Abstract
Purpose
Strongyloidiasis is mainly prevalent in developing countries with poor economic and sanitary conditions. The clinical manifestations of Strongyloides stercoralis infection are complex and diverse, lacking specificity, which can easily lead to misdiagnosis and delayed treatment.
Methods
An elderly male patient, repeated cough and expectoration for 4 years, with exacerbation and dyspnea for 10 days, was admitted to hospital. Sputum culture and smear were taken for examination. Nematode larvae were found under the microscope. Nematodes were also found in feces.
Results
Upon confirmation, the patient was diagnosed with a pulmonary infection caused by Strongyloides stercoralis. After treatment with albendazole, the symptoms improved, and the patient was discharged.
Conclusion
In this case report, combination of microscopic examination of sputum and alveolar lavage fluid and CT scan were used to quickly identify the cause of the patient, it provides a diagnostic basis and method for clinical treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Strongyloidiasis is mainly prevalent in developing countries with poor economic and sanitary conditions in warm and humid areas. For example, the infection rate of Strongyloides stercoralis in rural Argentina and Brazil is as high as 83.3% [1, 2] and 30.1% [3], respectively; the average infection rate of Strongyloides stercoralis in China is 0.122% [4]. Strongyloides populations in China are mainly distributed in southern regions with endemic characteristics. Strongyloides stercoralis infection has variety and complicated non-specific clinical manifestations [5]. Same time, the sporadic cases of Strongyloides stercoralis infection make this disease easy to be ignored clinically and lead to missed diagnosis [6]. A combination of microscopic examination of sputum and alveolar lavage fluid and CT scan to quickly identify the cause of the patient in this case report, it will provide a diagnostic basis and method for clinical treatment.
Case Report
An 82-year-old male farmer who lived in the countryside for a long time presented with a cough producing a small amount of yellow and white phlegm that was not easy to cough up. He was diagnosed with an acute exacerbation of chronic obstructive pulmonary disease at the local hospital and treated with aminophylline and methylprednisolone, which resulted in aggravation of the symptoms. On October 26, 2019, he was transferred to the emergency department of Qingyuan People’s Hospital, complaining of a 4-year history of cough and expectoration, which worsened with recurrence, and shortness of breath for the previous 10 days.
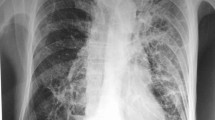
A chest computed tomography (CT) showed chronic bronchitis, emphysema, accompanied by inflammation in both lungs and consolidation in part of the right lower lobe; a small amount of bilateral pleural effusion; and calcification of the right upper lobe (Fig. 1A, B). Blood cell analysis: white blood cells 13.3 × 109/L; neutrophil ratio 91.9%; lymph node 2.9%; eosinophil 1%; erythrocyte 2.54 × 1012/L; hemoglobin 87 g/L; platelet 148 × 109 g/L. Serum biochemical results: potassium ion 2.80 mmol/L and sodium 131 mmol/L. The patient was transferred to the intensive care unit (ICU), area Ι, and treated for acute exacerbation of chronic obstructive pulmonary disease, severe hyponatremia, and hypokalemia.
Chest computed tomography (CT) scan on admission. A Both lungs exhibited increased texture, calcified lesions (arrows) were found in the upper lobe of the right lung. B Multiple patchy and flaky densities (arrow) were found in the upper lobes of both lungs, and partial changes were found in the lower lobes of both lungs. There was a small amount of effusion in the chest cavity
After 28 days, the patient got better, and transferred to the respiratory department. Sputum culture and smear examination were performed. Nematode larvae were seen in sputum smear under the microscope, and no eggs were found. Examination of fecal specimens revealed the presence of nematode larvae, but no eggs. Subsequent sputum and fecal samples were sent for examination and live nematode larvae were detected every time. Cerebrospinal fluid was found to be negative for parasites, a fecal occult blood test was negative, and the patient had normal blood eosinophils. A nematode infection was confirmed and microscopic examination, using the oil immersion objective (100×), of the larvae showed a bifurcated tail (Fig. 2A, B), which indicated an infection with Strongyloides stercoralis. The patient was treated with albendazole and developed hypotension and shock after the first day of treatment and was immediately transferred to the ICU. This was considered an anaphylactic shock caused by the release of large amounts of allergens following the death of the worms. The condition improved after boosting and anti-allergic treatment, and the patient was transferred to the respiratory department 2 days later. After treatment with albendazole 800 mg/day for 10 days, the number of parasites found in sputum samples was significantly reduced and there were no parasites in fecal samples or in the pleural effusion. The patient continued to take albendazole 2 weeks after discharge (1, 400 mg Po&Bid for a week; 2, follow-up; 3, 400 mg Po&Qn for a week; 4, follow-up) and was in good condition at follow-up until no live Strongyloides stercoralis larvae were detected in sputum and stool samples.
