Abstract
Purpose
Larval stages of trombiculid mites infest a wide variety of wild and domestic animals. The most common clinical signs related with the presence of these parasites are alopecia, crusts, erythema, excoriation, erosion, papules, pustules and vesicles. Most of trombiculid infestations may not be perceived by the clinician due to their small size. Although Ericotrombidium ibericense has been recorded on cats, it has not been found on dogs.
Methods
In August 2019, three domestic dogs presenting pruritic dermatological lesions in the ventral area of the body and interdigital spaces were presented at a veterinary clinic in Santarém, central Portugal. Trombiculid mites were extracted from the skin and preserved in 70% ethanol. Specimens were prepared in slides with Hoyer’s medium and observed with optical microscopy.
Results
After morphological examination of the specimens, mites were identified as E. ibericense (Acariformes: Trombiculidae).
Conclusions
Most of the trombiculids recorded in European clinical practice are generally identified as Neotrombicula autumnalis by default, since, in most cases, mites are not examined morphologically. This is the first record of E. ibericense in domestic dogs. More studies are needed to evaluate the distribution of these mites in Portugal. Veterinary clinicians must be aware of this parasitosis, as trombiculids can cause exuberant clinical signs, but are often misdiagnosed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trombiculid larvae, also known as “chigger mites”, parasitize a wide variety of terrestrial animals, including mammals, birds, reptiles, amphibians, and rarely invertebrates [1,2,3]. In addition, Neotrombicula autumnalis is a potential vector of different Rickettsia and Borrelia spp. of major zoonotic concern [4].
A wide variety of dermatological signs can be observed in domestic animals presenting with trombiculids, including alopecia, crusts, erythema, excoriation, erosions, papules, pustules and vesicles [5, 6] in ear margins, face, interdigital spaces and ventral abdomen [6, 7]. Nevertheless, the level of pruritus is variable and some animals may harbour a high number of specimens without any associated skin lesion or clinical sign [7], while others exhibit severe clinical signs even with a small number of infesting trombiculids [8].
Female trombiculids lay their eggs on the soil, mainly during late summer and autumn, although these arthropods can occur in other seasons [9]. Larvae hatch in approximately ten days and after that period they climb to low vegetation, look for warm-blooded hosts and feed on them for 2–10 days [8]. After this, engorged larvae return to the soil and molt to nymphal stages (proto-, deuto- and tritonymphs). Nymphs and adults feed small arthropods or their eggs [1].
More than 30 trombiculid species of the genus Ericotrombidium Vercammen-Grandjean 1966 are known [10], being common on the Mediterranean fauna [11]. Ericotrombidium ibericense was described on a lizard (Chalcides ocellatus) and a wood mouse (Apodemus sylvaticus) in Spain [10]. Later it was recorded on a domestic cat (Felis catus) in Portugal [12]. Nonetheless, to our best knowledge, this trombiculid species has not yet been described in domestic dogs (Canis familiaris) in the world.
The aim of this study was to identify to species level trombiculids collected from domestic dogs.
In August 2019, three indoor/outdoor domestic dogs presenting pruritic dermatological lesions in the ventral area and interdigital spaces associated to trombiculids were presented at a veterinary medical centre in Santarém, central Portugal.
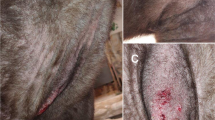
On the 9th of August 2019, a mongrel dog weighting 8.2 kg and aged 10.5-years-old was received for grooming. During visual inspection of the fur and skin, small orange mites were observed on the belly button, interdigital spaces and along the four legs (Fig. 1). The animal presented crusts, a small number of pustules and pruritus in a scale of 6/10 [13]. The dog lived mainly indoors and only had outdoor access when walking with his tutor. The animal was treated with one chewing tablet of 20 mg sarolaner (Simparica®; Zoetis). The animal was observed 5 weeks later and no parasites were observed.
On the 28th of August 2019, two indoor/outdoor mongrel dogs—a male weighting 25.5 kg and aged 2-years-old, and a female of 21.2 kg and 3-years-old—were presented for sterilization. During trichotomy, several mites were observed on both animals. The male presented severe clinical signs in the abdomen and lesions in the interdigital spaces with severe erythema, crusts, pustules and excoriations. This dog presented a pruritus of 7/10 (Fig. 2a) [13]. The female dog had fewer mites in the abdomen and interdigital spaces of posterior members, presenting crusts and small pustules, and a pruritus of 5/10 (Fig. 2b) [13]. The two dogs were treated with one chewing tablet of 80 mg sarolaner (Simparica®; Zoetis) after sterilization. Both animals were evaluated 12 days and 5 weeks later and no parasites were observed.
During clinical examination, mites were extracted from the skin with tweezers and preserved in 70% ethanol. Specimens were mounted on microscopic slides using Hoyer’s medium. Slides were dried in a thermostat at 37 °C for 3 days followed by the specimens’ examination (Fig. 3) with an Olympus BX50 microscope (Olympus Corporation, Tokyo, Japan). A photomicrograph of the scutum (Fig. 4) was taken with an Olympus DP10 digital camera (Olympus Corporation, Tokyo, Japan). Standard measurements (Table 1) were obtained with the use of an Olympus WH10X/22 microscope eyepiece (Olympus Corporation, Tokyo, Japan).
