Abstract
Introduction
The animal hookworm, Ancylostoma ceylanicum, is a dominant hookworm species of dogs and cats. However, it has increasingly been found infecting humans in Southeast Asia.
Purpose
We report an autochthonous case of A. ceylanicum in a suburban area of Selangor, Malaysia. A 66-year-old Indian lady who is an avid gardener presented with chronic diarrhea of 4 months’ duration.
Methods
The patient was examined clinically and colonoscopy was performed. Adult parasites obtained via colonoscopy were subjected to microscopy and molecular investigations.
Results
Clinical examinations were unremarkable, and blood investigation revealed normochromic normocytic anemia. Stool occult blood was positive but negative for ova, cyst and adult parasites. Colonoscopy performed showed multiple diverticulae and worm infestation from the terminal ileum to sigmoid colon. Morphological examination on the adult worms showed the specific characteristics of Ancylostoma species. Molecular investigations further confirmed the nematode as Ancylostoma ceylanicum. She was treated with albendazole 400 mg daily for 3 days with symptomatic improvements sustained 3 months later. It is suspected that the patient had ingested or contacted soil contaminated with filariform larvae while gardening.
Conclusion
Information on the A. ceylanicum infection in humans, especially in urban and suburban areas, is limited, necessitating further epidemiological and clinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The animal hookworm, Ancylostoma ceylanicum, is a dominant hookworm species of dogs and cats and is the second most common hookworm infecting humans in southeastern Asia [1, 2]. In 1966, investigations on hookworm infections in dogs, cats, and man carried out in Taiwan showed A. ceylanicum found in 2 of 12 aborigines and 3 of 128 Chinese of the general population [3]. Other sporadic reports included one case in Philippines [4], 16 positive out of 183 persons in Calcutta, India [5], 2 cases in Thailand’s temple communities [6] and 29.6% of the population in Cambodia (2012) [2]. In 2017, molecular identifications of human hookworms from certain parts of rural Lower Myanmar identified three sequences with high similarity of A. ceylanicum [7]. Recently. US-bound refugees living in Myanmar–Thailand border camps revealed that the prevalence of N. americanus hookworm was 25.4%, A. duodenale 0%, and A. ceylanicum 5.4% [8]. Besides Asia, A. ceylanicum is considered an emerging public health risk in tropical north Australia where the infection has been detected in domestic dogs in an indigenous community and present in soil samples at popular tourist locations [9]. Also, A. ceylanicum is endemic in the East Malaita region of Solomon Island, a Pacific Island nation located near to the east of Papua New Guinea [10].
Intestinal helminths infection in Malaysia had been reported with a wide prevalence of 0.7% up to 92.7% depending on the cohort area and demographics [11, 12]. Species identifying human hookworm infections among eight economically disadvantaged communities in rural areas of Peninsular Malaysia were determined molecularly and N. americanus was the most predominant hookworm identified, followed by A. ceylanicum [13]. In another study, molecular analysis was performed on 634 human and 105 domestic canine and feline fecal samples in Malaysia and revealed that most hookworm-positive individuals were infected with N. americanus, A. ceylanicum contributed 12.8% of single infections and 10.6% mixed infections with N. americanus. As for cats and dogs, more than half were positive for A. ceylanicum, followed by Ancylostoma caninum and Ancylostoma braziliense [14].
Although most hookworm studies were concentrated on rural area in Malaysia, recent reports indicate increasing prevalence among the urban population [15, 16]. Here, we report an autochthonous case of A. ceylanicum in a suburban area of Selangor, Malaysia.
