Abstract
We aimed to perform a combined analysis of cortical thickness and functional connectivity to explore their association with cognitive impairment in Parkinson’s disease (PD). A total of 53 PD and 15 healthy control subjects were enrolled. PD patients were divided into PD with normal cognition (PD-NC, n = 25), PD with mild cognitive impairment (PD-MCI, n = 11), and PD with dementia (PDD, n = 17). In some analyses, the PD-MCI and PDD groups were aggregated to represent “PD patients with cognitive impairment”. Cognitive status was assessed with the Mini-Mental State Examination (MMSE). Anatomical magnetic resonance imaging and resting-state functional connectivity analysis were performed in all subjects. First, surface-based morphometry measurements of cortical thickness and voxels with cortical thickness reduction were detected. Then, regions showing reduced thickness were analyzed for changes in resting-state functional connectivity in PD involving cognitive impairment. Our results showed that, compared with PD-NC, patients with cognitive impairment showed decreased cortical thickness in the left superior temporal, left lingual, right insula, and right fusiform regions. PD-MCI patients showed these alterations in the right lingual region. Widespread cortical thinning was detected in PDD subjects, including the left superior temporal, left fusiform, right insula, and right fusiform areas. We found that cortical thinning in the left superior temporal, left fusiform, and right temporal pole regions positively correlated with MMSE score. In the resting-state functional connectivity analysis, we found a decrease in functional connectivity between the cortical atrophic brain areas mentioned above and cognition-related brain networks, as well as an increase in functional connectivity between those region and the cerebellum. Alterations in cortical thickness may result in a dysfunction of resting-state functional connectivity, contributing to cognitive decline in patients with PD. However, it is more probable that the relation between structure and FC would be bidirectional,and needs more research to explore in PD cognitve decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a chronic neurodegenerative condition common in middle-aged and elderly subjects, affecting 8 to 18 in every 100,000 people every year (de Lau & Breteler, 2006). PD involves mainly the extrapyramidal system and manifests as motor symptoms, such as muscular rigidity, static tremor, bradykinesia, and postural gait disorder. Many PD patients also manifest non-motor symptoms, including autonomic dysfunction, psychiatric disturbances, sensory disorders, and cognitive impairment. Cognitive impairment in PD can range from mild cognitive impairment (PD-MCI) to dementia (PD-associated dementia, PDD). PD-MCI is an independent risk factor for PDD. PDD has been associated with increased risk of disability (Aarsland et al., 2000) and mortality(Levy et al., 2002), amounting to a heavy social and economic burden. Early screening for cognitive impairment and its biological markers is crucial to identify PD patients at higher risk of disease progression and worse outcomes.
In recent years, a number of magnetic resonance imaging (MRI) studies have shown promise for the prediction of cognitive function decline in PD. Previous MRI studies using voxel-based morphometry showed a correlation of grey matter atrophy with disease progression and cognitive impairment in PD (Ibarretxe-Bilbao et al., 2009, 2011). However, surface-based morphometry measurements of cortical thickness may be more sensitive than voxel-based morphometry for the identification of regional cortical thinning associated with PD (Pereira et al., 2012). Previous work found regional thinning in left temporal-occipital and right parietal-frontal areas in PD patients with cognitive disorder compared to patients without it (Pagonabarraga et al., 2013), while another study did not find this difference (Pereira et al., 2014).
Recently, several studies have examined so-called ‘resting state’ networks (Schindlbeck & Eidelberg, 2018; Wu & Hallett, 2013). Resting state networks are characterized by organized basal activity during rest or passive visual fixation and by low-frequency signal fluctuations. Analysis of these resting state networks suggest the existence of an underlying structural core of functional connectivity (FC) networks in the human brain (van den Heuvel & Hulshoff Pol, 2010). Previous work (Amboni et al., 2015; Baggio et al., 2015; Wolters et al., 2019; Zhan et al., 2018) showed that cognitive impairment in PD correlates with reduced connectivity in networks relevant to cognition, most prominently the default-mode, executive control, and salient networks. The default-mode network, including the hippocampus, medial temporal, posterior cingulate cortex, precuneus, medial prefrontal cortex, and inferior parietal lobule (Buckner et al., 2008), has been postulated to play roles in many cognitive processes, such as the theory of mind, social cognition, executive function, and episodic recall (Laird et al., 2011). Patients with PDD show disrupted connectivity in the default-mode network (Rektorova et al., 2012). Executive control networks are found in the dorsolateral prefrontal cortex and posterior parietal cortex. PD patients show alterations in executive control network functionality, leading to defects in focused attention, executive functions, and behavioral regulation (Dong et al., 2020). Salient networks include the insula and anterior cingulate cortex, integrating the up-down information from past experiences (Suzuki & Amaral, 1994). Disruptions in FC involving these networks may be useful biomarkers for identifying PD with cognitive impairment.
