Abstract
Internet addiction (IA) is a growing social concern and has been intensively studied in recent years. Previous imaging studies have shown that IA may impair brain structure and function, but with no robust conclusions. We conducted a systematic review and meta-analysis of neuroimaging studies in IA. Two separate meta-analyses were conducted for voxel-based morphometry (VBM) studies and resting-state functional connectivity (rsFC) studies. All meta-analyses were performed using two analysis methods activation likelihood estimation (ALE) and seed-based d mapping with permutation of subject images (SDM-PSI). The ALE analysis of VBM studies revealed less gray matter volume (GMV) in the supplementary motor area (SMA) (1176 mm3), anterior cingulate cortex (ACC) (one cluster size is 744 mm3 and the other is 688 mm3), and orbitofrontal cortex (OFC) (624 mm3) in subjects with IA. The SDM-PSI analysis showed less GMV in the ACC (56 voxels). The ALE analysis of rsFC studies showed stronger rsFC from posterior cingulate cortex (PCC) (880 mm3) or insula (712 mm3) to the whole brain in subjects with IA; however, the SDM-PSI analysis revealed no obvious rsFC alteration. These changes may underlie the core symptoms of IA, which include emotional regulation disorder, distraction, and impaired executive control. Our results reflect the common features of neuroimaging studies related to IA in recent years and may potentially help inform the development of more effective diagnostic and treatment approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the rapid advances in information technology, the internet has become a popular and convenient tool for socializing and entertainment(Loh & Kanai, 2016). However, some people may experience difficulties in controlling their internet use behaviors, which can eventually lead to internet addiction (IA) (Stevens et al., 2020; Weinstein & Lejoyeux, 2010). According to a meta-analysis, the estimated average prevalence rate of IA is 7.02%, which is projected to increase over time(Pan et al., 2020).
Previous reports have highlighted several negative consequences of IA. IA adversely affects the quality of life (physical discomfort, loss of energy, emotional disturbance, poor academic performance) (Alsalameh et al., 2019; Aznar-Díaz et al., 2020; Karaer & Akdemir, 2019), causes social problems (dysfunctional social relations and decreased productivity) (Byington & Schwebel, 2013; Cerniglia et al., 2017; Marzilli et al., 2020; Recupero, 2021), and is also associated with a range of symptoms (craving, impulsiveness, response inhibition) (Cudo & Zabielska-Mendyk, 2019; Dong et al., 2018; Lee et al., 2018; Liu et al., 2014; Loh & Kanai, 2016; Ma et al., 2019), cognitive problems (attention capacities, memory processes, and social cognition) (Firth et al., 2019), and psychiatric disorders (depression, anxiety, attention deficit and hyperactivity disorder) (Jorgenson et al., 2016; Wang et al., 2017; Weinstein & Lejoyeux, 2010). Given the myriad adverse effects of IA, research on the corresponding brain changes is a key imperative. Unraveling the structural and functional alterations underlying IA may provide useful insights into its diagnosis and treatment.
Magnetic resonance imaging (MRI) is an advanced non-invasive technique that can reveal brain alterations under physiological or pathophysiological conditions(Yousaf et al., 2018). The voxel-based morphometry (VBM) method can be used to quantify the gray matter volume (GMV), reflecting the cerebral structure alterations(Takeuchi & Kawashima, 2017). Resting-state functional connectivity (rsFC) provides a measure of the macroscopic functional network of the human brain(Fingelkurts et al., 2005). Currently, VBM and rsFC are commonly utilized in IA-related neuroimaging studies(Weinstein & Lejoyeux, 2020; Weinstein et al., 2017), however with inconsistent conclusions. VBM studies have revealed less GMV in the anterior cingulate cortex (ACC)(Lee et al., 2018), dorsolateral prefrontal cortex(Wang et al., 2016), and supplementary motor area (SMA)(Jin et al., 2016). Besides, rsFC studies have revealed stronger connectivity in the insula(Ko et al., 2015), posterior cingulate cortex (PCC)(Seok & Sohn, 2018), and precuneus(Lee et al., 2021), and less connectivity in the SMA(Lee et al., 2021), inferior parietal lobule(Ding et al., 2013), and superior frontal gyrus(Y. Zhang et al. 2016a, b). Despite several overlapping structures, the results have been largely inconsistent. For example, in one study, the posterior insula and SMA showed less rsFC(Y. Zhang et al. 2016a, b), but in another study, these structures showed stronger rsFC(Zhang et al., 2016a, b). Some VBM studies showed less GMV in subjects with IA(Choi et al., 2017; He et al., 2021; Lee et al., 2018, 2021; C. Wang et al. 2016, 2021a, b; Yuan et al. 2011), while other studies have yielded opposite results(Han et al., 2012; Lee et al., 2019; Seok & Sohn, 2018; Sun et al., 2014; Yoon et al., 2017). Thus, performing a systematic review and meta-analysis of available evidence is important to draw definitive conclusions regarding the IA-induced brain structural and functional alterations.
Compared to a single study, meta-analyses provide more comprehensive estimation of research results by integrating data from all available relevant studies(Cortese et al., 2016). The two most widely utilized neuroimaging analysis methods are activation likelihood estimation (ALE) and seed-based d mapping (SDM)(Pezzoli et al., 2021; Santangelo et al., 2019). Previous meta-analyses have commonly used a single analysis method(Solly et al., 2022) or frequently focused on a single problem such as internet game disorder(Yao et al., 2017). However, IA, as a social problem, is very different from clinical diseases in its modality and speed of change. In the contemporary digital era, the coverage and diversity of internet have widely expanded. Therefore, we believe that a combination of analytic approaches can enable more comprehensive characterization of this condition. By synthesizing VBM and rsFC studies in IA patients, we aimed to explore common and specific neurological alterations in IA from the perspective of brain structure and function. Compared to previous studies that generally used only one method, we used both methods to provide more reliable results.
Methods
Search strategy
A comprehensive literature search of VBM and rsFC studies of IA was conducted in the PubMed, Cochrane Library, and Web of Science databases to retrieve studies published as of November 2022. The following search strategy was used: (“internet addiction disorder” OR “social media addiction” OR “smartphone addiction” OR “internet gaming disorder” OR “gaming addiction” OR “mobile phone dependence” OR “internet communication addiction” OR “problematic smartphone use”) AND (“voxel-based morphometry” OR “VBM” OR “gray matter” OR “grey matter”).