Case Report
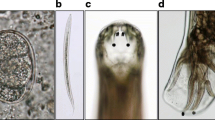
A 66-year-old Indian lady, who is an avid gardener, presented with chronic diarrhea (Bristol Type 6 and 7) for the last 4 months before she visited our center. There was no mucus or blood in the stool detected macroscopically. She has type 2 diabetes mellitus (HbA1c 9.6%) and hypertension. She has no family history of gastrointestinal pathology and is a non-smoker. Clinical examinations were unremarkable, and investigations revealed normochromic normocytic anemia (hemoglobin 10.3 g/dL) with no eosinophilia. Stool occult blood was positive but negative for ova, cyst and adult parasites. Colonoscopy was performed to rule out colorectal carcinoma. It showed multiple diverticulae and worm infestation from the terminal ileum to the sigmoid. Ten worms were visualized, with three extracted for identification (Fig. 1). Morphological examination of the adult worms showed the specific characteristics of Ancylostoma species (teeth) (Fig. 2). For species identification, the adult worm was subjected to a polymerase chain reaction (PCR) targeting the internal transcribed spacer-2 (ITS-2) ribosomal RNA gene [17]. In brief, the worm was mechanically grounded with a sterile scalpel using a vortex. The homogenate was then digested with proteinase K, followed by genomic DNA extraction using a commercial kit (MachereyNagel, Neumann-Neander, Duren, Germany). DNA amplification was performed, and the positive amplicon was subjected to DNA sequencing. Homology search using the National Center for Biotechnology Information (NCBI) reference sequences with Basic Local Alignment Search Tool (BLAST) confirmed the species as A. ceylanicum with 99.7% identity to the reference sequences deposited in the GenBank (GenBank accession number JN164659.1, JF960369.1, LC036567.1). Our observed consensus ITS-2 sequence was available in GenBank under the accession number MT925626. She received albendazole 400 mg once daily for 3 days with symptomatic improvements and hemoglobin returning to a healthy 12.6 g/dL at 3 months and 13.2 g/dL at 6 months.
Discussion
Ancylostoma ceylanicum is a hookworm of cats and dogs that have long been known to have established patent enteric infections in humans and has been considered the most neglected among all human hookworm species [18]. However, it is still impossible to differentiate eggs of all hookworm to species level based on the light microscopic techniques during direct fecal examination [19]. With the advent of PCR methods, differentiation between all hookworm species in humans, dogs, and cats is now feasible.
Ancyclostoma ceylanicum has been experimentally tested on human volunteers. In Thailand, the main signs and symptoms of the human volunteer include itching, urticaria-like skin lesions on the arm, becoming nodular in 1 day and developing blebs with edema. This symptom disappeared on the 6th day; there was irritation of the throat and dry cough at night after 12 days; hookworm eggs first appeared in the feces 32 days after the infection. The eosinophil count rose to a maximum of 58% on the 28th day, while chest X-rays remained negative. Post-treatment with tetrachloroethylene was given and revealed a total of 87 adults A. ceylanicum [20]. Another human volunteer study was published after a year where three human volunteers were infected orally and another three percutaneously with A. ceylanicum obtained from a cat. Oral infections became apparent in 18–26 days, at which time the two subjects in whom the adult worms were counted, 43% and 61%, had matured into the adult stage, respectively. However, less than 5% of larvae given percutaneously developed and the creeping eruption was not seen, indicating the more effective way of hookworm maturation in human hosts could be the oral route [21]. Heavy infection can result in bloody diarrhea and iron deficiency anemia, but unlike the other human hookworms, it can also cause severe enteritis and cognitive impairment [22]. Our patient experienced chronic diarrhea and anemia with colorectal carcinoma and helminths infestation low on the list, inexplicably resulting in inadequate social history.
Molecular diagnostic investigations can be used to confirm the diagnosis and allowed the identification of A. ceylanicum. The cytochrome c oxidase subunit 1 (cox 1) sequence of A. ceylanicum was phylogenetically analyzed. The results demonstrated that isolates of A. ceylanicum were divided into two distinct clusters, which might suggest potential haplotype-linked differences in zoonotic, epidemiological and pathobiological characteristics [23]. Recently, a novel, species-specific, real-time PCR-based assay for detecting A. ceylanicum has been developed to identify the species of hookworms [24].
In Malaysia, a case of A. ceylanicum infection was detected by endoscopy in a 58-year-old woman who lives in a rural area where uncontrolled populations of stray and semi-domesticated dogs are common [25]. Similarly, a Japanese patient who returned from a visit to Thailand and Lao People's Democratic Republic (PDR) was diagnosed with A. ceylanicum infection [26]. Another 25-year-old Japanese biologist who stayed in a rainforest in Malaysia for 4 weeks collecting spiders developed abdominal pain and diarrhea in the third week. A total of 11 adult worms were expelled after treatment with pyrantel pamoate and were identified as A. ceylanicum based on morphological characteristics and DNA sequences of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene [27]. A recent study on hookworm infections among migrant workers in Malaysia was conducted. The identified PCR amplicons revealed 81.0% identified as Necator americanus, 16.7% as Ancylostoma spp. and 2.4% as mixed infections of both species. All eight Ancylostoma spp. were confirmed to be Ancylostoma duodenale, albeit no A. ceylanicum was identified through this study [28].