Previous imaging studies have found alterations in cortical thickness and FC in patients with PD-MCI. However, their conclusions are based on separated studies and none of them combined both analyses at different stages of PD cognitive impairment. In the present study, we analyzed neuroimaging data of PD patients in two steps: (1) surface-based morphometry measurements of cortical thickness and voxels with cortical thickness reduction were detected; then (2) regions of interest as defined in step 1 were analyzed to search for abnormalities in FC between voxels with reduced cortical thickness and whole-brain voxels in resting-state FC analysis. We expect that our study will guide the discovery of neuroimaging markers and underlying mechanisms related to cognitive impairment in PD.
Methods
Participants
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University. All participants provided written informed consent for their anonymized clinical data to be analyzed and published for research purposes.
The flow chart of this study is shown in Fig. 1. Study subjects were enrolled from a series of outpatient clinics and from the neurology department of the First Affiliated Hospital of Kunming Medical University between June 2020 and October 2021 in this cross-sectional study. PD patients were diagnosed according to the 2015 criteria of the International Parkinson’s and Motor Disorders Association (Postuma et al., 2015).
Studies have shown that MMSE seems to be more suitable for cognitive screening of PD population in China, especially for illiterate, primary school education, which can screen out more PD patients with cognitive dysfunction (Wang et al., 2014).We believe that China’s social and cultural background is different from that of western countries, and the illiteracy rate among the elderly is high. In clinical studies, it should be taken into account that the understanding and execution ability for Cognitive assessment Scale of subjects with low education level, and those with low education level tend to have higher prevalence of other physical diseases, which may affect test scores more easily. So, The overall cognitive status was assessed by the Mini-Mental State Examination (MMSE) in both PD patients and healthy controls in this study. And those are considered cognitively decline according to their educational level: (1) Illiteracy: the MMSE score < 19. (2) Primary schools: the MMSE score < 23. (3) Middle school and above: MMSE score < 27 (Wang et al., 2014). PD patients were stratified into three groups: those with normal cognition (PD-NC), PD-MCI, and PDD. In some analyses, the PD-MCI and PDD groups were aggregated to represent “PD patients with cognitive impairment”. The diagnoses of PD-MCI or PDD were made according to the Diagnostic Criteria by the Movement Disorder Society Task Force Guidelines (Emre et al., 2007; Litvan et al., 2012). Patients who did not satisfy the criteria for PD-MCI or PDD were defined as PD without cognitive impairment.
Patients were excluded if they presented any of the following: atypical or secondary symptoms associated with PD, including multiple progressive supranuclear palsy, multiple system atrophy, corticobasal ganglionic degeneration, vascular parkinsonism, or neuroleptic agent-related parkinsonism; previous history of additional neurological or psychological disorders; any MRI-associated contraindications; left-handedness; or history of intracranial surgery for PD.
As healthy controls, individuals were recruited who had normal cognitive function, as assessed by the MMSE no psychological or neurological disorder; and no current chronic condition.
Neuropsychological tests
Study assessments were performed during ‘ON’ medication conditions (best motor), without stopping any ongoing medication. Motor symptom severity was assessed using the Hoehn and Yahr (H-Y) scale (Goetz et al., 2004) and the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III) (Goetz, 2010). Drug intake was recorded, and levodopa-equivalent daily dose in patients with PD was calculated as recommended (Tomlinson et al., 2010).