The following terms were also used to retrieve relevant studies: (“internet addiction disorder” OR “social media addiction” OR “smartphone addiction” OR “internet gaming disorder” OR “gaming addiction” OR “mobile phone dependence” OR “internet communication addiction” OR “problematic smartphone use”) AND (“magnetic resonance imaging” OR “functional magnetic resonance imaging” OR “fMRI”) AND (“functional connectivity”). In addition, the reference lists of the retrieved articles and relevant literature reviews were manually screened to identify relevant studies.
Study inclusion criteria
Original studies were included if: (1) IA was diagnosed according to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) or other quantitative assessment tools or both.; (2) VBM or seed-based rsFC analysis was utilized; (3) the comparison was conducted between subjects with IA (IAs) and healthy controls (HCs) without substance abuse, drug addiction, or other mental illnesses; (4) results were reported as coordinates in Montreal Neurological Institute (MNI) or Talairach space; (5) study design was cross-sectional comparative studies.
Exclusion criteria were: (1) Neither VBM study nor rsFC study; (2) analysis was performed on predefined regions of interest (ROI) rather than on the whole brain; (3) longitudinal or interventional design but without baseline comparisons; (4) no comparisons with HCs; (5) research based on task state.
Studies that compared different IA groups in the same article with a single HCs group were coded differently. Since there is no specific quality evaluation tool for neuroimaging meta-analysis, we referred to the Newcastle-Ottawa scale (NOS) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm). The detailed items and scores of included studies are listed in Supplementary Tables S1 and S2. This study was registered with PROSPERO (CRD42021277662). The whole process was carried out according to the registered content, and the specific protocol can be obtained by contacting the corresponding author.
Data extraction
Two authors independently retrieved the articles, and any disagreement regarding the selection of the articles was reviewed by a third experienced author and a final decision was made by consensus. The titles, abstracts, and full-texts of the screened articles were sequentially reviewed by researchers to determine their eligibility for inclusion. Selected articles were conserved through the Endnotes data manager. Then, two authors independently extracted the required data from each paper that met the inclusion criteria. The detailed data extracted from the articles are summarized in Table 1. Finally, we divided the coordinates included in the VBM and rsFC studies into two groups: IAs > HCs group and IAs < HCs group. These coordinates were summarized in an individual document for further analysis.
Data analysis
For a more reliable and comprehensive study, the meta-analysis procedure was performed by using the two most commonly used coordinate-based neuroimaging meta-analysis methods: ALE and SDM.
ALE meta-analysis
First, we used the GingerALE (http://www.brainmap.org/)(Raimo et al., 2021; Turkeltaub et al., 2002) to analyze the VBM studies and rsFC studies separately. The ALE technique is a voxel-based method for finding convergence across neuroimaging experiment coordinates. The revised version of the ALE algorithm treats foci as 3-dimensional Gaussian probability distributions centered at the given coordinates(Eickhoff et al., 2009, 2012). To quantify convergence among included studies, a random effect analysis was conducted(Eickhoff et al., 2012). Based on the sample size and random effect model, the likelihood of consensus among different experiments was attained. A modeled activation (MA) map was computed by merging the probability distributions of foci included in each study. Then, the union of all MA maps at the voxel level was performed to generate the final ALE map with ALE scores. Based on recommendations(Eickhoff et al., 2016), ALE maps were set with a threshold at p < 0.05 using a cluster level family-wise error (cFWE) with a cluster forming threshold at voxel level p < 0.001. The p values in our analyses were generated with 5000 permutations(Eickhoff et al., 2009, 2012; Turkeltaub et al., 2012).
SDM meta-analysis
We then repeated the meta-analysis using the seed-based d mapping with permutation of subject images (SDM-PSI) (version 6.21, https://www.sdmproject.com). The major change of the new SDM-PSI method is the provision of multiple comparison correction (MCC), which improves the reliability of the results. The main advantage of this method is that it considers studies with both positive, negative, and non-significant results, leading to more accurate estimates than other methods that ignore non-significant results. First, the significant peak coordinates and the associated t-values were extracted. Second, as a preprocessing step, the lower and upper effect size limits were estimated. A 20 mm full-width half maximum (FWHM) anisotropic Gaussian kernel and 2 mm voxel size was used for preprocessing(Albajes-Eizagirre et al., 2019). Third, the maximum likelihood estimation (MLE) of the most likely effect size and its standard error was performed according to the standard SDM-PSI parameters(Albajes-Eizagirre et al., 2019). Fourth, the significant results were corrected using the family-wise error (FWE) correction method based on threshold-free cluster enhancement (TFCE)(Albajes-Eizagirre et al., 2019). The corrected p-value threshold was 0.05. Furthermore, cluster sizes > 20 voxels were used as a supplement to reduce the possibility of false-positive results.
Heterogeneity, sensitivity analysis, meta-regression analysis, and publication bias
The heterogeneity among the included studies was assessed by calculating the I2 statistic. To verify the stability and reliability of the results, the Jackknife sensitivity analysis was performed for each meta-analysis, by repeating the same analysis after sequential exclusion of one dataset at a time. Then, meta-regression analysis was performed to examine the relationship between relevant factors and brain changes. Finally, the effect of potential publication bias on the results of the meta-analysis was assessed by creating funnel plots based on the SDM-PSI software, which calculated effect sizes by Hedge’s g. A scatter plot resembling an asymmetrically inverted funnel is considered indicative of lack of significant publication bias(Sterne et al., 2011).
Results
Characteristics of the included studies
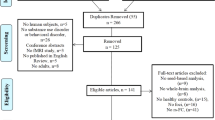
A total of 556 publications were retrieved on database search, of which 463 were retained after removal of duplicate publications. After reviewing the titles and abstracts, 108 articles were reviewed to assess their eligibility for inclusion. After full text reviews, 14 studies that were not VBM studies, 7 that were not rsFC studies, 10 that did not include healthy controls, 25 that did not report whole brain results, and 2 studies that did not report coordinates were excluded. Finally, 19 VBM studies enrolling 440 IAs and 444 HCs, and 31 rsFC studies enrolling 1042 IAs and 909 HCs were included. The demographic and clinical characteristics of the study population in the included studies are summarized in Tables 1 and 2. Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram(Liberati et al., 2009).