It is suspected that our patient had ingested or contacted soil contaminated with filariform larvae while gardening in an area where stray cats and dogs are commonly seen. It is known that both A. ceylanicum and A. braziliense are found abundantly among dogs and cats in Malaysia [29]. However, a molecular survey had identified A. ceylanicum as being the predominant species among urban stray dogs. In contrast, rural dogs had a higher prevalence of A. caninum than A. ceylanicum, while both species demonstrated equal distribution among dogs in shelters [30]. Furthermore, a molecular survey on helminth eggs excreted in the faeces of stray cats, dogs, and soil samples collected from Klang Valley, a central-west region of Peninsular Malaysia, indicated that A. ceylanicum was dominant in both faecal and soil samples in the area [31].
Hookworm infection is well associated with iron deficiency anemia. Its infection can be easily treated with a common anthelminthic drug such as albendazole [32]. Although albendazole is still an effective treatment as presented in the current case, there is a need to acknowledge that gastrointestinal nematodes are becoming more resistant to the available antihelmintic drugs. Also, there is no new anthelmintic on the market for quite some years [33]. Nevertheless, vaccine using recombinant antigens of A. ceylanicum has been explored on animal models, and the immunization with a vaccine cocktail resulted in a 33.5% worm burden reduction in hamsters [34].
More zoonotic hookworm species can cross species and mature into adults other than their regular hosts, and it is expected that emerging infectious diseases will be of zoonotic origin [35]. For example, in South Korea, Jung et al. [36] reported a 60-year-old man who underwent a colonoscopy and recovered a juvenile female worm with three pairs of teeth. DNA sequencing showed 100% identity with A. caninum, a species of dog hookworm. A systematic review approach on research studies and case reports on human A. ceylanicum infections in Southeast Asia and the Pacific has been published. Therefore, a One Health approach to zoonotic hookworm control in populations in places where this zoonosis is still endemic is strongly recommended [37].
Conclusion
The epidemiology of A. ceylanicum is poorly known, especially in urban and suburban areas in Peninsular and Malaysian Borneo, necessitating further epidemiological and clinical studies. Furthermore, on a larger scale, the “hotspots” of zoonotic hookworm infection need to be identified in Asia and Pacific regions, and appropriate prevention and control measures should be implemented to counter the disease emergence soon.
References
Traub RJ (2013) Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int J Parasit 43:1009–1015. https://doi.org/10.1016/j.ijpara.2013.07.006
Inpankaew T, Schär F, Dalsgaard A, Khieu V, Chimnoi W, Chhoun C, Sok D, Marti H, Muth S, Odermatt P, Traub RJ (2014) High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg Infect Dis 20:976–982. https://doi.org/10.3201/eid2006.131770
Yoshida Y, Okamoto K, Chiu JK (1968) Ancylostoma ceylanicum infection in dogs, cats, and man in Taiwan. Am J Trop Med Hyg 17:378–381. https://doi.org/10.4269/ajtmh.1968.17.378
Velasquez CC, Cabrera BC (1968) Ancylostoma ceylanicum (Looss, 1911) in a Filipino woman. J Parasit 54:430–431
Chowdhury AB, Schad GA (1972) Ancylostoma ceylanicum: a parasite of man in Calcutta and environs. Am J Trop Med Hyg 21:300–301. https://doi.org/10.4269/ajtmh.1972.21.300
Traub RJ, Inpankaew T, Sutthikornchai C, Sukthana Y, Thompson RA (2008) PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet Parasit 155:67–73. https://doi.org/10.1016/j.vetpar.2008.05.001
