Abstract
Introduction
Copepods of the genus Hatschekia Poche, 1902 are parasitic on the gills of marine actinopterygians. Currently, about 151 species of this genus have been reported in marine ecosystems and only few occur in South Atlantic Ocean.
Methods
Fifty specimens of A. virginicus from Angra dos Reis, off the State of Rio de Janeiro, Brazil, were parasitized by copepods on the gills. Parasites were fixed and preserved in 70% ethanol. Morphological observations were based on light and scanning electron microscopy.
Results
Hatschekia nagasawai n. sp. can be distinguished from all congeners by the combination of the following characters: (1) presence of two pointed processes on the proximal (first) segment of antennule, (2) cephalothorax octagonal to ovoid, (3) absence of processes on the intercoxal sclerite of legs 1 and 2, (4) trunk without lobes at the postero-lateral margins. Other species of Hatschekia and their hosts previously collected off Brazil were analysed and discussed.
Conclusions
This is the first report of a representative of the family Hatschekiidae Kabata, 1979 parasitizing a species of Anisotremus. The number of species of Hatschekia reported in the South Atlantic Ocean was increased to five, including the new species; however, the diversity of hatschekiid copepods in this oceanographic region is still underestimated, most likely being higher than what is currently known.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Siphonostomatoid copepods of the family Hatschekiidae Kabata, 1979 represent one of the most diverse groups of crustacean parasites, found on the gills of marine actinopterygian fish [1, 2]. Currently, this family comprises about 170 species, belonging to nine genera namely, Bassettithia Wilson, 1922, Brachihatschekia Castro-Romero & Baeza-Kuroki, 1989, Congericola Beneden, 1854, Hatschekia Poche, 1902, Laminohatschekia Boxshall, 1989; Mihbaicola Uyeno, 2013, Prohatschekia Nunes-Ruivo, 1954, Pseudocongericola Yü, 1933 and Wynnowenia Boxshall, 1987 [1,2,3]. Hatschekia is the most diverse genus and since the last systematic revision by Jones [4] that reduced the number of valid species from 100 to 68, 83 other species were described worldwide [1, 2, 5,6,7,8,9].
Surveys on hatschekiid copepods from marine fish in the Southwestern Atlantic are scarce and, similarly, the related taxonomic knowledge is fragmentary [10]. In this sense, only two species of Hatschekia have been reported so far in this region, parasitizing gills of their hosts, i.e., H. conifera Yamaguti, 1939 from the Atlantic pomfret Brama brama (Bonnaterre), off Mar del Plata and San Matías gulf, Argentina and H. quadrabdominalis Yü, 1933 from the Atlantic bigeye Priacanthus arenatus (Cuvier), off Rio de Janeiro, Brazil [10, 11]. In addition, there are three undetermined species reports of Hatschekia, one on the Porkfish Anisotremus virginicus (Linnaeus), one on the Coney Cephalopholis fulva (Linnaeus) and one on the Bluewing searobin Prionotus punctatus (Bloch), off Rio de Janeiro, Brazil [10, 12].
Anisotremus virginicus (Perciformes: Haemulidae) is the most common species of the genus on the Brazilian coast [13]. This species occurs in costal zones from USA to Brazil (including Gulf of Mexico and Caribbean Sea), commonly inhabiting reefs and rocky bottoms and feeding on a variety of invertebrates, mainly mollusks, echinoderms, annelids and crustaceans [13, 14]. To date, five species of copepods have been reported parasitizing A. virginicus in the Atlantic Ocean, namely, Caligus atromaculatus Wilson, 1913, C. haemulonis Krøyer, 1863, C. longipedis Bassett-Smith, 1898, C. xystercus Cressey, 1991 and Lernanthropus amplitergum Pearse, 1951, as well as an unidentified species of Hatschekia [12].
During parasitological studies on specimens of A. virginicus from the Brazilian coastal zone, some parasitic copepods were recovered from their gills. Detailed morphological analysis of these parasites revealed that they represented a new species of Hatschekia, which is described herein. In addition, other species of Hatschekia previously reported on actinopterygians in the Brazilian coast were re-examined and discussed.