Meta-analyses of VBM studies
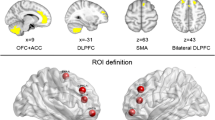
The ALE meta-analysis of 19 VBM studies revealed less GMV in the bilateral SMA, bilateral ACC, and left orbitofrontal cortex (OFC) (Fig. 2A; Table 3). Four clusters with significant differences were identified: the size of cluster 1 was 1176 mm3, belonging to Brodmann area (BA) 6, z-value was 4.60; the size of cluster 2 was 744 mm3, belonging to BA32/24, z-value was 4.76; the size of cluster 3 was 688 mm3, belonging to BA 24/32/33, z-value was 4.53; the size of cluster 4 was 624 mm3, belonging to BA 10, z-value was 4.57. It is worth noting that both cluster 2 and cluster 3 were within the ACC, and the sum of the two clusters exceeded cluster 1. The SDM-PSI meta-analysis showed less GMV in the left ACC, which was consistent with ALE method (Fig. 2B; Table 3). One cluster with significant difference was found, and this cluster contained 56 voxels, belonging to BA 24, p-value was 0.041.
Results of neural alterations in IA. (A) The results of the meta-analysis of all VBM studies using ALE method. Compared with HCs, IAs showed less GMV in the bilateral SMA, bilateral ACC, and left OFC. (B) The results of the meta-analysis of all VBM studies using SDM-PSI method. Compared with HC, IAs showed less GMV in the left ACC. (C) The results of the meta-analysis of all rsFC studies using ALE method. Compared with HCs, IA showed stronger rsFC from PCC or insula to the whole brain. Abbreviations: VBM, voxel-based morphometry; ALE, activation likelihood estimation; HCs, healthy controls; GMV, gray matter volume; IA, internet addiction; SMA, supplementary motor area; ACC, anterior cingulate cortex; OFC, orbitofrontal cortex; SDM-PSI, seed-based d mapping with permutation of subject images; rsFC, resting-state functional connectivity; PCC, posterior cingulate cortex
Meta-analyses of rsFC studies
The analysis of 31 rsFC studies using ALE revealed significantly stronger whole brain connectivity in the left PCC and insula in IAs (Fig. 2C; Table 4). The significant difference was observed in one of the clusters having a size of 880 mm3 and belonging to BA 31 with a z-value of 5.15, and cluster 2 having a size of 712 mm3 and belonging to BA 13 with a z-value of 4.90. However, the SDM-PSI meta-analysis after the added strict FWE correction demonstrated no cluster with significantly different rsFC (Fig. 2C; Table 4).
Combined with the results of VBM and rsFC studies, it was found that the cingulate gyrus region demonstrates significant structure and function alterations in IA subjects.
Heterogeneity, sensitivity analysis, meta-regression analysis, and publication bias
The results showed no significant heterogeneity among the studies (VBM: I2 = 42%; rsFC: I2 = 1%). In jackknife analysis, the lesser GMV in ACC (18/19), SMA (17/19), and OFC (17/19) was reproducible, and the stronger rsFC in ACC (29/31) and insula (28/31) was reproducible. The meta-regression analysis of rsFC studies showed no significant association of the altered brain regions with age. Analysis of the VBM studies showed a negative correlation between GMV changes in SMA and age (p = 0.0008), suggesting that the older the IAs, the smaller the GMV of SMA. To assess the potential effect of publication bias on the meta-analysis results, we established a funnel plot based on SDM software. For the VBM study (Fig. 3), the funnel plot was symmetrical and the test based on SDM-PSI software was not statistically significant (p > 0.05), indicating no publication bias. For the rsFC study (Fig. 4), the funnel plot was also symmetrical and the SDM-PSI software assessment also showed no potential publication bias (p > 0.05).
Discussion
To the best of our knowledge, this is the first meta-analysis that combined ALE with SDM methods to investigate neural alterations in subjects with IA across modalities (VBM and rsFC). The VBM meta-analysis showed that subjects with IAs had reduced GMV in the SMA, ACC, and OFC. Moreover, the rsFC meta-analysis showed that IA was associated with stronger whole brain connectivity in the left PCC and insula. Among them, the structural change of the ACC was the most robust, which was verified with both ALE and SDM-PSI methods.
The findings of the current meta-analysis were consistent with those of previous neuroimaging studies. Meng(Meng et al., 2015) quantitatively summarized task-related functional magnetic resonance imaging (fMRI) studies by using a coordinate-based meta-analysis and found that patients with internet gaming disorder showed higher brain activation in the ACC. A systematic review and meta-analysis of VBM studies by Qin(Qin et al., 2020) highlighted that behavioral addiction could lead to ACC and SMA alterations. Yao(Yao et al., 2017) showed hyperactivation in the ACC and less GMV in the ACC, orbitofrontal and premotor cortices, confirming both structural and functional changes in subjects with internet gaming disorder. However, there are some inconsistencies between our results and the above studies. For example, abnormalities in the fusiform gyrus, dorsolateral prefrontal cortex, caudate, and other regions were found in the meta-analysis for task-related fMRI studies, while GMV changes in putamen and dorsolateral prefrontal cortex were shown in the meta-analysis for VBM studies. There may be two reasons for these inconsistencies. First, compared with the resting state, meta-analysis of task state studies may lead to different results due to failure to standardize the task contents. Second, the previous meta-analysis used the old version of SDM software without MCC, resulting in more significant results. For this reason, we chose to analyze the resting state studies and used the latest version of the analysis method. Moreover, we used two meta-analysis methods to obtain more robust results.