Identification of the mites to genus was based on two recently published keys [14, 15]. Identification to species level was performed with the use of the last revision of the genus [10], based on the following traits: distance between anterolateral scutal setae (AW), distance between posterolateral scutal setae (PW), distance between sensillary bases (SB), distance from the level of sensillary bases to extreme anterior margin of the scutum (ASB), distance from the level of sensillary bases to extreme posterior margin of the scutum (PSB), SD = ASB + PSB, distance from the level of posterolateral scutal setae to extreme posterior margin of the scutum (P-PL), distance from anterolateral to posterolateral scutal setae on one side (AP), length of anteromedian seta of the scutum (AM), length of anterolateral setae of the scutum (AL), length of posterolateral setae of the scutum (PL), length of sensillum (S), length of humeral idiosomal setae (H), length of leg I, including coxa (pa), length of leg II, including coxa (pm), length of leg III, including coxa (pp), Ip = pa + pm + pp, number of dorsal idiosomal setae (DS), number of ventral idiosomal setae (VS) and NDV = DS + VS + number of humeroventral setae (Fig. 5).
Measurements of the scutum of Ericotrombidium sp. Adapted from Stekolnikov et al [15]. AW distance between anterolateral scutal setae, PW distance between posterolateral scutal setae, SB distance between sensillary bases, ASB distance from the level of sensillary bases to extreme anterior margin of the scutum, PSB distance from the level of sensillary bases to extreme posterior margin of the scutum, P-PL distance from the level of posterolateral scutal setae to extreme posterior margin of the scutum, AP distance from anterolateral to posterolateral scutal setae on one side, AM length of anteromedian seta of the scutum, AL length of anterolateral setae of the scutum, PL length of posterolateral setae of the scutum, S length of sensillum
All observed specimens presented a rectangular scutum (Fig. 4), with flagelliform sensilla branched in distal half, dorsal idiosomal setae arranged as 2H-8-6-6-4-2-2, 7 branched setae and nude subterminala on palpal tarsus, three-pronged palpal claw, branched galeal seta, branched palpal femoral, genual and ventral tibial seta, nude dorsal and lateral palpal tibial setae.
All performed measurements are presented in Table 1. The obtained values were in agreement with those registered by Stekolnikov et al. for E. ibericense from a domestic cat from Portugal [12]. Thus, all examined specimens were identified as E. ibericense.
Trombiculids are important in human and veterinary medicine, since they can be vectors of different pathogenic agents [16]. These ectoparasites are distributed worldwide, having been mostly reported in wild animals, with fewer reports in domestic dogs [17, 18].
Although attacks of chigger mites in Europe were mostly recorded during autumn, they can also take place in mid-summer because the temperate climate of Mediterranean area provides better conditions for the development of these arthropods in comparison with northern European regions [19].
Pruritus is the most common clinical sign reported during trombiculiasis and associated with several dermatological manifestations. Pruritus level is variable and based on characteristics of the host allergic reaction caused by the salivary secretion of biting chiggers [8].
During clinical practice, only a few clinicians alert to the occurrence of these mites. Usually, those who detect trombiculids identify them as N. autumnalis by default [11], leading to incorrect diagnosis. Under microscopic magnification it is possible to observe that E. ibericense differs from N. autumnalis by its rectangular scutum and by the presence of eight setae in the first row of dorsal idiossomal setae vs. nearly pentagonal scutum and six setae in the first row of N. autumnalis [11].
Amongst trombiculids and besides this species, Neotrombicula inopinata and E. ibericense have already been recorded on cats from Portugal [6, 11], as well as Straelensia cynotis in a dog [20]. In the present work we report the presence of E. ibericense in dogs in Portugal for the first time, a fact which highlights that clinicians must be alert for the different trombiculid mites affecting dogs and include them in differential diagnosis when analysing cases of pruritus and allergic reactions in domestic animals. Since several trombiculid species attacking pets are known in Portugal, further studies are needed concerning the distribution of lesions on hosts and their relation with different chigger species, drug efficacy and geographical dispersion.