Materials and Methods
Fifty specimens of A. virginicus (total length 20.3–40.5; mean ± standard deviation 27.2 ± 5.1 cm) from the coast of Angra dos Reis (23° 01′ 21ʺ S, 44° 19′ 13ʺ W), State of Rio de Janeiro, Southeastern Brazil, were purchased from local fishermen in April 2011 and July 2012. Copepods were collected from the host gills, fixed and preserved in 70% ethanol until morphological analysis. For microscopical observation, specimens were cleared in 85% lactic acid and the appendages were dissected and examined using the wooden slide procedure described by Humes and Gooding [15]. Drawings were made using an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan) with a drawing tube attached. For analysis using scanning electron microscopy (SEM), three female specimens were dehydrated through a graded ethanol series, dried in hexamethyldisilazane, coated with gold and examined in a Jeol JSM 6510 LV scanning electron microscope, operating at 30 kV. Measurements were based on ten adult females, given as the ranges followed by mean and one standard deviation inside parentheses, all in micrometers. For identification and comparison purposes, the following specimens of Hatschekia deposited in the Coleção Helmintológica do Instituto Oswaldo Cruz (acronym CHIOC) and in the Coleção de Crustacea do Museu Nacional do Rio de Janeiro (acronym MNRJ) were observed: H. quadrabdominalis (MNRJ15338; MNRJ 15339) from P. arenatus reported by Tavares et al. [16]; Hatschekia sp. (MNRJ-23992) from A. virginicus reported by Paschoal et al. [12]; Hatschekia sp. (CHIOC-35358) from P. punctatus reported by Bicudo et al. [17]. The morphological terminology used herein follows Huys and Boxshall [18], and the measurement were taken according to the methodology proposed by Uyeno and Nagasawa [19]. Ecological terminology adopted for parasites is according to Bush et al. [20]. Host identification was based on Menezes and Figueiredo [13], and nomenclature and classification were updated according to Froese and Pauly [14]. Type specimens were deposited in the collection of the Museu de Zoologia da Universidade de São Paulo (acronym MZUSP), Brazil.
Order Siphonostomatoida Burmeister, 1835
Family Hatschekiidae Kabata, 1979
Genus Hatschekia Poche, 1902
Hatschekia nagasawai n. sp.
Hatschekia sp. of Paschoal et al. [12]
Type host: The Porkfish Anisotremus virginicus (Linnaeus, 1758) (Perciformes: Haemulidae).
Site on host: Gill filaments.
Type locality: Angra dos Reis (23° 01′ 21ʺ S 44° 19′ 13ʺ W), State of Rio de Janeiro, Brazil.
Prevalence and intensity: 50% (25 infected fish out of 50 examined); mean of 6.3 copepods per infected fish (range 1–20).
Type material: Holotype female (MZUSP-42555) and nine paratype females (MZUSP-42556).
ZooBank registration: urn:lsid:zoobank.org:pub:A9112F17-667F-46BD-9E69-2205356EE7D5.
Etymology: The new species is named after Prof. Kazuya Nagasawa from Japan, for his contributions to our knowledge of parasitic copepods.
Description (Figs. 1, 2, 3, 4).
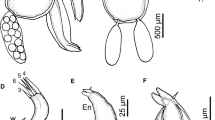
Hatschekia nagasawai n. sp. (adult female). A Habitus, dorsal view, p1 = leg 1, p2 = leg 2, p3 = leg 3, p4 = leg 4; B details of cephalothorax, dorsal view, a1 = antennule, a2 = antenna, ch = dorsal chitinous markings, ma = maxilla; C posterior part of trunk, ventral view, ti = internal tissues; D caudal ramus, ventral view; E egg sacs, lateral view
Adult Female
Body elongate (Fig. 1A), comprising distinct cephalothoracic head, short neck and long cylindrical trunk; external cuticular layer with grooves. Body length 929–1100 (977 ± 55.2) mm, excluding caudal rami. Cephalothorax forming octagonal to ovoid segment (Fig. 1A, B), shorter than wide 190–200 (194 ± 3.9) × 210–264 (228 ± 16.2); its dorsal surface bearing chitinous marks resembling horseshoe outline, symmetrically divided in two sides by median longitudinal thick bar, with bifid extremities; each side with pointed process at anterior part and two median transversal slender bars connecting to median thick one; posterior part deep, with middle bar branching towards horseshoe outline (Fig. 1B). Trunk fusiform, longer than wide, 700–864 (759 ± 56.5) × 203–258 (222 ± 17.1), lacking posterolateral lobes or processes, with anterior narrow and short “neck”; expanding to maximum width at region slightly posterior to second legs level and gradually narrowing to posterior end; internal tissues separated from cuticle and apparently sheathed with cuticular membrane. Urosome (Fig. 1C) comprising fused genital complex and abdomen, wider than long 46–62 (52 ± 6.2) × 57–67 (61 ± 4.3). Caudal ramus (Fig. 1D) fused to urosome, longer than wide 16–23 (20 ± 2.5) × 12–20 (16 ± 2.9), with six naked setae: five distal and one lateral. Egg sacs (Fig. 1E) shorter than trunk, mean of 5.0 eggs per sac, range from three to six eggs per sac (n = 5).