Comparison between ALE and SDM
ALE and SDM are the most common applications for performing neuroimaging meta-analysis, but there are no clear standards for the processing of neuroimaging meta-analysis, and the scope of application of these two commonly used tools is not well defined. The main operation for applying ALE software is the input of coordinates, a process without much manual intervention, avoiding additional errors. In addition, the latest version of the ALE software has greater restrictions on reporting results, so that only clusters size larger than 400 are finally presented. Moreover, the ALE software has always provided FWE correction, which enhances the robustness of the results. However, ALE does not have a specific algorithm to distinguish between function and structure, and the results of both modalities converge using the same approach. In contrast, the advantage of SDM software is that it provides analysis tools for a variety of modes, covering fMRI, VBM, Tract-based spatial statistics (TBSS), among others, and the matching algorithms are selected for various modes(Albajes-Eizagirre et al., 2019). SDM also provides global analysis, linear regression, publication bias analysis, and other functions to meet the needs of diverse studies. However, previous versions of the SDM software failed to provide corrections, reducing the credibility of the results. While newer versions of the SDM software do provide corrections, the software creators believe that the corrections may be too conservative(Albajes-Eizagirre et al., 2019). In the current study, we observed consistency between ALE and SDM in the meta-analysis for the VBM study, but the results were still ambiguous in the meta-analysis for the rsFC study. We consider that this may be caused by the overly strict correction, as stated by the creators of the SDM software. Another possible reason is that the seed points of the original studies were not entirely consistent, as mentioned in the review by Müller and others(Müller et al., 2015). Nevertheless, we believe that resting-state studies are preferable materials for meta-analysis when compared with task-state studies, and there is a paucity of studies based on the amplitude of low-frequency fluctuation (ALFF), regional homogeneity (ReHo), and other indicators for IA; therefore, we opted for rsFC as the main target of resting-state meta-analysis. Although the seed points of the original study were not completely consistent, they all belonged to the prefrontal striatal circuits, and our results were also consistent with the previous meta-analysis; therefore, we believe that the meta-analysis of rsFC had a certain significance.
The cingulate gyrus
The cingulate gyrus is known to be a key part of the limbic system. It is responsible for emotional control, action-outcome learning, and memory(Rolls, 2019). The ACC receives information from the OFC about rewarding and non-rewarding outcomes and links rewards to behavior; in addition, the ACC is also related to emotions(Rolls, 2019). The ACC is closely related to the interaction between cognitive control and reward-related networks(Gläscher et al., 2012). Moreover, the ACC was also shown to play a key role in modulating task switching based on task demands or possible rewards(Rushworth et al., 2004). Therefore, it can be inferred that ACC changes in IAs may be related to poor decision-making ability and task-switching ability. In addition, the ACC projects to the thalamus through the globus pallidus substantia nigra and ventral striatum, which is related to cognitive control processes, such as error detection and emergency inhibitory control(Feil et al., 2010). This circuit plays an essential role in the assessment of consequences and error detection of addictive behaviors(Volkow et al., 2013). A characteristic feature of addiction is that excessive exposure to the addictive target or environment reduces the sensitivity of the brain’s reward system. This process is believed to be mediated by the dopamine circuit, and the striatum is a key region of this dopamine circuit(Solinas et al., 2019). The ACC mediates reward signals, and the projection area is also closely related to the addiction circuit, which may be a critical area involved in the addiction process.
On the other hand, the PCC receives spatial and action-related information from parietal cortical areas. It participates in visual-spatial and sensorimotor processes. Because the PCC has outputs to the hippocampal system, it is also involved in memory(Rolls, 2019; Rolls & Wirth, 2018). Therefore, decreased attention and poor executive ability in IAs may be associated with abnormal PCC. The PCC has been shown to be associated with the self-reference function(Bush et al., 2000). Dysfunction within and between these circuits may cause disturbances in human emotional behavior(Zhou et al., 2011). The enhanced rsFC of PCC in IAs may be a compensatory expression.
PCC and ACC have been frequently referred to in the process of addiction(Zilverstand et al., 2018), and this view is strongly supported by the current study. Alterations in the cingulate gyrus may be a key neurobiological basis for IA, as these regions were consistently identified in our cross-modal and cross-method meta-analysis.
SMA
The GMV of SMA in IAs was significantly less than that in HCs. The SMA is critical for motor planning and execution, especially voluntary action, corresponding to the process of response inhibition associated with addiction(Shirota et al., 2019). According to a review by Volkow, SMA is involved in the inferior frontal gyrus circuit associated with response inhibition(Volkow et al., 2013). Furthermore, the region projected from SMA into the globus pallidus is the output portion of the dopaminergic pathway between the inferior frontal gyrus and thalamus, through which the basal ganglia regulate other prefrontal subcortical circuits(Feil et al., 2010). The dysfunction of this circuit in IAs is liable to affect their reward-related dopaminergic nervous system and may further lead to addiction. There is growing evidence that motor control decline may be age-related, with cortical motor networks such as SMA contributing to motor performance(Seidler et al., 2010). This suggests that the effect of SMA on motor performance changes with age, which may explain the negative correlation between GMV in SMA and age observed in the meta-regression analysis.
OFC
Our VBM meta-analysis also showed abnormal GMV of OFC in subjects with IAs. The OFC, as a part of the prefrontal cortex, is involved in executive function. It is connected to some striatal regions including the caudate and nucleus accumbens, which associate the OFC with the reward function(Schoenbaum et al., 2006). Moreover, this circuit is highly involved in decision-making and regulation of impulsivity(Volkow et al., 2013). The OFC abnormalities may interfere with addicts’ action plans, leading them to prefer immediate rewards over delayed gratification(Volkow et al., 2013). Dysfunction of this circuit has been widely reported in substance-dependent individuals and is thought to play an important role in guiding the perceived outcomes of decision-making and subsequent behaviors(Feil et al., 2010).
Insula
The insula is the part of the cerebral cortex that plays a crucial role in substance use behaviors such as interoception, decision-making, anxiety, pain perception, cognition, mood, threat recognition, and conscious urges(Ibrahim et al., 2019). Clinical studies have demonstrated that the volume of insula gray matter decreases in addicts(Zhang et al., 2021), that destruction of the insula can cause nicotine addicts to stop smoking(Naqvi et al., 2007), and that insula destruction can also affect gambling behavior in gambling addicts(Clark et al., 2014). The insula cortex has now been identified by clinical studies as a key target for addiction treatment(Ibrahim et al., 2019). Our study also confirms this, while reaffirming the similarity between IA and drug addiction.