Aung WPP, Htoon TT, Tin HH, Sanpool O, Jongthawin J, Sadaow L, Phosuk I, Ropai R, Intapan PM, Maleewong W (2017) First molecular identifications of Necator americanus and Ancylostoma ceylanicum infecting rural communities in lower Myanmar. Am J Trop Med Hyg 96:214–216. https://doi.org/10.4269/ajtmh.16-0610
O’Connell EM, Mitchell T, Papaiakovou M, Pilotte N, Lee D, Weinberg M, Sakulrak P, Tongsukh D, Oduro-Boateng G, Harrison S, Williams SA (2018) Ancylostoma ceylanicum Hookworm in Myanmar Refugees, Thailand, 2012–2015. Emerg Infect Dis 24:1472–1478. https://doi.org/10.3201/eid2408.180280
Smout FA, Skerratt LF, Butler JR, Johnson CN, Congdon BC, Thompson RA (2017) The hookworm Ancylostoma ceylanicum: An emerging public health risk in Australian tropical rainforests and Indigenous communities. One Health 3:66–69. https://doi.org/10.1016/j.onehlt.2017.04.002
Bradbury RS, Hii SF, Harrington H, Speare R, Traub R (2017) Ancylostoma ceylanicum hookworm in the Solomon Islands. Emerg Infect Dis 23:252–257. https://doi.org/10.3201/eid2302.160822
Norhayati M, Fatmah MS, Yusof S, Edariah AB (2003) Intestinal parasitic infections in man: a review. Med J Malaysia 58:296–305
Ngui R, Aziz S, Chua KH, Aidil RM, Lee SC, Tan TK, Sani MM, Arine AF, Rohela M, Lim YA (2015) Patterns and risk factors of soil-transmitted helminthiasis among orang Asli subgroups in peninsular Malaysia. Am J Trop Med Hyg 93:361–370. https://doi.org/10.4269/ajtmh.13-0677
Ngui R, Lee SC, Tan TK, Roslan MA, Lim YAL (2012) Molecular identification of human hookworm infections in economically disadvantaged communities in Peninsular Malaysia. Am J Trop Med Hyg 86:837–842. https://doi.org/10.4269/ajtmh.2012.11-0446
Ngui R, Lim YAL, Traub R, Mahmud R, Mistam MS (2012) Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLoS Negl Trop Dis 6:e1522. https://doi.org/10.1371/journal.pntd.0001522
Jamaiah I, Rohela M (2005) Prevalence of intestinal parasites among members of the public in Kuala Lumpur, Malaysia. Southeast Asian J Trop Med Public Health 36:68–71
Asady, Ismail S, Jalil MA, Pakeer O (2019). Soil transmitted helminth infection among children admitted to Hospital Tengku Ampuan Afzan, Kuantan, Pahang. IIUM Med J Malaysia 18(2). https://journals.iium.edu.my/kom/index.php/imjm/article/view/89
Ngui R, Lee SC, Tan TK, Muhammad Aidil R, Lim YAL (2012) Genetic characterization of human hookworm infections in rural and remote areas of Peninsular Malaysia. Am J Trop Med Hyg 86:837–842. https://doi.org/10.4269/ajtmh.2012.11-0446
Conlan JV, Sripa B, Attwood S, Newton PN (2011) A review of parasitic zoonoses in a changing Southeast Asia. Vet Parasit 182:22–40. https://doi.org/10.1016/j.vetpar.2011.07.013
Conlan JV, Khamlome B, Vongxay K, Elliot A, Pallant L, Sripa B, Blacksell SD, Fenwick S, Thompson RC (2012) Soil-transmitted helminthiasis in Lao People’s Democratic Republic: a community-wide cross-sectional study of humans and dogs in a mass drug administration environment. Am J Trop Med Hyg 86:624–634. https://doi.org/10.4269/ajtmh.2012.11-0413
Arebkul S, Radomyos P, Viravan C (1970) Experimental infection of Ancylostoma ceylanicum in man. J Med Assoc Thailand 53:190–194
Yoshida Y, Okamoto K, Chiu JK (1971) Experimental infection of man with Ancylostoma ceylanicum Looss. 1911. Chinese J Microbiol 4:157–167
Thompson RCA (2015) Neglected zoonotic helminths: Hymenolepis nana, Echinococcus canadensis and Ancylostoma ceylanicum. Clin Microbiol Infect 21:426–432. https://doi.org/10.1016/j.cmi.2015.01.004
Ngui R, Mahdy MA, Chua KH, Traub R, Lim YAL (2013) Genetic characterization of the partial mitochondrial cytochrome oxidase c subunit I (cox 1) gene of the zoonotic parasitic nematode, Ancylostoma ceylanicum from humans, dogs and cats. Acta Trop 128:154–157. https://doi.org/10.1016/j.actatropica.2013.06.003