Rostrum absent. Antennule (Fig. 2A) indistinctly five segmented, 112–150 (134 ± 12.5) long; armature formula: 7, 6, 3, 1, 10 + 1 aesthetasc; proximal segment bearing two pointed processes (possibly modified setae) on posterior margin (Figs. 2A, 4A). Antenna (Figs. 2B, 4B) with three segments: proximal (coxa) unarmed, mid (basis) ornamented with surface pits and terminal claw large, without armature. Antenna 189–221 (197 ± 13.3) long; lengths of proximal, mid and terminal segments 30–36 (32 ± 2.1), 85–107 (94 ± 7.6) and 65–78 (70 ± 4.5), respectively. Parabasal papilla (Fig. 2B) blunt, thumb-like with annulated surface. Oral cone robust. Mandible (Fig. 2C) styliform, with two sharp teeth. Maxillule (Figs. 2D, 4C) bilobate, both lobes armed with two tapering setae of unequal sizes. Maxilla (Figs. 2E, 4D) with four segments: proximal unarmed; second rod-like with one basal seta and two rows of blunt, fine spinules on anterior surface; third elongate with one distal seta. Terminal segment small, with one short seta and bifid claw. Maxilliped absent.
Legs 1 and 2 biramous, each joined by bar-like intercoxal sclerite. Exopods represented by two incompletely fused segments and two-segmented endopods. All setae on rami naked and thick. Spine and seta formula as follows:
Protopod | Exopod | Endopod | |
|---|---|---|---|
Leg 1 | 1–1 | 1–0; 5 | 0–0; 5 |
Leg 2 | 1–0 | 1–0; 5 | 0–1; 4 |
Leg 1 (Fig. 3A) 80–96 (85 ± 7.3) long, with coxa and basis fused forming large protopod and retaining trace of suture. Protopod 40–56 (50 ± 6.5) long, with large inner seta and slender outer seta. Exopod longer than endopod, 37–41 (39 ± 1.8) and 27–32 (29 ± 2.2) long, respectively. Leg 2 (Fig. 3B) 111–130 (121 ± 8.1) long, with coxa and basis fused to form protopod, retaining trace of suture. Protopod 48–62 (56 ± 4.7) long, with slender basal outer seta. Exopod longer than endopod, 54–63 (57 ± 3.1) and 42–46 (43 ± 1.8) long, respectively. Intercoxal sclerites of legs 1 and 2 (Figs. 3C, D) slender, without processes. Protopods and rami of legs 1 and 2 ornamented with rows of blunt and fine spinules on anterior surface.
Leg 3 (Figs. 3E, 4E) represented by two simple naked setae, located at middle of trunk with slightly swollen base. Leg 4 (Figs. 3F, 4F) represented by small naked seta located at posterior 1/4 of trunk with slightly swollen base.
Adult Male
Unknown
Remarks
The adult female specimens examined in the present study were assigned to Hatschekia based on the following features: (1) legs 1 and 2 biramous, (2) legs 3 and 4 represented by 1 or more setae and (3) egg-sacs uniseriate [1]. Of the 151 nominal species currently assigned to Hatschekia, H. nagasawai n. sp. can be clearly differentiated from 143 based on the presence of a pointed processes on the proximal (first) segment of antennule. The remaining eight species namely, H. balistae Nuñes-Ruivo, 1954, H. bodiani Nuñes-Ruivo, 1954, H. cluthae (Scott, 1902), H. couardi Nuñes-Ruivo, 1954, H. gerro Leigh-Sharpe, 1936, H. labracis (van Beneden, 1871), H. pseudobalistesi Uyeno & Nagasawa, 2010 and H. pygmaea Scott & Scott, 1913, can be easily differentiated from H. nagasawai n. sp. by the presence of one pointed process on the proximal segment of the antennule (vs. two in the new species) [4, 21,22,23]. Details on host and geographic occurrence of the previously mentioned eight species can be found in Table 1.