Discussion
Strongyloides stercoralis is a facultative parasite with complicated life cycle. Parasitic adults mainly infect the small intestine of the host (such as humans, cats, dogs). Larvae can invade important organs such as the lung and brain stem causing strongyloidiasis [7]. This disease is mainly prevalent in warm, humid tropical and subtropical regions, with sporadic infections in temperate and frigid regions [8]. It is classified as an opportunistic pathogenic parasite, and infection is common in southern China [4]. Humans can show three disease phenotypes after being infected with Strongyloides stercoralis: (1) mild infections, due to an effective immune response, infections can be cleared, and asymptomatic carriers can be found; (2) persistent, chronic infections (up to several decades); (3) disseminated severe infections [9]. Larvae can invade the lungs, liver, kidneys, and other organs causing diffuse tissue damage and even death due to severe exhaustion [10]. Severe strongyloidiasis has a very high fatality rate and lacks specific symptoms, signs, and imaging findings [11, 12].
The parasitic cycle of Strongyloides stercoralis can be completed in the host and cause infection. The free-living cycle of Strongyloides stercoralis is carried out in the soil, and the larvae invade human skin and mucosa to cause infection, the migration of a large number of infectious larvae in the intestine can bring intestinal bacteria into the bloodstream and cause sepsis [11, 13]. Strongyloides stercoralis is associated with hyperinfection syndrome which multiple organ failure. It is high prevalence risk among achlorhydria and patients immunosuppressed due to any cause (leprosy, corticosteroid therapy, HIV, HTLV-I, anti-tumour necrosis factor-alpha therapy), organ or bone marrow transplant patients [14,15,16,17]. It can also infect immunocompetent patients, although rare. Farmers often come into contact with the soil as part of their work. Additionally, some rural areas have poor living environments where, for example, manure water that has not been treated is used to irrigate the land. At the same time, peasants usually work with bare hands and feet and the skin is in direct contact with the soil, exposing it to the excrements of insect carriers which may contain filamentous larvae, increasing the possibility of infection [18].
In the present case, there was a slight increase in white blood cells with normal eosinophils levels on routine blood analysis. Strongyloides is unique among the nematodes for its autoinfection cycle within the human host. The host response normally controls the parasite leading to a chronic, smoldering infection. Strongyloides infection is associated with the expansion of Tregs, which suppress host expulsion of the parasites. Tregs can blunt Th2 responses including production of IL-5 [19]. Eosinophils play an important role in the immunity of the body against parasitic infection and can act as antigen presenting cells (APC) in allergy. Eosinophils aggregate in corresponding parts of parasitic infection or allergy, and release eosinophils. Toxins kill invading pathogens. Parasitic infection usually activates the body’s Th2 lymphocytes and produces IL-5, IgE and eosinophilia, of which IL-5 is a cytokine involved in the differentiation, activation and proliferation of eosinophils. The parasite is difficult to be phagocytosed by phagocytes due to its large size. When the antibody IgE encapsulates the parasite, eosinophils can kill it through the high-affinity FcεRI channel. Strongyloides stercoralis antigens can activate eosinophils, which further activate T cells for specific immune responses. Eosinophils play a role in antigen presentation in the Th2-mediated primary and secondary immune responses in Strongyloides sternum, suggesting that eosinophils are an essential factor between innate and adaptive immunity. Therefore, eosinophils play an important role in preventing Strongyloides infection. Eosinophilia may be a sign of poor prognosis. The level of eosinophils in severely infected patients is lower than that in asymptomatic carriers, which may be related to the increase in Th2 cell apoptosis caused by eosinophilia [20,21,22].