Limitations
Some limitations of this meta-analysis should be considered while interpreting the results. First, we used the ALE model, which is currently the most widely used and well-known coordinate-based meta-analysis method, but since ALE meta-analysis was performed on the whole brain mask, results can only be obtained from the whole. The results obtained by SDM were structurally consistent with ALE but showed no significant results in rsFC. This FWE correction can be an overly conservative strategy in some cases and lead to false-negative results(Albajes-Eizagirre et al., 2019). Second, our meta-analysis included a small number of studies and our results may have been affected by potential publication bias because we did not include gray literature. Future studies will require larger sample sizes. Third, due to the small number of studies, a more detailed investigation of various subgroups of IA, such as internet game disorder, smartphone addiction, etc., was not conducted. More studies are required to facilitate subgroup analysis. Fourth, adequate information on clinical variables was not available for all studies. Further studies should include psychological behavioral information and pharmacological interventions. Fifth, the studies did not evaluate sex-based differences; moreover, the study population in the included studies mainly consisted of Asians and we could not assess the effect of genetic, ethnic, and cultural factors. Sixth, it is difficult to exclude the heterogeneity of research methods (including software, thresholds, and magnetic field strength). Our study reports the results of two methods, which can be subsequently validated using more novel methods.
Conclusion
We conclude that patients with IA possess structural and resting-state brain alterations compared to HCs, which involve the cingulate gyrus, SMA, and OFC. Our results reflect the common characteristics of neuroimaging studies related to IA in recent years, and has certain guiding significance for subsequent research.
Code Availability
Not applicable.
Data Availability
Available from the corresponding author upon request.
References
Albajes-Eizagirre, A., Solanes, A., Vieta, E., & Radua, J. (2019). Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. Neuroimage, 186, 174–184. https://doi.org/10.1016/j.neuroimage.2018.10.077.
Alsalameh, A. M., Harisi, M. J., Alduayji, M. A., Almutham, A. A., & Mahmood, F. M. (2019). Evaluating the relationship between smartphone addiction/overuse and musculoskeletal pain among medical students at Qassim University. J Family Med Prim Care, 8(9), 2953–2959. https://doi.org/10.4103/jfmpc.jfmpc_665_19.
Aznar-Díaz, I., Romero-Rodríguez, J. M., García-González, A., & Ramírez-Montoya, M. S. (2020). Mexican and spanish university students’ internet addiction and academic procrastination: Correlation and potential factors. PLoS One, 15(5), e0233655. https://doi.org/10.1371/journal.pone.0233655.
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends In Cognitive Sciences, 4(6), 215–222. https://doi.org/10.1016/s1364-6613(00)01483-2.
Byington, K. W., & Schwebel, D. C. (2013). Effects of mobile internet use on college student pedestrian injury risk. Accident Analysis And Prevention, 51, 78–83. https://doi.org/10.1016/j.aap.2012.11.001.
Cerniglia, L., Zoratto, F., Cimino, S., Laviola, G., Ammaniti, M., & Adriani, W. (2017). Internet addiction in adolescence: Neurobiological, psychosocial and clinical issues. Neuroscience And Biobehavioral Reviews, 76(Pt A), 174–184. https://doi.org/10.1016/j.neubiorev.2016.12.024.
Choi, J., Cho, H., Kim, J. Y., Jung, D. J., Ahn, K. J., Kang, H. B., & Kim, D. J. (2017). Structural alterations in the prefrontal cortex mediate the relationship between internet gaming disorder and depressed mood. Scientific Reports, 7(1), 1245. https://doi.org/10.1038/s41598-017-01275-5.
Clark, L., Studer, B., Bruss, J., Tranel, D., & Bechara, A. (2014). Damage to insula abolishes cognitive distortions during simulated gambling. Proc Natl Acad Sci U S A, 111(16), 6098–6103. https://doi.org/10.1073/pnas.1322295111.
Cortese, S., Castellanos, F. X., Eickhoff, C. R., D’Acunto, G., Masi, G., Fox, P. T., & Eickhoff, S. B. (2016). Functional decoding and Meta-analytic connectivity modeling in adult Attention-Deficit/Hyperactivity disorder. Biological Psychiatry, 80(12), 896–904. https://doi.org/10.1016/j.biopsych.2016.06.014.
Cudo, A., & Zabielska-Mendyk, E. (2019). Cognitive functions in internet addiction - a review. Psychiatria Polska, 53(1), 61–79. https://doi.org/10.12740/pp/82194.
Ding, W. N., Sun, J. H., Sun, Y. W., Zhou, Y., Li, L., Xu, J. R., & Du, Y. S. (2013). Altered default network resting-state functional connectivity in adolescents with internet gaming addiction. PLoS One, 8(3), e59902. https://doi.org/10.1371/journal.pone.0059902.
Dong, G., Wang, L., Du, X., & Potenza, M. N. (2018). Gender-related differences in neural responses to gaming cues before and after gaming: Implications for gender-specific vulnerabilities to internet gaming disorder. Soc Cogn Affect Neurosci, 13(11), 1203–1214. https://doi.org/10.1093/scan/nsy084.
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F., & Fox, P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage, 59(3), 2349–2361. https://doi.org/10.1016/j.neuroimage.2011.09.017.
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., & Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. https://doi.org/10.1002/hbm.20718.
Eickhoff, S. B., Nichols, T. E., Laird, A. R., Hoffstaedter, F., Amunts, K., Fox, P. T., & Eickhoff, C. R. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage, 137, 70–85. https://doi.org/10.1016/j.neuroimage.2016.04.072.
Feil, J., Sheppard, D., Fitzgerald, P. B., Yücel, M., Lubman, D. I., & Bradshaw, J. L. (2010). Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neuroscience And Biobehavioral Reviews, 35(2), 248–275. https://doi.org/10.1016/j.neubiorev.2010.03.001.
Fingelkurts, A. A., Fingelkurts, A. A., & Kähkönen, S. (2005). Functional connectivity in the brain–is it an elusive concept? Neuroscience And Biobehavioral Reviews, 28(8), 827–836. https://doi.org/10.1016/j.neubiorev.2004.10.009.