It should be observed that the absence of lobes in the postero-lateral margins of trunk in the new species is also a characteristic of H. cluthae and H. pygmaea. However, H. nagasawai n. sp. differs from H. cluthae by the maxillule with three setae and bifid tip (vs. four setae and bilobate in the new species) and by the presence of a tubercle with subapical conical outgrowth on Leg 3 (vs. lacking in the new species) [22]. Moreover, H. nagasawai n. sp. differs from H. pygmaea by the segmented neck region (vs. not segmented in the new species) [22].
In addition, the new species differs from H. balistae and H. pseudobalistesi by the absence of processes on the intercoxal sclerite of legs 1 and 2 [21, 23], as well as from H. labracis, H. couardi and H. bodiani by the cephalothorax without protrusions [4, 21, 22]. Finally, it differs from H. gerro by the postero-lateral margins of trunk not extending beyond the abdomen [4].
Other Species of Hatschekia Collected from Actinopterygians off Brazil
Currently, there are more than 200 species of copepods reported parasitizing fish in Brazil, about 93 of them belong to the Order Siphonostomatoida, and the Perciformes is the most studied group of hosts [10, 26,27,28,29]. Regarding hatschekiid copepods, only four species are known from fish in Brazil, accounting for 4.3% of the total known siphonostomatoid copepods in the country. Unfortunately, these previous reports of hatschekiid in Brazil were based on non-taxonomic studies [10, 12, 16, 17], which may put the validity of these specific identifications in question. Therefore, in the present study, the material related to the reports of these four hatschekiid species from Brazil was taxonomically verified and the most important remarks are presented as follows:
-
Hatschekia quadrabdominalis from Priacanthus arenatus (Priacanthidae): this copepod was originally described based on specimens from the Redcoat Sargocentron rubrum (Forskål) (Holocentriformes, Holocentridae) in China [30]. Subsequently, Jones [4] reported this species on P. arenatus from the coast of Venezuela and proposed that H. curvata Yamaguti & Yamasu, 1959 should be a synonym of H. quadrabdominalis based on the curved trunk, shared by both. Recently, Izawa [8] redescribed H. curvata and remarked that the specimens studied by Jones [4] were not conspecific to H. quadrabdominalis, since they had an intercoxal bar with prominent triangular processes in both legs 1 and 2, which are absent in H. quadrabdominalis and, thus, revalidated H. curvata [8, 30]. According to Izawa [8], the material referred to as H. quadrabdominalis by Jones [4] is closely related to H. curvata, but differences in the intercoxal processes of legs 1 and 2 justified the proposal of a new taxon, namely H. priacanthis, to accommodate the specimens studied by Jones [4]. Therefore, only two species of Hatschekia are reported from Priacanthidae worldwide: H. curvata parasitizing the Glasseye Heteropriacanthus cruentatus (Lacepède) in Japan and H. priacanthis Izawa, 2016 from P. arenatus off Puerto la Cruz, Venezuela [8]. In Brazil, the presence of H. quadrabdominalis on P. arenatus off the State of Rio de Janeiro was reported by Tavares et al. [16], which was a possible taxonomic misidentification, based on the previous discussion. After observation of the specimens studied by Tavares et al. [16] (MNRJ15338; MNRJ 15,339), it was possible to conclude that they have curved trunk, intercoxal bar 1 and 2 with prominent triangular processes, and exopod and endopod of leg 1 with 3 + 2 setal elements, and leg 2 with 2 + 2 setal elements. On this evidence, H. quadrabdominalis does not occur in Brazil, and the species reported by Tavares et al. [16] is H. priacanthis.