The filamentous larvae of Strongyloides stercoralis are very similar to hookworm [23]. The esophagus of the filamentous larvae of Strongyloides stercoralis is about half of the body length and the tail is bifurcated; while the esophagus of hookworm filamentous larvae is about one third of the body length, and the tail is thin. Furthermore, Strongyloides stercoralis has smaller eggs, and some eggs contain embryonic larvae. In contrast, hookworm eggs usually contain 4–8 egg cells. Studies have shown that using SSU rRNA as the target gene to detect the DNA of Strongyloides stercoralis in fecal specimens with real-time PCR has good specificity and sensitivity [24].
The clinical manifestations of Strongyloides stercoralis infection are complex and diverse, lacking specificity, which can easily lead to misdiagnosis and delayed treatment. The key to diagnosis is to find the pathogen. Treatment with ivermectin is the first choice for Strongyloides stercoralis. However, albendazole, which is slightly less effective, is more commonly used in China. Compared to the treatment of other helminth infections, the treatment of strongyloidiasis is more difficult, because it is difficult to completely remove the worms, and it is necessary to combine treatment with serological tests to determine whether the infection is cleared.
Availability of Data and Materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Taranto NJ, Bonomi de Filippi H, Orione O (1993) Prevalence of Strongyloides stercoralis infection in childhood. Oran, Salta, Argentina. Bol Chil Parasitol 48(3–4):49–51
Puthiyakunnon S, Boddu S, Li Y, Zhou X, Wang C, Li J, Chen X (2014) Strongyloidiasis—an insight into its global prevalence and management. PLoS Negl Trop Dis 8(8):e3018. https://doi.org/10.1371/journal.pntd.0003018
Mendonca SC, Goncalves-Pires Mdo R, Rodrigues RM, Ferreira A Jr, Costa-Cruz JM (2006) Is there an association between positive Strongyloides stercoralis serology and diabetes mellitus? Acta Trop 99(1):102–105. https://doi.org/10.1016/j.actatropica.2006.06.006
Wang C, Xu J, Zhou X, Li J, Yan G, James AA, Chen X (2013) Strongyloidiasis: an emerging infectious disease in China. Am J Trop Med Hyg 88(3):420–425. https://doi.org/10.4269/ajtmh.12-0596
Krolewiecki A, Nutman TB (2019) Strongyloidiasis: a neglected tropical disease. Infect Dis Clin North Am 33(1):135–151. https://doi.org/10.1016/j.idc.2018.10.006
Montes M, Sawhney C, Barros N (2010) Strongyloides stercoralis: there but not seen. Curr Opin Infect Dis 23(5):500–504. https://doi.org/10.1097/QCO.0b013e32833df718
Page W, Judd JA, Bradbury RS (2018) The unique life cycle of Strongyloides stercoralis and implications for public health action. Trop Med Infect Dis. https://doi.org/10.3390/tropicalmed3020053
Lodh N, Caro R, Sofer S, Scott A, Krolewiecki A, Shiff C (2016) Diagnosis of Strongyloides stercoralis: detection of parasite-derived DNA in urine. Acta Trop 163:9–13. https://doi.org/10.1016/j.actatropica.2016.07.014
Nutman TB (2017) Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology 144(3):263–273. https://doi.org/10.1017/S0031182016000834
Altintop L, Cakar B, Hokelek M, Bektas A, Yildiz L, Karaoglanoglu M (2010) Strongyloides stercoralis hyperinfection in a patient with rheumatoid arthritis and bronchial asthma: a case report. Ann Clin Microbiol Antimicrob 9:27. https://doi.org/10.1186/1476-0711-9-27
Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG (2018) Soil-transmitted helminth infections. Lancet 391(10117):252–265. https://doi.org/10.1016/S0140-6736(17)31930-X
Woodring JH, Halfhill H 2nd, Berger R, Reed JC, Moser N (1996) Clinical and imaging features of pulmonary strongyloidiasis. South Med J 89(1):10–19. https://doi.org/10.1097/00007611-199601000-00002
Cohen R, Finn T, Babushkin F, Shapiro M, Uda M, Grossman T (2018) Streptococcus pyogenes bacteremia and toxic shock syndrome related to Strongyloides stercoralis hyperinfection: a case report. J Med Case Rep 12(1):346. https://doi.org/10.1186/s13256-018-1885-7