Rapid eye movement sleep behavior disorder (RBD) was diagnosed based on the Rapid Eye Movement Sleep Behavior Disorder screening questionnaire (RBDSQ) (Bušková et al., 2019). Across the 10 items of the RBDSQ, the total possible score is 13, and a score > 5 was considered suggestive of RBD (Bušková et al., 2019). Patients’ roommates were asked whether the patient had nightmares, talked or moved while sleeping. The severity of depression in PD patients was assessed using the Hamilton Depression Scale (HAMD) (Broen et al., 2015), while severity of anxiety was assessed using the Hamilton Anxiety Scale (HAMA) (Kummer et al., 2010).
Image acquisition and processing
Image acquisition
All subjects underwent MRI exams on a 3.0-T whole body scanner (Discovery 750w, GE Healthcare, USA) at the Imaging Department of the First Affiliated Hospital of Kunming Medical University, using standard head coils as transmitting and receiving coils. Participants lay on their back with their head on a foam pad, and they wore rubber earplugs to reduce noise. They were instructed to breathe calmly and not to think about anything specific or fall asleep. T1-weighted, T2-weighted or fluid attenuated inversion recovery MRI was performed, as required, in order to exclude any pathological condition. The MRI parameters were as follows: repetition time = 8.2 ms, echo time = 3.2 ms, turn angle = 12°, inversion time = 450 ms, matrix = 256 × 256, field-of-view = 256 mm × 256 mm, and slice thickness = 1 mm. In addition, RS-fMRI images were obtained using an echo planar imaging using the follow parameters: 36 slices, slice thickness: 3 mm, no gap, voxel size: 3.5 × 3.5 × 4 mm, volumes: 240, repetition time: 2000ms, Echo time: 30ms, turn angle: 90°, field-of-view: 224 mm, matrix: 64 × 64.
Image processing
Cortical thickness
The analysis of surface-based morphometry relied on FreeSurfer software version 6.0.0 (http://surfer.nmr.mgh.harvard.edu/). The main steps of the analysis were as follows. First, MRIcron software was used to convert the Digital Imaging and Communications in Medicine (DICOM) format of 3D-T1 images into the 4D-Neuroimaging Informatics Technology Initiative (NIfTI) format for subsequent processing and analysis. Then, using Linux Ubuntu, NIfTI data were imported into FreeSurfer 6.0.0 for automatic processing. This automatic processing included head movement correction, removal of structures external to the brain tissue (such as the skull), automatic conversion into Talairach space, automatic segmentation of subcortical structure, gray normalization, mosaic of gray matter boundary, automatic topological correction, surface deformation of brain tissue, surface expansion after the cortical images were generated, and transformation to a spherical distribution template. The cortical thickness, defined as the distance from the grey/white matter boundary to the corresponding pial surface, was calculated for each brain region after processing with a Gaussian smoothing nucleus (full width at half maximum = 10). Finally, all reconstructed data sets were visually inspected to ensure accuracy of registration, skull dissection, segmentation, and cortical surface reconstruction.
Resting-state functional connectivity
We used the CONN toolsbox for SPM version 17 (http://www.nitrc.org/projects/conn, RRID: SCR_009550) for the analysis of resting-state data. The first 10 images were discarded to eliminate T1 equilibrium artifacts in the time series, and preprocessed using the CONN preprocessing pipeline: (1) The remining images were corrected for slice timing using the middle slice as a reference, realigned to remove head motion. (2)The T1-weighted anatomical image was coregistered to the mean functional image using a rigid-body transformation, then the structural images were segmented into gray matter, WM, and CSF. (3) The data were normalized to the MNI space to obtain anatomical accuracy across participants. (4)Then, resampling of the normalized RS-fMRI data to 3 × 3 × 3-mm voxels and spatial smoothing with an isotropic 8-mm FWHM Gaussian kernel. (5)Linear detrending and temporal bandpass (0.01–0.08 Hz) filtering were then used to remove low-frequency drifts and physiological high-frequency noise. (6) Then, we did nuisance linear regression with the white matter, cerebrospinal fluid, and Friston-24 head motion parameters (including 6 head motion parameters and their historical effects as well as the 12 corresponding squared items) .