-
Hatschekia sp. from Prionotus punctatus (Triglidae): of the valid species of Hatschekia, only H. prionoti Pearse, 1947 was reported parasitizing a Triglidae fish, i.e., the Northern searobin Prionotus carolinus (Linnaeus) from North Carolina, USA [4]. Subsequently, Bicudo et al. [17] reported the presence of Hatschekia sp. on the gills of P. punctatus off Rio de Janeiro, Brazil. After analysis of the material studied by Bicudo et al. [17] (CHIOC-35358), we concluded that the specimens have an almost circular cephalothorax, trunk cylindrical, genital complex as wide as trunk and antenna with strongly recurved hook. These features are also present in H. prionoti [4], suggesting its conspecificity with the specimens found on P. punctatus, studied by Bicudo et al. [17]. However, the study of additional specimens is necessary to confirm this assertion, since it was not possible to observe some appendages (e.g., antennule; mandible; maxilla; legs) in the present analysis, due to the poor preservation of the deposited material.
-
Hatschekia sp. from Cephalopholis fulva (Serranidae): currently, 17 species of Hatschekia have been reported parasitizing serranid fish worldwide [6, 8]. Of these, seven occur in the Atlantic Ocean namely, H. albirubra Wilson, 1913 from the Kelp bass Paralabrax cf. clathratus (Girard) in West Indies [4, 31]; H. cadenati Nuñes-Ruivo, 1954 from the Bluespotted seabass Cephalopholis taeniops (Valenciennes) and the Goldblotch grouper Epinephelus costae (Steindachner) in Senegal [21]; H. cernae Goggio, 1905 from E. costae, the White grouper E. aeneus (Geoffroy Saint-Hilaire) and the Dusky grouper E. marginatus (Lowe) in the Mediterranean and Angola [4, 32]; H. delamarei Nuñes-Ruivo, 1954 from the Mottled grouper Mycteroperca rubra (Bloch) in Senegal [21]; H. insolita Wilson, 1913 from the Rock hind E. adscensionis (Osbeck) and the Black grouper M. bonaci (Poey) in Jamaica, Caribbean Islands, Venezuela and USA [4]; H. petiti Nuñes-Ruivo, 1954 from E. aeneus in Senegal [21]; and H. uncata Wilson, 1913 from E. adscensionis in West Indies [4, 31]. In Brazil, Luque and Tavares [9] reported Hatschekia sp. on the gills of C. fulva off Rio de Janeiro. Previously, only H. cadenati was reported in Cephalopholis serranids in the world. Unfortunately, the material studied by Luque and Tavares [9] in Brazilian could not be assessed since it was not deposited in any collection.
-
Hatschekia sp. from Anisotremus virginicus (Haemulidae): the new species described in the present study was previously reported as Hatschekia sp. by Paschoal et al. [12], parasitizing the gills of the present type host off Rio de Janeiro, Brazil. This conclusion was achieved after the analysis of the material studied by Paschoal et al. [12] (MNRJ-23992), which confirmed its morphological similarity with the new species. Therefore, the specimens deposited by Paschoal et al. [12] under accession number MNRJ-23992, at the Museu Nacional do Rio de Janeiro, Brazil, should be considered vouchers of H. nagasawai n. sp.
Discussion
The discovery of H. nagasawai n. sp. on A. virginicus represents the second report of a Hatschekia species parasitizing fish belonging to Haemulidae. In this sense, only the congener Hatschekia linearis Wilson, 1913 has been reported on gill filaments of the following haemulid fish: the barred grunt Conodon nobilis (Linnaeus), the white margate Haemulon album Cuvier, the Tomtate grunt Haemulon aurolineatum Cuvier, the bluestriped grunt Haemulon sciurus (Shaw) and the white grunt Haemulon plumierii (Lacepède), all from the Caribbean Sea [4, 12]. According to Jones [4], H. linearis has a unique combination of features that separate it from all congeners (obviously including H. nagasawai n. sp.). This combination consists of the following characteristics: (1) trunk seven to nine times long than the cephalothorax, narrowing anteriorly to form a neck; (2) bases of legs 1 and 2 swollen laterally and exceeding the trunk margins; and (3) lack of lobes on the postero-lateral trunk margins. Suárez-Morales et al. [33] also reported Hatschekia parasitizing the gills of Haemulon sciurus and Haemulon plumierii in the Mexican Caribbean, but the report refers to unidentified species. Even though only few species of Hatschekia have been reported as parasites of haemulid fish, the real biodiversity of such copepods on the referred host taxa would probably be much higher, since Haemulidae represents one of the most diverse families within the Perciformes [34] and the present findings now include the genus Anisotremus as potential host for these parasites. In fact, the efforts related to taxonomic studies on Hatschekia parasitizing haemulids are still underdeveloped, contributing to the previously depicted scenario.