Darlong J (2016) Strongyloides hyper infection in a steroid dependent leprosy patient. Leprosy Rev 87(4):536–542
Gupta V, Bhatia S, Mridha AR, Das P, Khanna N (2017) Strongyloides stercoralis hyperinfection: an often missed but potentially fatal cause of anemia and hypoalbuminemia in leprosy patients on long-term steroid therapy. Indian J Dermatol Venereol Leprol 83(3):381. https://doi.org/10.4103/ijdvl.IJDVL_347_16
Prabha N, Chhabra N (2018) Prevention of Strongyloides stercoralis hyperinfection in leprosy patients on long-term steroid therapy. Indian J Dermatol Venereol Leprol 84(6):709–711. https://doi.org/10.4103/ijdvl.IJDVL_574_17
Ortega-Diaz M, Carretero MP, Navarro JAM, Sanchez TA, Arroyo RA, Prats EC, Ortiz PD, Ramon MA, Rodriguez MTJ, Zahonero LM (2020) Immunosuppression as a trigger for hyperinflammatory syndrome due to Strongyloides stercoralis in membranous nephropathy. Nefrologia 40(3):345–350. https://doi.org/10.1016/j.nefro.2019.04.007
Laoraksawong P, Sanpool O, Rodpai R, Thanchomnang T, Kanarkard W, Maleewong W, Kraiklang R, Intapan PM (2018) Current high prevalences of Strongyloides stercoralis and Opisthorchis viverrini infections in rural communities in northeast Thailand and associated risk factors. BMC Public Health 18(1):940. https://doi.org/10.1186/s12889-018-5871-1
Malpica L, White AC Jr, Leguia C, Freundt N, Barros N, Chian C, Antunez EA, Montes M (2019) Regulatory T cells and IgE expression in duodenal mucosa of Strongyloides stercoralis and human T lymphotropic virus type 1 co-infected patients. PLoS Negl Trop Dis 13(6):e0007415. https://doi.org/10.1371/journal.pntd.0007415
Gao LW, Jiao AX, Wu XR, Zhao SY, Ma Y, Liu G, Yin J, Xu BP, Shen KL (2017) Clinical characteristics of disseminated cryptococcosis in previously healthy children in China. BMC Infect Dis 17(1):359. https://doi.org/10.1186/s12879-017-2450-5
Winnicki W, Eder M, Mazal P, Mayer FJ, Sengölge G, Wagner L (2018) Prevalence of Strongyloides stercoralis infection and hyperinfection syndrome among renal allograft recipients in Central Europe. Sci Rep 8(1):15406. https://doi.org/10.1038/s41598-018-33775-3
Vasquez-Rios G, Pineda-Reyes R, Pineda-Reyes J, Marin R, Ruiz EF, Terashima A (2019) Strongyloides stercoralis hyperinfection syndrome: a deeper understanding of a neglected disease. J Parasit Dis 43(2):167–175. https://doi.org/10.1007/s12639-019-01090-x
Forrer A, Khieu V, Schar F, Vounatsou P, Chammartin F, Marti H, Muth S, Odermatt P (2018) Strongyloides stercoralis and hookworm co-infection: spatial distribution and determinants in Preah Vihear Province, Cambodia. Parasit Vectors 11(1):33. https://doi.org/10.1186/s13071-017-2604-8
Nadir E, Grossman T, Ciobotaro P, Attali M, Barkan D, Bardenstein R, Zimhony O (2016) Real-time PCR for Strongyloides stercoralis—associated meningitis. Diagn Microbiol Infect Dis 84(3):197–199. https://doi.org/10.1016/j.diagmicrobio.2015.11.015
Funding
This study was supported by grants from the Natural Science Foundation of China (Grant no. 31770183), Guangdong Provincial Bureau of Traditional Chinese Medicine (Grant no. 20201407) and Qingyuan People’s Hospital Medical Scientific Research Fund Projects (Grant nos. 20190209 and 3303035).
Author information
Authors and Affiliations
Contributions
QH, LX and LC wrote and edited the manuscript. YT and GZ conducted the literature review. YL and ZZ were part of the medical team responsible for the patient. HL and BF provided expert consultation during the management of the patient and in the elaboration of this manuscript. All the authors critically evaluated the manuscript and accepted the final edition of it. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, L., He, Q., Chen, L. et al. Pulmonary Infection Caused by Strongyloides stercoralis. Acta Parasit. 67, 1044–1048 (2022). https://doi.org/10.1007/s11686-022-00527-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00527-3