Statisticcal analysis
Analysis of clinical data
SPSS version 25.0 (IBM, Armonk, NY, USA) was used for analysis of clinical data. For normally distributed data, the mean ± standard deviation (SD) was reported. Differences across groups were assessed for significance using multivariate analysis of variance, post-hoc comparisons were further conducted with the Bonferroni correction. For skewed data, the median and 25–75% percentiles were reported, and differences were assessed for significance using the Mann-Whitney U test (two groups) or Kruskal-Wallis test (more than two groups). Frequencies were expressed as percentages, and differences were assessed using the chi-squared or Fisher tests. P < 0.05 was considered statistically significant. The multiple linear regression was used to analyze the factors associated with MMSE score.
Image data analysis
Statical Analysis of T1-image
The “mri_glmfit” routine in Freesurfer was used for cortical thickness analysis and correlation analysis. Vertex-by-vertex comparison was performed with a general linear model, where age and education were included as covariables. All results were corrected for multiple comparisons using a pre-cached cluster-wise Monte-Carlo simulation. We first set the statistical significance to a voxel level of p < 0.01. Then, multiple comparisons were corrected with cluster-based Monte-Carlo simulation with 10,000 permutations, and we searched for significant clusters with a cluster-level of p < 0.05.
Statical analysis of resting-state functional magnetic resonance image
According to the changes in the cortical thickness among the groups, we extracted the corresponding MNI coordinates as regions of interest (ROIs), and the radius was set to 10 mm. Then, the FC was analyzed between each ROI and whole brain voxels using the SPM12-based CONN toolbox. The mean blood oxygen level-dependent time series was computed across all voxels within each ROI. Bivariate correlation analyses were used to determine the linear association of the blood oxygen level-dependent time series between each pair of sources, and a Fisher Z transformation was applied. Individual seed-to-voxel maps were entered into a second-level analysis. A whole-brain height threshold of P < 0.001 (uncorrected) was used to identify areas showing significant changes in FC. A false discovery rate-corrected threshold of P < 0.05 at this height threshold was applied for all reported clusters.
Results
Demographic and clinical characteristics of study patients
15 healthy control subjects and 53 PD patients were recruited (25 PD-NC, 11 PD-MCI, and 17 PDD). Sex, H-Y grading, HAMA, HAMD, and RBD were similar among the PD groups ( Table 1,P > 0.05 ). PDD groups(69.3 ± 10.3) were older compared with healthy controls (43.4 ± 10.2), PD-NC (59.2 ± 10.8) and the PD-MCI (64.9 ± 10.0) (Table 1, P < 0.05 ). Compared to PD-NC and PD-MCI ,PDD have longer disease duration (respectively 2 (1.75, 3.6), 1 (1,5), 5 (2, 7)) and higher levodopa-equivalent daily dose (respectively 358.52 ± 182.72, 282.29 ± 132.3, 467.67 ± 181.46) (Table 1, P < 0.05). Besides, MMSE scores decreased in the trend controls > PD-NC > PD-MCI > PDD (respectively 28.87 ± 0.99, 26.92 ± 2.74, 21.91 ± 3.48, 15.41 ± 5.13), and differences between groups were significant (Table 1,P < 0.05). And UPDRSIII scores increased in PD-NC < PD-MCI < PDD (respectively 22 (18,32.5), 36 (15,47), 43 (28.5,51.5) (Table 1, P < 0.05 ). The results of our multiple linear regression analysis showed that age and education level had an impact on MMSE, but gender and LEDD did not (Supplementary Table 1).
Analysis of images
Healthy controls versus PD patients with cognitive impairment
Cortical thickness
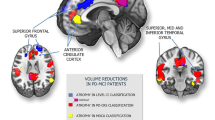
PD patients with cognitive impairment (PD-MCI and PDD) showed reduced cortical thickness in the left insula, left parstriangularis, right superior temporal, and right lingual compared with healthy controls (Table 2; Fig. 2 A).
Comparison of cortical thickness in different brain areas among group. (A) Cortical thickness was higher in healthy controls (HC) than in PD-CI patients. (B) Cortical thickness was higher in PD-NC than in PD-CI patients. (C) PD-NC patients showed greater cortical thickness than the PD-MCI group. (D) The PD-NC group showed greater cortical thickness than the PDD group
The red-blue color bar on the figure shows the logarithmic scale of the P value (-log10). Red is positive, blue is negative
Resting-state functional connectivity
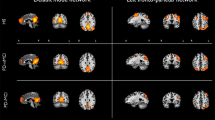
When we defined the left insula MNI coordinates as the ROI, PD patients with cognitive impairment showed markedly higher connectivity between the left insula and several brain areas (vermis 10, left cerebellum 9, vermis 9, and brain-stem) compared with the healthy control group (Table 3; Fig. 3 A).