According to Lee et al. [6], some species of Hatschekia remain poorly described, mainly as a consequence of featureless bodies and small vestigial appendages. However, a series of interspecific differences that is useful for taxonomic diagnosis have been found in these small appendages, e.g., segmentation and armature formula of antennule, presence/absence of parabasal papilla, ornamentation of mandible and maxillule, as well as structure and ornamentation of legs [4, 6, 35]. In addition to these morphological features, the morphometry of female body and of some appendages are strongly recommended to be used in Hatschekia spp. descriptions [2, 6, 19, 35]. In the present study, it was observed that the presence of one or two setal elements modified as pointed processes, on the proximal segment of the antennule, was shared by only eight Hatschekia congeners (see Table 1), reiterating the taxonomic importance of these small appendages within the genus, as well as the need of descriptions including these minute details.
Most of the Hatschekia spp. are reported in the North Pacific Ocean and, according to Uyeno and Nagasawa [36], this taxon achieves high biodiversity in Japanese waters, where 37% of the total known species occur. The Japanese Archipelago, located in East Asia, is surrounded by four sea regions (Northwestern Pacific Ocean, East China Sea, Sea of Japan, and Sea of Okhotsk) extending over an area of 3,000 km [37], and supporting 3,875 species of fish [14]. Such high biodiversity of potential hosts well justifies the also high species richness of Hatschekia along the Japanese marine territory, as asserted by Uyeno and Nagasawa [36]. In contrast, in the Atlantic coast of South America that includes Brazil, Argentina and Uruguay extends for more than 10,000 km and supports 2,184 species of fish [14], only 5 species of Hatschekia have been reported, accounting for only 3.3% of the global generic diversity. These data emphasize that much more effort should be directed to the study of fish-parasitic copepods in South Atlantic, since the biodiversity of hatschekiids in this area is still neglected and most likely higher than that currently known.
References
Boxshall GA, Halsey SH (2004) An introduction to copepod diversity. The Ray Society, London
Uyeno D, Ali AH (2013) Parasitic copepods from two species of commercial fishes collected off Iraq, with description of Hatschekia shari n. sp. Syst Parasitol 86(3): 301–312. https://doi.org/10.1007/s11230-013-9446-3
Uyeno D (2013) A new genus and species of hatschekiid copepod (Siphonostomatoida) from groupers (Actinopterygii: Serranidae) collected off the Ryukyu Archipelago. Japan Syst Parasitol 84(1):89–95. https://doi.org/10.1007/s11230-012-9395-2
Jones JB (1985) A revision of Hatschekia Poche, 1902 (Copepoda: Hatschekiidae), parasitic on marine fishes. N Z J Zool 12(2):213–271
El-Rashidy HH, Boxshall GA (2011) Two new species of parasitic copepods (Crustacea) on two immigrant fishes from the Red Sea of family Siganidae. Syst Parasitol 79(3):175–193. https://doi.org/10.1007/s11230-011-9298-7
Lee S, Lee W, Boxshall GA (2013) Seven new species of Hatschekia Poche, 1902 (Copepoda: Siphonostomatoida: Hatschekiidae) parasitic on fishes New Caledonia, and a redescription of H. cadenati Nunes-Ruivo, 1954. Zoosystema 35(3): 377–413. https://doi.org/10.5252/z2013n3a3
Moon SY, Kim IH (2013) Copepods of Hatschekiidae (Copepoda, Siphonostomatoida) new to Korean fauna, with description of a new species. Ocean Sci J 48(1):19–34. https://doi.org/10.1007/s12601-013-0002-5
Izawa K (2016) Some new and known species of Hatschekia Poche, 1902 (Copepoda, Siphonostomatoida, Hatschekiidae) parasitic on the branchial lamellae of Japanese actinopterygian fishes belonging to Perciformes, with revision of the known species of the genus. Crustaceana 82(9):209–238. https://doi.org/10.1163/15685403-00003503
Walter TC, Boxshall G (2021) World of Copepods Database. Hatschekia Poche, 1902. WoRMS. http://www.marinespecies.org/aphia.php?p=taxdetails&id=135591. Accessed 5 Jan 2022
