, Andersson M, Peťko B (2016) First molecular identification of Babesia gibsoni in dogs from Slovakia, central Europe. Ticks Tick Borne Dis 7:54–59. https://doi.org/10.1016/j.ttbdis.2015.08.004
Boozer AL, Macintire DK (2003) Canine babesiosis. Vet Clin North Am Small Anim Pract 33:885–904. https://doi.org/10.1016/S0195-5616(03)00039-1
Brandao LP, Hagiwara MK, Myiashiro SI (2003) Humoral immunity and reinfection resistance in dogs experimentally inoculated with Babesia canis and either treated or untreated with imidocarb dipropionate. Vet Parasitol 114:253–265. https://doi.org/10.1016/S0304-4017(03)00130-4
Furlanello T, Fiorio F, Caldin M, Lubas G, Solano-Gallego L (2005) Clinicopathological findings in naturally occurring cases of babesiosis caused by large form Babesia from dogs of northeastern Italy. Vet Parasitol 134:77–85. https://doi.org/10.1016/j.vetpar.2005.07.016
Eichenberger RM, Riond B, Willi B, Hofmann-Lehmann R, Deplazes P (2016) Prognostic markers in acute Babesia canis infections. J Vet Intern Med 3:174–182. https://doi.org/10.1111/jvim.13822
Mathe A, Vörös K, Papp L, Reiczigel J (2006) Clinical manifestations of canine babesiosis in Hungary (63 cases). Acta Vet Hung 54:367–385. https://doi.org/10.1556/avet.54.2006.3.7
Matijatko V, Kiš I, Torti M, Brkljačić M, Kučer N, Rafaj RB, Grden D, Živičnjak T, Mrljak V (2009) Septic shock in canine babesiosis. Vet Parasitol 162:263–270. https://doi.org/10.1016/j.vetpar.2009.03.011
Carli E, Tasca S, Trotta M, Furlanello T, Caldin M, Solano-Gallego L (2009) Detection of erythrocyte binding IgM and IgG by flow cytometry in sick dogs with Babesia canis canis or Babesia canis vogeli infection. Vet Parasitol 162:51–57. https://doi.org/10.1016/j.vetpar.2009.02.002
Kirtz G, Leschnik M, Hooijberg E, Tichy A, Leidinger E (2012) In-clinic laboratory diagnosis of canine babesiosis (Babesia canis canis) for veterinary practitioners in Central Europe. Tierarztl Prax Ausg K Kleintiere Heimtiere 40:87–94. https://doi.org/10.1055/s-0038-1623628
Rafaj RB, Kuleš J, Selanec J, Vrkić N, Zovko V, Zupančič M, Trampuš Bakija A, Matijatko V, Crnogaj M, Mrljak V (2013) Markers of coagulation activation, endothelial stimulation, and inflammation in dogs with babesiosis. J Vet Intern Med 27:1172–1178. https://doi.org/10.1111/jvim.12146
Thongsahuan S, Chethanond U, Wasiksiri S, Saechan V, Thongtako W, Musikacharoen T (2020) Hematological profile of blood parasitic infected dogs in Southern Thailand. Vet World 13:2388–2394. https://doi.org/10.14202/vetworld.2020.2388-2394
Schetters TP, Kleuskens JAGM, Van De Crommert J, De Leeuw PWJ, Finizio AL, Gorenflot A (2009) Systemic inflammatory responses in dogs experimentally infected with Babesia canis; a haematological study. Vet Parasitol 162:7–15. https://doi.org/10.1016/j.vetpar.2009.02.012
Makinde MO, Bobade PA (1994) Osmotic fragility of erythrocytes in clinically normal dogs and dogs infected with parasites. Res Vet Sci 57:343–348. https://doi.org/10.1016/0034-5288(94)90128-7
Kumar P, Kumar A (2018) Haemato-biochemical changes in dogs infected with babesiosis. Int J Chem Stud 4:25–28
Shah SA, Sood NK, Tumati SR (2011) Haemato-biochemical changes in natural cases of canine babesiosis. Asian J Anim Sci 5:387–392. https://doi.org/10.3923/ajas.2011.387.392
Andoni E, Rapti D, Postoli R, Zalla P (2012) Hematologic changes in dogs naturally infected with Babesia. Albanian J Agric Sci 11:155–158
Schetters TH, Moubri K, Précigout E, Kleuskens J, Scholtes NC, Gorenflot A (1997) Different Babesia canis isolates, different diseases. Parasitology 115:485–493. https://doi.org/10.1017/S0031182097001686
Matijatko V, Mrljak V, Kiš I, Kučer N, Foršek J, Živičnjak T, Romić Ž, Šimec Z, Ceron J (2007) Evidence of an acute phase response in dogs naturally infected with Babesia canis. Vet Parasitol 144:242–250. https://doi.org/10.1016/j.vetpar.2006.10.004
Tvedten H (2010) Laboratory and clinical diagnosis of anemia. In: Weiss DJ, Wardrop KJ (eds) Schalm’s veterinary hematology, 6th edn. Blackwell Publishing Ltd, Ames, Iowa, pp 152–161
Casati S, Sager H, Gern L, Piffaretti JC (2006) Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med 13:65–70
Massung RF, Slater KG (2003) Comparison of PCR assays for detection of the agent of human granulocytic Ehrlichiosis, Anaplasma phagocytophilum. J Clin Microbiol 41:717–722. https://doi.org/10.1128/JCM.41.2.717-722.2003
Rishniw M, Barr SC, Simpson KW, Frongillo MF, Franz M, Alpizar JLD (2006) Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet Parasitol 135:303–314. https://doi.org/10.1016/j.vetpar.2005.10.013
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Altschul SF, Gish W, Miller W, Myers EW (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Weiss DJ, Souza CD (2010) Monocytes and macrophages and their disorders. In: Weiss DJ, Wardrop KJ (eds) Schalm’s veterinary hematology, 6th edn. Blackwell Publishing Ltd, Ames, Iowa, pp 298–306
Russell KE (2010) Platelet kinetics and laboratory evaluation of thrombocytopenia. In: Weiss DJ, Wardrop KJ (eds) Schalm’s veterinary hematology, 6th edn. Blackwell Publishing Ltd, Ames, Iowa, pp 576–585
Rafaj RB, Matijatko V, Kiš I, Kučer N, Živičnjak T, Lemo N, Žvorc Z, Brkljačić M, Mrljak V (2009) Alterations in some blood coagulation parameters in naturally occurring cases of canine babesiosis. Acta Vet Hung 57:295–304. https://doi.org/10.1556/avet.57.2009.2.10
Acknowledgements
Authors want to thank doc. MVDr. Dagmar Mudronova, PhD. for offering her expertise and helping with results processing.
Funding
This work was supported by research grant VEGA project 2/0014/21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No conflicts of interest have been declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the first and last names of all authors were interchanged and have now been corrected.
Appendix
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turna, H., Vichova, B., Miterpakova, M. et al. Clinical and Hematologic Findings in Babesia canis Infection in Eastern Slovakia. Acta Parasit. 67, 1329–1334 (2022). https://doi.org/10.1007/s11686-022-00584-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00584-8