Firth, J., Torous, J., Stubbs, B., Firth, J. A., Steiner, G. Z., Smith, L., & Sarris, J. (2019). The “online brain”: How the internet may be changing our cognition. World Psychiatry, 18(2), 119–129. https://doi.org/10.1002/wps.20617.
Gläscher, J., Adolphs, R., Damasio, H., Bechara, A., Rudrauf, D., Calamia, M., & Tranel, D. (2012). Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc Natl Acad Sci U S A, 109(36), 14681–14686. https://doi.org/10.1073/pnas.1206608109.
Han, D. H., Lyoo, I. K., & Renshaw, P. F. (2012). Differential regional gray matter volumes in patients with on-line game addiction and professional gamers. Journal Of Psychiatric Research, 46(4), 507–515. https://doi.org/10.1016/j.jpsychires.2012.01.004.
He, Q., Turel, O., Wei, L., & Bechara, A. (2021). Structural brain differences associated with extensive massively-multiplayer video gaming. Brain Imaging Behav, 15(1), 364–374. https://doi.org/10.1007/s11682-020-00263-0.
Ibrahim, C., Rubin-Kahana, D. S., Pushparaj, A., Musiol, M., Blumberger, D. M., Daskalakis, Z. J., & Le Foll, B. (2019). The Insula: A brain stimulation target for the treatment of addiction. Frontiers In Pharmacology, 10, 720. https://doi.org/10.3389/fphar.2019.00720.
Jin, C., Zhang, T., Cai, C., Bi, Y., Li, Y., Yu, D., & Yuan, K. (2016). Abnormal prefrontal cortex resting state functional connectivity and severity of internet gaming disorder. Brain Imaging Behav, 10(3), 719–729. https://doi.org/10.1007/s11682-015-9439-8.
Jorgenson, A. G., Hsiao, R. C., & Yen, C. F. (2016). Internet addiction and other behavioral addictions. Child And Adolescent Psychiatric Clinics Of North America, 25(3), 509–520. https://doi.org/10.1016/j.chc.2016.03.004.
Karaer, Y., & Akdemir, D. (2019). Parenting styles, perceived social support and emotion regulation in adolescents with internet addiction. Compr Psychiatry, 92, 22–27. https://doi.org/10.1016/j.comppsych.2019.03.003.
Ko, C. H., Hsieh, T. J., Wang, P. W., Lin, W. C., Yen, C. F., Chen, C. S., & Yen, J. Y. (2015). Altered gray matter density and disrupted functional connectivity of the amygdala in adults with internet gaming disorder. Progress In Neuropsychopharmacology And Biological Psychiatry, 57, 185–192. https://doi.org/10.1016/j.pnpbp.2014.11.003.
Lee, D., Namkoong, K., Lee, J., & Jung, Y. C. (2018). Abnormal gray matter volume and impulsivity in young adults with internet gaming disorder. Addiction Biology, 23(5), 1160–1167. https://doi.org/10.1111/adb.12552.
Lee, D., Namkoong, K., Lee, J., & Jung, Y. C. (2019). Preliminary evidence of altered gray matter volume in subjects with internet gaming disorder: Associations with history of childhood attention-deficit/hyperactivity disorder symptoms. Brain Imaging Behav, 13(3), 660–668. https://doi.org/10.1007/s11682-018-9872-6.
Lee, D., Namkoong, K., Lee, J., & Jung, Y. C. (2021). Dorsal striatal functional connectivity changes in internet gaming disorder: A longitudinal magnetic resonance imaging study. Addiction Biology, 26(1), e12868. https://doi.org/10.1111/adb.12868.
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., & Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Bmj, 339, b2700. https://doi.org/10.1136/bmj.b2700.
Liu, G. C., Yen, J. Y., Chen, C. Y., Yen, C. F., Chen, C. S., Lin, W. C., & Ko, C. H. (2014). Brain activation for response inhibition under gaming cue distraction in internet gaming disorder. Kaohsiung Journal Of Medical Sciences, 30(1), 43–51. https://doi.org/10.1016/j.kjms.2013.08.005.
Loh, K. K., & Kanai, R. (2016). How Has the Internet Reshaped Human Cognition? Neuroscientist, 22(5),506–520. https://doi.org/10.1177/1073858415595005
Ma, S. S., Worhunsky, P. D., Xu, J. S., Yip, S. W., Zhou, N., Zhang, J. T., & Fang, X. Y. (2019). Alterations in functional networks during cue-reactivity in internet gaming disorder. J Behav Addict, 8(2), 277–287. https://doi.org/10.1556/2006.8.2019.25.
Marzilli, E., Cerniglia, L., Ballarotto, G., & Cimino, S. (2020). Internet addiction among young adult University students: The Complex interplay between Family Functioning, Impulsivity, Depression, and anxiety. International Journal Of Environmental Research And Public Health, 17(21), https://doi.org/10.3390/ijerph17218231.
Meng, Y., Deng, W., Wang, H., Guo, W., & Li, T. (2015). The prefrontal dysfunction in individuals with internet gaming disorder: A meta-analysis of functional magnetic resonance imaging studies. Addiction Biology, 20(4), 799–808. https://doi.org/10.1111/adb.12154.
Müller, K. W., Janikian, M., Dreier, M., Wölfling, K., Beutel, M. E., Tzavara, C., & Tsitsika, A. (2015). Regular gaming behavior and internet gaming disorder in european adolescents: Results from a cross-national representative survey of prevalence, predictors, and psychopathological correlates. European Child And Adolescent Psychiatry, 24(5), 565–574. https://doi.org/10.1007/s00787-014-0611-2.
Naqvi, N. H., Rudrauf, D., Damasio, H., & Bechara, A. (2007). Damage to the insula disrupts addiction to cigarette smoking. Science, 315(5811), 531–534. https://doi.org/10.1126/science.1135926.
Pan, Y. C., Chiu, Y. C., & Lin, Y. H. (2020). Systematic review and meta-analysis of epidemiology of internet addiction. Neuroscience And Biobehavioral Reviews, 118, 612–622. https://doi.org/10.1016/j.neubiorev.2020.08.013.