Luque JL, Tavares LER (2007) Checklist of Copepoda associated with fishes from Brazil. Zootaxa 1579: 1–39. https://doi.org/10.11646/zootaxa.1579.1.1
Cantatore DMP, Braicovich PE, Alarcos AJ, Lanfranchi AL, Rossin MA, Vales DG, Timi JT (2012) New records of parasitic copepods (Crustacea, Copepoda) from marine fishes in the Argentinean Sea. Acta Parasitol 57(1):83–89. https://doi.org/10.2478/s11686-012-0003-z
Paschoal F, Cezar AD, Luque JL (2015) Checklist of metazoan associated with grunts (Perciformes, Haemulidae) from the Nearctic and Neotropical regions. Check List 11(1):1501. https://doi.org/10.15560/11.1.1501
Menezes NA, Figueiredo JL (1980) Manual de Peixes Marinhos do Sudeste do Brasil. IV. Teleostei (3). Museu de Zoologia da Universidade de São Paulo, São Paulo
Froese R, Pauly D (2021) FishBase. World Wide Web electronic publication. http://www.fishbase.org. Accessed 5 Jan 2022
Humes AG, Gooding RU (1964) A method for studying the external anatomy of copepods. Crustaceana 6:238–240. https://doi.org/10.1163/156854064x00650
Tavares LER, Luque JL, Neto SLB (2001) Hatschekia quadrabdominalis Yu, 1933 (Copepoda, Hatschekiidae), a parasite of Priacanthus arenatus (Cuvier, 1829) (Osteichthyes, Priacanthidae) in the Brazilian coast. Rev Bras Zoociências 3(1):129–131
Bicudo AJ, Tavares LE, Luque JL (2005) Metazoários parasitos da cabrinha Prionotus punctatus (Bloch, 1793) (Osteichthyes: Triglidae) do litoral do Estado do Rio de Janeiro. Brasil Rev Bras Parasitol Vet 14(1):27–33
Huys R, Boxshall GA (1991) Copepod evolution. The Ray Society, London
Uyeno D, Nagasawa K (2009) Redescription of four species of Hatschekia (Copepoda: Siphonostomatoida: Hatschekiidae) parasitic on tetraodontiform fishes from Japan. Zootaxa 2110:1–21. https://doi.org/10.11646/zootaxa.2110.1.1
Bush JO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83(4):575–583. https://doi.org/10.2307/3284227
Nuñes-Ruivo LP (1954) Parasites de poissons de mer ouest-Africains récoltés par M.J. Cadenat. III. Copépodes (2°note). Genres Prohatschekia n. gen. et Hatschekia Poche. Bull Inst fr Afr Noire 16(2):479–505
Kabata Z (1979) Parasitic Copepoda of British Fishes. The Ray Society, London
Uyeno D, Nagasawa K (2010) The copepod genus Hatschekia Poche, 1902 (Siphonostomatoida: Hatschekiidae) from triggerfishes (Pisces: Tetraodontiformes: Balistidae) from off the Ryukyu Islands, Japan, with descriptions of eleven new species. Zootaxa 2478:1–40. https://doi.org/10.11646/zootaxa.2478.1.1
Papoutsoglou SE (1976) Metazoan parasites of fishes from Saronicos Gulf, Athens. Greece Thalassagraphica 1(1):69–102
Leigh-Sharpe WH (1936) New parasitic Copepoda from Naples. Parasitology 28(1):63–71. https://doi.org/10.1017/s0031182000022253
Luque JL, Pavanelli G, Vieira F, Takemoto R, Eiras J (2013) Checklist of Crustacea parasitizing fishes from Brazil. Check List 9(6):1449–1470. https://doi.org/10.15560/9.6.1449
Couto JV, Paschoal F (2021) Two new species of Colobomatus Hesse, 1873 (Crustacea: Philichthyidae) parasitic in the interorbital canals of Holocentrus spp. (Holocentriformes: Holocentridae) in the South Atlantic Ocean. Syst Parasitol 98(5–6):753–764. https://doi.org/10.1007/s11230-021-10009-1
Canel D, Levy E, Soares IA, Braicovich PE, Haimovici M, Luque JL, Timi JT (2019) Stocks and migrations of the demersal fish Umbrina canosai (Sciaenidae) endemic from the subtropical and temperate Southwestern Atlantic revealed by its parasites. Fish Res 214:10–18. https://doi.org/10.1016/j.fishres.2019.02.001
Oliveira BDL, Fernandes LFL, Rocha GM, Malanski ACGS, Paschoal, F (2020) Occurrence of Caligus asperimanus Pearse, 1951 (Copepoda: Caligidae) parasitic Lutjanus spp. (Perciformes: Lutjanidae) in the western South Atlantic. Rev Bras Parasitol Vet 29(2): e018219. https://doi.org/10.1590/s1984-29612020001
Yu SC (1933) Chinese parasitic copepods collected by H.W. Wu, with descriptions of new genera and species. Bull Fan Mem Inst Biol 4(4):111–139.