Differences in functional connectivity among group
(A) When the left insula and right lingual regions were considered seed, the PD-CI group showed markedly higher connectivity than healthy controls. (B) When the right insula was considered seed, the PD-CI group presented markedly lower connectivity than PD-NC patients. (C) When the right lingual region was considered a seed, PD-MCI patients showed markedly higher connectivity than PD-NC patients. (D) When the left superior temporal, right insula, and right fusiform were considered seed, PDD patients showed markedly abnormal connectivity than PD-NC patients
More yellow or purple color indicates stronger functional connectivity
When the right lingual was defined as the ROI, PD patients with cognitive impairment presented higher connectivity between the right lingual and left superior parietal lobule compared with healthy controls. No regions showed decreased connectivity to the right lingual region (Table 3; Fig. 3 A). Other regions did not show significant FC differences between healthy controls and PD patients with cognitive impairment.
Comparison of PD patients with or without cognitive impairment
Resting-state functional connectivity
When the right insula MNI coordinates were defined as the ROI, patients with cognitive impairment showed decreased FC between right insula and the following brain regions: right superior parietal lobule, lateral occipital cortex (right superior division), supramarginal gyrus (right posterior division), right postcentral gyrus, supramarginal gyrus (right anterior division), and lateral occipital cortex (left superior division) (Table 3; Fig. 3B). No regions showed increased FC with the right insula. Other regions did not show significant FC differences between patients with or without cognitive impairment.
Resting-state functional connectivity
Compared to PD-NC patients, PD-MCI patients showed higher resting-state FC between the right lingual and the following brain regions: right cerebellum crus 2, left cerebellum crus 2, and vermis 7 (Table 3; Fig. 3 C). No regions of reduced FC to the right lingual region were detected in PD-MCI patients relative to PD-NC patients.
PD-NC versus PDD
Cortical thickness
Widespread cortical thinning was detected in PDD compared to PD-NC, including the left superior temporal, left fusiform, right insula, and right fusiform areas (fusiform1 and 2) (Table 2; Fig. 2D). Resting-state functional connectivity.
Resting-state functional connectivity
Based on the left superior temporal as ROI, the PDD group showed higher connectivity between the left superior temporal and several brain areas (left cerebellum crus 1 and 2) and decreased connectivity between the left superior temporal and right superior parietal lobule and right postcentral gyrus, compared with the PD-NC group (Table 3; Fig. 3D).
When the right insula was defined as the ROI, PDD patients showed decreased right insula FC to the cingulate gyrus (anterior division) and right paracingulate gyrus (Table 3; Fig. 3D). No regions showed increased connectivity to the right insula.
When the right fusiform 1 was defined as the ROI, the PDD group showed decreased right fusiform FC to the superior temporal gyrus (left anterior division), left planum temporale, left Heschl’s gyrus, left central opercular cortex, and left planum polare compared with the PD-NC group (Table 3; Fig. 3D). No regions showed increased connectivity to the right fusiform 1 region in the PDD group.
When the left fusiform was considered the ROI, the PD-NC and PDD groups showed similar FC.
Correlation analysis
MMSE scores of PD patients positively correlated with cortical thickness in the left superior temporal, left fusiform, and right temporal pole areas (Table 4; Fig. 4). H-Y score negatively correlated with cortical thickness in the left transverstemporal, left superior temporal, and right precuneus areas (Table 4; Fig. 5 A). The UPDRSIII score negatively correlated with cortical thickness in the left superior temporal, left insula, right superior temporal, and right fusiform areas (Table 4; Fig. 5B).