References
Wharton GW, Fuller HS (1952) A manual of the chiggers. Mem Entomol Soc Wash 4:1–185
Stekolnikov AA, Bavani MM, Rafinejad J, Saboori A (2019) A new species of chigger mite (Acariformes: Trombiculidae: Leeuwenhoekiinae) collected from a scorpion in Iran. Int J Acarol 45:341–346
Mendoza-Roldan JA, Colella V, Lia RP, Nguyen VL, Barros-Battesti DM, Iatta R, Dantas-Torres F, Otranto D (2019) Borrelia burgdorferi (sensu lato) in ectoparasites and reptiles in southern Italy. Parasit Vectors 12:35
Mendoza-Roldan JA, Modry D, Otranto D (2020) Zoonotic parasites of reptiles: a crawling threat. Trends Parasitol. https://doi.org/10.1016/j.pt.2020.04.014
Tudor P, Fernoaga C, Tudor N (2015) Trombiculosis in cats due to Neotrombicula autumnalis (Acari: Trombiculidae) larvae: the first report in Romania. J Anim Plant Sci 25:1496–1498
Ramilo DW, Monteiro C, Carreira M, Pereira da Fonseca I, Cardoso L (2019) First report of Neotrombicula inopinata infestation in domestic cats from Portugal. Vet Parasitol 267:1–3
Schöler A, Maier WA, Kampen H (2006) Multiple environmental factor analysis in habitats of the harvest mite Neotrombicula autumnalis (Acari: Trombiculidae) suggests extraordinarily high euryoecious biology. Exp Appl Acarol 39:41–62
Santibáñez P, Palomar AM, Portillo A, Santibáñez A, Oteo JA (2015) The role of chiggers as human pathogens. In: Samie A (ed) An overview of tropical diseases. InTech, London, pp 173–202
Guarneri C, Chokoeva AA, Wollina U, Lotti T, Tchernev G (2017) Trombiculiasis: not only a matter of animals! Wien Med Wochenschr 167:70–73
Vercammen-Grandjean PH, Langston RL (1976) The Chigger mites of the World (Acarina: Trombiculidae et Leeuwenhoekiidae) Leptotrombidium complex. Section A. Section B. Section C, vol III. George Williams Hooper Foundation, San Francisco
Stekolnikov AA, Santibáñez P, Palomar AM, Oteo JA (2014) Neotrombicula inopinata (Acari: Trombiculidae)—a possible causative agent of trombiculiasis in Europe. Parasit Vectors 7:90
Stekolnikov AA, Waap H, Gomes J, Antunes T (2016) Chigger mites of the genus Ericotrombidium (Acariformes: Trombiculidae) attacking pets in Europe. Vet Parasitol 221:60–63
Hill PB, Lau P, Rybníček J (2007) Development of an owner-assessed scale to measure the severity of pruritus in dogs. Vet Dermatol 18:301–308
Stekolnikov AA (2018) Taxonomy and distribution of African chiggers (Acariformes, Trombiculidae). Eur J Taxon 395:1–233
Stekolnikov AA, Saboori A, Shamsi M, Hakimitabar M (2019) Chigger mites (Acariformes: Trombiculidae) of Iran. Zootaxa 4549:1–66
Fernández-Soto P, Pérez-Sánchez R, Encinas-Grandes A (2001) Molecular detection of Ehrlichia phagocytophila genogroup organisms in larvae of Neotrombicula autumnalis (Acari: Trombiculidae) captured in Spain. J Parasitol 87:1482–1483
Poliana T (2012) Trombiculidae harvest mites (Neotrombicula autumnalis) infestation in dog in winter season—a case report. Scientif Works C Series Vet Med 58:306–311
Areso-Apesteguía M, Areso-Portell JB, Halaihel-Kassab N, Garcia Salinas MJ (2019) Severe trombiculiasis in hunting dogs infested with Neotrombicula inopinata (Acari: Trombiculidae). J Med Entomol 56:1389–1394
Leone F, Di Bella A, Vercelli A, Cornegliani L (2013) Feline trombiculosis: a retrospective study in 72 cats. Vet Dermatol 24:535–e126
Seixas F, Travassos PJ, Pinto ML, Correia J, Pires MA (2006) Dermatitis in a dog induced by Straelensia cynotis: a case report and review of the literature. Vet Dermatol 17:81–84
Acknowledgements
We are grateful to the staff of São Francisco de Assis Veterinary Clinic who provided the specimens for our study. The authors also acknowledge Zoetis Portugal for the medicines (Simparica®) provided during this project. DWR holds the FCT post-doctoral grant SFRH/BPD/115202/2016.
Funding
This work was supported by CIISA - Centro de Investigação Interdisciplinar em Sanidade Animal, Project UIDP/CVT/00276/2020, funded by FCT, by Project UIDB/CVT/00772/2020, also funded by FCT, and by the Ministry of Science and Higher Education of the Russian Federation (project No. AAAA-A19-119020790133–6, to AAS).
Author information
Authors and Affiliations
Contributions
David W. Ramilo: conceptualization; data curation; formal analysis; investigation; methodology; writing, original draft. Pedro Costa: conceptualization; data curation; formal analysis; investigation; methodology; writing, original draft. Alexandr A. Stekolnikov: formal analysis; visualization; writing, review and editing. João Martinho Cláudio: data curation; formal analysis; investigation; methodology; resources. Ana Mafalda Lourenço: formal analysis; Isabel Pereira da Fonseca: supervision; writing, review and editing. Luís Cardoso: supervision; writing, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data obtained in the present work is available in the manuscript.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
David W. Ramilo and Pedro Costa: Joint first authors.
Rights and permissions
About this article
Cite this article
Ramilo, D.W., Costa, P., Stekolnikov, A.A. et al. First report of Ericotrombidium ibericense in domestic dogs. Acta Parasit. 66, 253–258 (2021). https://doi.org/10.1007/s11686-020-00247-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-020-00247-6