Pezzoli, S., Sánchez-Valle, R., Solanes, A., Kempton, M. J., Bandmann, O., Shin, J. I., & Radua, J. (2021). Neuroanatomical and cognitive correlates of visual hallucinations in Parkinson’s disease and dementia with Lewy bodies: Voxel-based morphometry and neuropsychological meta-analysis. Neuroscience And Biobehavioral Reviews, 128, 367–382. https://doi.org/10.1016/j.neubiorev.2021.06.030.
Qin, K., Zhang, F., Chen, T., Li, L., Li, W., Suo, X., & Gong, Q. (2020). Shared gray matter alterations in individuals with diverse behavioral addictions: A voxel-wise meta-analysis. J Behav Addict, 9(1), 44–57. https://doi.org/10.1556/2006.2020.00006.
Raimo, S., Cropano, M., Trojano, L., & Santangelo, G. (2021). The neural basis of gambling disorder: An activation likelihood estimation meta-analysis. Neuroscience And Biobehavioral Reviews, 120, 279–302. https://doi.org/10.1016/j.neubiorev.2020.11.027.
Recupero, P. R. (2021). Homicide and the internet. Behavioral Sciences & The Law, 39(2), 216–229. https://doi.org/10.1002/bsl.2509.
Rolls, E. T. (2019). The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct, 224(9), 3001–3018. https://doi.org/10.1007/s00429-019-01945-2.
Rolls, E. T., & Wirth, S. (2018). Spatial representations in the primate hippocampus, and their functions in memory and navigation. Progress In Neurobiology, 171, 90–113. https://doi.org/10.1016/j.pneurobio.2018.09.004.
Rushworth, M. F., Walton, M. E., Kennerley, S. W., & Bannerman, D. M. (2004). Action sets and decisions in the medial frontal cortex. Trends In Cognitive Sciences, 8(9), 410–417. https://doi.org/10.1016/j.tics.2004.07.009.
Santangelo, G., Raimo, S., Cropano, M., Vitale, C., Barone, P., & Trojano, L. (2019). Neural bases of impulse control disorders in Parkinson’s disease: A systematic review and an ALE meta-analysis. Neuroscience And Biobehavioral Reviews, 107, 672–685. https://doi.org/10.1016/j.neubiorev.2019.09.041.
Schoenbaum, G., Roesch, M. R., & Stalnaker, T. A. (2006). Orbitofrontal cortex, decision-making and drug addiction. Trends In Neurosciences, 29(2), 116–124. https://doi.org/10.1016/j.tins.2005.12.006.
Seidler, R. D., Bernard, J. A., Burutolu, T. B., Fling, B. W., Gordon, M. T., Gwin, J. T., & Lipps, D. B. (2010). Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neuroscience And Biobehavioral Reviews, 34(5), 721–733. https://doi.org/10.1016/j.neubiorev.2009.10.005.
Seok, J. W., & Sohn, J. H. (2018). Altered Gray Matter volume and resting-state connectivity in individuals with internet gaming disorder: A voxel-based morphometry and resting-state functional magnetic resonance imaging study. Frontiers In Psychiatry, 9, 77. https://doi.org/10.3389/fpsyt.2018.00077.
Shirota, Y., Hanajima, R., Ohminami, S., Tsutsumi, R., Ugawa, Y., & Terao, Y. (2019). Supplementary motor area plays a causal role in automatic inhibition of motor responses. Brain Stimulation, 12(4), 1020–1026. https://doi.org/10.1016/j.brs.2019.03.002.
Solinas, M., Belujon, P., Fernagut, P. O., Jaber, M., & Thiriet, N. (2019). Dopamine and addiction: What have we learned from 40 years of research. J Neural Transm (Vienna), 126(4), 481–516. https://doi.org/10.1007/s00702-018-1957-2.
Solly, J. E., Hook, R. W., Grant, J. E., Cortese, S., & Chamberlain, S. R. (2022). Structural gray matter differences in problematic usage of the internet: A systematic review and meta-analysis. Molecular Psychiatry, 27(2), 1000–1009. https://doi.org/10.1038/s41380-021-01315-7.
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., & Higgins, J. P. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj, 343, d4002. https://doi.org/10.1136/bmj.d4002.
Stevens, C., Zhang, E., Cherkerzian, S., Chen, J. A., & Liu, C. H. (2020). Problematic internet use/computer gaming among US college students: Prevalence and correlates with mental health symptoms. Depression And Anxiety, 37(11), 1127–1136. https://doi.org/10.1002/da.23094.
Sun, Y., Sun, J., Zhou, Y., Ding, W., Chen, X., Zhuang, Z., & Du, Y. (2014). Assessment of in vivo microstructure alterations in gray matter using DKI in Internet gaming addiction. Behavioral And Brain Functions, 10, 37. https://doi.org/10.1186/1744-9081-10-37.
Takeuchi, H., & Kawashima, R. (2017). [Voxel-Based morphometry and cognitive function]. Brain And Nerve, 69(5), 547–556. https://doi.org/10.11477/mf.1416200781.
Turkeltaub, P. E., Eden, G. F., Jones, K. M., & Zeffiro, T. A. (2002). Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage, 16(3 Pt 1), 765–780. https://doi.org/10.1006/nimg.2002.1131.
Turkeltaub, P. E., Eickhoff, S. B., Laird, A. R., Fox, M., Wiener, M., & Fox, P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping, 33(1), 1–13. https://doi.org/10.1002/hbm.21186.
Volkow, N. D., Wang, G. J., Tomasi, D., & Baler, R. D. (2013). Unbalanced neuronal circuits in addiction. Current Opinion In Neurobiology, 23(4), 639–648. https://doi.org/10.1016/j.conb.2013.01.002.
Wang, B. Q., Yao, N. Q., Zhou, X., Liu, J., & Lv, Z. T. (2017). The association between attention deficit/hyperactivity disorder and internet addiction: A systematic review and meta-analysis. Bmc Psychiatry, 17(1), 260. https://doi.org/10.1186/s12888-017-1408-x.