Wilson CB (1913) Crustacean parasites of West Indian fishes and land crabs. Proc U S Natl Mus 44(1950):189–277. https://doi.org/10.5479/si.00963801.44-1950.189
Capart A (1959) Copépodes parasites. Résultats Scientifiques de l'Expédition Océanographique Belge dans les Eaux Côtieres Afrricaines de l'Atlantique Sud (1948–1949). Bull Inst R Sci Nat Belg 3(5): 59–126
Suárez-Morales E, Reyes-Lizama C, González-Solís D (2010) Parasitic copepods from reef grunts (Teleostei, Haemulidae) with description of a new species of Lernanthropus (Siphonostomatoida, Lernanthropidae) from the Mexican Caribbean. Acta Parasitol 55:167–176. https://doi.org/10.2478/s11686-010-0025-3
Nelson JS, Grande TC, Wilson MVH (2016) Fishes of the World. John Wiley & Sons, Hoboken
Uyeno D, Nagasawa K (2010) Three new species of Hatschekia Poche, 1902 (Copepoda: Siphonostomatoida: Hatschekiidae) parasitic on boxfishes (Pisces: Tetraodontiformes: Aracanidae and Ostraciidae) in Japanese waters. Syst Parasitol 75:147–158. https://doi.org/10.1007/s11230-009-9226-2
Uyeno D, Nagasawa K (2013) The genus Hatschekia (Copepoda: Hatschekiidae) from pufferfishes (Tetraodontiformes: Tetraodontidae) off the Ryukyu Islands, Japan, with descriptions of four new species and a redescription of H. pholas. Folia Parasitol 60(1):61–74. https://doi.org/10.14411/fp.2013.008
Nagasawa K (2015) Parasitic copepods of marine fish cultured in Japan: a review. J Nat Hist 49:45–48. https://doi.org/10.1080/00222933.2015.1022615
Acknowledgements
We are thankful to Dra. Cristiana Serejo and Dra. Irene Cardoso, Curators at the Museu Nacional do Rio de Janeiro, Brazil, and Dr. Marcelo Knoff, Curator at the Coleção Helmintológica do Instituto Oswaldo Cruz, Brazil, for lending vouchers of Hatschekia spp. Thanks are also due to Dr. Daysuke Uyeno from Kagoshima University, Japan, for providing literature. Fabiano Paschoal was supported by Universidade Castelo Branco Rio de Janeiro (Brazil). José L. Luque was supported by a research fellowship from the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico do Brazil (CNPq).
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable institutional, national, and international guidelines for the care and use of animals were followed. The authors declare that the animals used in the work were acquired dead in fish markets and there is no need for approval by the Research Ethics Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paschoal, F., Couto, J.V., Pereira, F.B. et al. A New Species of Hatschekiid Copepod (Crustacea: Hatschekiidae) Parasitic on the Porkfish Anisotremus virginicus (Linnaeus, 1758) (Actinopterygii: Haemulidae), with Notes on Previously Known Species of Hatschekia Poche, 1902 Collected from Actinopterygians off Brazil. Acta Parasit. 67, 1126–1135 (2022). https://doi.org/10.1007/s11686-022-00551-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00551-3