Correlation of cortical thickness in different brain areas with Hoehn and Yahr (H-Y) scale (A) and the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III) (B). The red-blue color bar on the figure shows the logarithmic scale of the P value (-log10). Red indicates positive correlation, blue indicates negative corralation; L, left; R, right
Discussion
In this work, we explored associations among cerebral cortical thickness, FC, and cognitive impairment in PD. By combining structural and functional connectivities, our findings suggest that alterations in cortical thickness and resting-state FC may contribute to cognitive decline in PD. In the present study, We used regions presenting cortical atrophy as ROIs for the first time, show that atrophy in cortical thickness may result in dysfunction of resting-state functional connectivity. In addition, we found increased connectivity between the cortical atrophic brain areas and the cerebellar regions in PD with cognitive impairment, which suggests that the cerebellum in particular may be involved in cognitive dysfunction in PD. Furthermore, we associated the decrease in cortical thickness with disease progression in PD patients.
Our data on cortical thickness in PD-associated cognitive impairment are consistent with previous studies showing widespread temporal, frontal, and occipital lobe cortical thinning (Chen et al., 2020; Hwang et al., 2013; Lee et al., 2014; Mak et al., 2015; Trufanov et al., 2013). We also found widespread cortical thinning, involving the superior temporal, lingual, fusiform, and insula regions, which points to impairment in multiple cognitive domains. The temporal lobe is closely related to memory and also affects executive function and visuospatial function through the frontotemporal and occipito-temporal loops (Gratwicke et al., 2015). Lingual and fusiform regions are posterior visual cortical components, and their atrophy correlates with visual perceptual dysfunction (Goldman et al., 2014). Insular lesions in human patients have been shown to impair performance on tasks requiring cognitive flexibility (Hodgson et al., 2007). Our results found that cortical thinning in the left superior temporal, left fusiform, and right temporal pole areas positively correlated with global cognitive decline. This is consistent with the regions known to be involved in higher-order cognitive functions. Previous work (Zarei et al., 2013) also found a positive correlation between MMSE scores and cortical thickness in the anterior temporal, dorsolateral prefrontal, posterior cingulate, fusiform, and occipitotemporal cortex. In addition, longitudinal studies (Ibarretxe-Bilbao et al., 2012; Williams-Gray et al., 2009) have linked gradual thinning in the temporal-occipital cortex to deterioration that can eventually lead to dementia. These considerations suggest that the decrease in cortical thickness may be a promising marker for predicting cognitive decline in PD (Zarei et al., 2013).
The underlying mechanisms behind the cortical thinning that we and others have observed remains unclear, and several hypotheses have been formulated to explain it. First, progression of PD is associated with progressive cortico-striatal circuitry dysfunction, which can be tracked by monitoring glucose metabolism in cortical regions using positron emission tomography (Braak et al., 2003). Thus, dysfunction of projections between cortico-striatal regions may lead to chronic “disuse” of the cortex, leading in turn to cortical thinning. Another hypothesis is that abnormal phosphorylation of α-synuclein and Tau occurs at cortical synapses in PD patients with cognitive dysfunction (González-Redondo et al., 2014), which contributes to cortical mitochondrial dysfunction, leading to neuronal death and, potentially, cortical atrophy (Ferrer, 2009). A third hypothesis is that cortical thinning arises not from neuronal death but from cellular shrinkage and reduction in dendritic arborization (Morrison & Hof, 1997). Indeed, in PD-MCI, the topographic distribution of cortical thinning is consistent with the regions where hypometabolism and hypoperfusion occur (Abe et al., 2003). This leads to reduced size of neuronal cell bodies, reduced dendritic arborization and/or loss of presynaptic terminals, which can result in cortical atrophy (Braak et al., 2005; Pellicano et al., 2012).