Wang, C., Zhang, Z., Che, L., Wu, Y., Qian, H., & Guo, X. (2021a). The gray matter volume in superior frontal gyrus mediates the impact of reflection on emotion in internet gaming addicts. Psychiatry Res Neuroimaging, 310, 111269. https://doi.org/10.1016/j.pscychresns.2021a.111269.
Wang, X., Cheng, B., Wang, S., Lu, F., Luo, Y., Long, X., & Kong, D. (2021b). Distinct grey matter volume alterations in adult patients with panic disorder and social anxiety disorder: A systematic review and voxel-based morphometry meta-analysis. Journal Of Affective Disorders, 281, 805–823. https://doi.org/10.1016/j.jad.2020.11.057.
Wang, Y., Zou, Z., Song, H., Xu, X., Wang, H., d’Oleire Uquillas, F., & Huang, X. (2016). Altered Gray Matter volume and White Matter Integrity in College students with mobile phone dependence. Frontiers In Psychology, 7, 597. https://doi.org/10.3389/fpsyg.2016.00597.
Weinstein, A., & Lejoyeux, M. (2010). Internet addiction or excessive internet use. American Journal Of Drug And Alcohol Abuse, 36(5), 277–283. https://doi.org/10.3109/00952990.2010.491880.
Weinstein, A., & Lejoyeux, M. (2020). Neurobiological mechanisms underlying internet gaming disorder Dialogues Clin Neurosci, 22(2),113–126. https://doi.org/10.31887/DCNS.2020.22.2/aweinstein
Weinstein, A., Livny, A., & Weizman, A. (2017). New developments in brain research of internet and gaming disorder. Neuroscience And Biobehavioral Reviews, 75, 314–330. https://doi.org/10.1016/j.neubiorev.2017.01.040.
Yao, Y. W., Liu, L., Ma, S. S., Shi, X. H., Zhou, N., Zhang, J. T., & Potenza, M. N. (2017). Functional and structural neural alterations in internet gaming disorder: A systematic review and meta-analysis. Neuroscience And Biobehavioral Reviews, 83, 313–324. https://doi.org/10.1016/j.neubiorev.2017.10.029.
Yoon, E. J., Choi, J. S., Kim, H., Sohn, B. K., Jung, H. Y., Lee, J. Y., & Kim, Y. K. (2017). Altered hippocampal volume and functional connectivity in males with internet gaming disorder comparing to those with alcohol use disorder. Scientific Reports, 7(1), 5744. https://doi.org/10.1038/s41598-017-06057-7.
Yousaf, T., Dervenoulas, G., & Politis, M. (2018). Advances in MRI methodology. International Review Of Neurobiology, 141, 31–76. https://doi.org/10.1016/bs.irn.2018.08.008.
Yuan, K., Qin, W., Wang, G., Zeng, F., Zhao, L., Yang, X., & Tian, J. (2011). Microstructure abnormalities in adolescents with internet addiction disorder. PLoS One, 6(6), e20708. https://doi.org/10.1371/journal.pone.0020708.
Zhang, J. T., Yao, Y. W., Li, C. S., Zang, Y. F., Shen, Z. J., Liu, L., & Fang, X. Y. (2016a). Altered resting-state functional connectivity of the insula in young adults with internet gaming disorder. Addiction Biology, 21(3), 743–751. https://doi.org/10.1111/adb.12247.
Zhang, M., Gao, X., Yang, Z., Wen, M., Huang, H., Zheng, R., & Zhang, Y. (2021). Shared gray matter alterations in subtypes of addiction: A voxel-wise meta-analysis. Psychopharmacology (Berl), 238(9), 2365–2379. https://doi.org/10.1007/s00213-021-05920-w.
Zhang, Y., Mei, W., Zhang, J. X., Wu, Q., & Zhang, W. (2016b). Decreased functional connectivity of insula-based network in young adults with internet gaming disorder. Experimental Brain Research, 234(9), 2553–2560. https://doi.org/10.1007/s00221-016-4659-8.
Zhou, Y., Lin, F. C., Du, Y. S., Qin, L. D., Zhao, Z. M., Xu, J. R., & Lei, H. (2011). Gray matter abnormalities in internet addiction: A voxel-based morphometry study. European Journal Of Radiology, 79(1), 92–95. https://doi.org/10.1016/j.ejrad.2009.10.025.
Zilverstand, A., Huang, A. S., Alia-Klein, N., & Goldstein, R. Z. (2018). Neuroimaging impaired response inhibition and salience attribution in Human Drug Addiction: A systematic review. Neuron, 98(5), 886–903. https://doi.org/10.1016/j.neuron.2018.03.048.
Acknowledgements
The authors would like to thank Drs. Wu-Xun Cui, Si-Jie Xiu, from the Department of Radiology of Tangdu Hospital, as well as Dr. Xiao-Cheng Wei from MR Research China of GE Healthcare for their outstanding technical support. Our gratitude also goes for Profs. Jin-Lian Li, Liang-Wei Chen, and Jun-Ling Zhu from the Department of Radiology of Tangdu Hospital for helpful comments on the manuscript.
Funding
This work was supported by the Hovering Program of Fourth Military Medical University (axjhww to WW), and the Talent Foundation of Tangdu Hospital (2018BJ003 to WW).
Author information
Authors and Affiliations
Contributions
WW and RW, Conceptualization; JTS, BH and TQC, Design experiments; JTS and BH, Data curation; TQC and ZHC, Data extraction; YXS Software; YTL, YXS and ZHC, Data analysis; RW, Supervision; JTS and BH, Visualization; JTS, Writing-original draft; WW, Writing-review & editing.
Corresponding authors
Ethics declarations
This study was a systematic review of the previously published studies and did not use original human or animal data.
Conflict of interest
None of the authors have a conflict of interest to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing-Ting Sun, Bo Hu and Tian-Qi Chen contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, JT., Hu, B., Chen, TQ. et al. Internet addiction-induced brain structure and function alterations: a systematic review and meta-analysis of voxel-based morphometry and resting-state functional connectivity studies. Brain Imaging and Behavior 17, 329–342 (2023). https://doi.org/10.1007/s11682-023-00762-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-023-00762-w