Papaiakovou M, Pilotte N, Grant JR, Traub RJ, Llewellyn S, McCarthy JS, Krolewiecki AJ, Cimino R, Mejia R, Williams SA (2017) A novel, species-specific, real-time PCR assay for the detection of the emerging zoonotic parasite Ancylostoma ceylanicum in human stool. PLoS Negl Trop Dis 11:e0005734. https://doi.org/10.1371/journal.pntd.0005734
Ngui R, Lim YAL, Ismail WHW, Lim KN, Mahmud R (2014) Zoonotic Ancylostoma ceylanicum infection detected by endoscopy. Am J Trop Med Hyg 91:86–88. https://doi.org/10.4269/ajtmh.13-0756
Kaya D et al (2016) Ancylostoma ceylanicum hookworm infection in Japanese traveler who presented chronic diarrhea after return from Lao People’s Democratic Republic. Parasitol Int 65:737–740. https://doi.org/10.1016/j.parint.2016.07.001
Yoshikawa M, Ouji Y, Hirai N, Nakamura-Uchiyama F, Yamada M, Arizono N, Akamatsu N, Yoh T, Kaya D, Nakatani T, Kikuchi E (2018) Ancylostoma ceylanicum, novel etiological agent for traveler’s diarrhea—report of four Japanese patients who returned from Southeast Asia and Papua New Guinea. Trop Med Health 46:1–6. https://doi.org/10.1186/s41182-018-0087-8
Sahimin N, Lim YAL, Douadi B, Khalid MKNM, Wilson JJ, Behnke JM, Zain SNM (2017) Hookworm infections among migrant workers in Malaysia: molecular identification of Necator americanus and Ancylostoma duodenale. Acta Trop 173:109–115. https://doi.org/10.1016/j.actatropica.2017.06.011
Yoshida Y, Okamoto K, Matsuo K, Kwo EH, Retnasaba-Pathy A (1973) The occurrence of Ancylostoma braziliense (de Faria, 1910) and Ancylostoma ceylanicum (Looss, 1911) in Malaysia. Southeast Asian J Trop Med Public Health 4:498–503
Mahdy MA, Lim YAL, Ngui R, Fatimah MS, Choy SH, Yap NJ, Al-Mekhlafi HM, Ibrahim J, Surin J (2012) Prevalence and zoonotic potential of canine hookworms in Malaysia. Parasites Vectors 5:88. https://doi.org/10.1186/1756-3305-5-88
Tun S, Ithoi I, Mahmud R, Samsudin NI, Heng CK, Ling LY (2015) Detection of helminth eggs and identification of hookworm species in stray cats, dogs and soil from Klang Valley. Malaysia PloS One 10:e0142231. https://doi.org/10.1371/journal.pone.0142231
Hsu YC, Lin JT (2012) Intestinal infestation with Ancylostoma ceylanicum. N Engl J Med 366:e20. https://doi.org/10.1056/NEJMicm1101717
Zajíčková M, Nguyen LT, Skálová L, Stuchlíková LR, Matoušková P (2019) Anthelmintics in the future: current trends in the discovery and development of new drugs against gastrointestinal nematodes. Drug Discov Today 25:430–437. https://doi.org/10.1016/j.drudis.2019.12.007
Wiśniewski M, Łapiński M, Daniłowicz-Luebert E, Jaros S, Długosz E, Wędrychowicz H (2016) Vaccination with a cocktail of Ancylostoma ceylanicum recombinant antigens leads to worm burden reduction in hamsters. Acta Parasitol 61:556–561. https://doi.org/10.1515/ap-2016-0074
Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P (2008) Global trends in emerging infectious diseases. Nature 451:990–993. https://doi.org/10.1038/nature06536
Jung BK, Lee JY, Chang T, Song H, Chai JY (2020) Rare case of enteric Ancylostoma caninum hookworm infection, South Korea. Emerg Infect Dis 26:181–183. https://doi.org/10.3201/eid2601.191335
Stracke K, Jex AR, Traub RJ (2020) Zoonotic ancylostomiasis: an update of a continually neglected zoonosis. Am J Trop Med Hyg. https://doi.org/10.4269/ajtmh.20-0060
Acknowledgements
We thank the staff at the Gastroenterology Unit and Microbiology Laboratory, Clinical Trial Centre, Faculty of Medicine, UiTM for their kind support and assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Writing and identification of the worms: CCH; extracting worms via colonoscopy from patient: ARR; molecular identification of the worms: RN.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heo, C.C., Rafiz, A.R. & Ngui, R. A Case of Zoonotic Ancylostoma ceylanicum Infection in a Suburban Area of Selangor, Malaysia. Acta Parasit. 67, 564–568 (2022). https://doi.org/10.1007/s11686-021-00478-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00478-1