Although the atrophy changes in our patients were relatively limited, they affected cortical regions in areas important for information integration (Achard et al., 2006; Tononi et al., 1998). Analysis of normal cerebral cortical thickness revealed the existence of a group of posteromedial cortical areas that form dense interconnected brain networks (Hagmann et al., 2008). Executive control, default-mode, and salient networks are thought to be mainly related to cognitive function, and these networks depend on, and regulate each other, to ensure normal cognitive function (Pievani et al., 2011). Cortical atrophy of brain regions at important nodes leads to disruption of brain network connections and dysfunction, which gives rise to cognitive impairment in PD ((Tononi et al., 1998). This observation was confirmed in our resting-state FC analysis using atrophic brain regions as ROIs. Our results showed that PD patients with cognitive impairment showed weak FC in insula with executive control and default-mode networks. The PDD group showed weak FC in superior temporal with executive control and default-mode networks; weak FC in insula with the salient network; and weak FC in fusiform with default-mode and executive control networks. The insula is an important node of the salient and ventral attention networks. Moreover, dysfunction in the loops of midbrain ventral tegmental area and the insula contributes to executive dysfunction in PD (Oades & Halliday, 1987), and specific disruption of projections to the insular cortex contribute to worsening executive impairment and PDD. This may result because insula cannot effectively recruit other cognitive networks, such as the fronto-parietal network (Gratwicke et al., 2015). In this way, our study and literature establish strong correlations between atrophy of the insula cortex and progression to PDD. Similarly, our work supports the idea that atrophy of the temporal lobe, lingual, and fusiform regions disrupts the default-mode, executive control, and salient networks, thereby contributing to cognitive impairment in PD.
In our study, PD patients with cognitive dysfunction presented stronger FC between the cortical atrophic brain areas and the cerebellum than healthy controls or PD-NC patients. This implicates the cerebellum may be particularly involved in adaptive changes that occur in PD with cognitive dysfunction in response to local cortical atrophic change. The cerebellum is known to influence motor activity and cognition through the cerebello-thalamo-cortical circuit (Middleton & Strick, 2001). Our patients showed alterations in FC involving voxels related to executive control and default-mode networks, and these alterations likely affect executive ability, visuospatial function, memory, and attention (Li et al., 2018). To overcome these defects in executive ability and visuospatial function, PD patients may compensate by strengthening their abilities in certain functions (e.g. volition, planning, and purposive action) and in self-referential processing (e.g. recollection of past experiences). This may explain why our PD patients with cognitive impairment showed increased cerebellar FC with executive control and default-mode networks. Similarly, we previously found that PD patients with visual disorders showed stronger FC involving the cerebellar vermis X, visual networks and default-mode networks than patients without visuospatial disorders (Yin et al., 2021). Patients with visual disorders also showed increased FC within the intracerebellar network between the vermis X and left cerebellum 8, and left cerebellum 9 (Yin et al., 2021). The connectivity in another work showed stronger connectivity between the cerebellum and frontoparietal networks in PDD (Zhan et al., 2018). These observations strongly implicate the cerebellum in cognitive dysfunction in PD. Further work should examine whether PD patients strengthen FC between the cerebellum and other networks as a compensatory mechanism.
Our data indicate that the decrease in mean cortical thickness in superior temporal, precuneus, insula, and fusiform regions may be useful for assessing PD severity, confirming previous observations ((Burton et al., 2004; Mak et al., 2015; Zarei et al., 2013). PD symptoms and disease severity are typically assessed using the UPDRS score and H-Y staging ((Chen et al., 2016). UPDRS-III scores in PD patients negatively correlate with the thickness of the left and right fusiform gyrus and left temporal pole (Zarei et al., 2013), while H-Y stage negatively correlates with the thickness of the posterior cingulate cortex and the temporal cortex (Gao et al., 2018). The anatomical distribution of these cortical thinning is consistent with changes in cortical glucose metabolism and perfusion decreases observed in the early stage of the disease, which gradually extend to multiple cortical regions as the disease progresses (Borghammer et al., 2010; González-Redondo et al., 2014). These results further illustrate the feasibility of using MRI biomarkers to assess the pathophysiology of PD motor dysfunction.
Our study presents some limitations. First, despite the rigorous exclusion of MRI data affected by head motion and other preprocessing steps to reduce motion artifacts, such artifacts may still have influenced the results. Second, due to motor dysfunction and poor cooperation of PD patients, comprehensive cognitive assessment was not performed, and the scale used to evaluate cognitive function is relatively simple, so our study could not provide more detailed understanding of the characteristics and progression of cognitive dysfunction. Third, the sample was relatively small, and the cross-sectional design prevented us from determining causal relationships. Our findings should be verified and extended in large, longitudinal investigations.
Conclusion
Alterations in cortical thickness may disrupt resting-state FC and thereby contribute to cognitive decline in PD. However, it is more probable that the relation between structure and FC would be bidirectional,and needs more research to explore in PD cognitve decline. Our results may guide the discovery of neuroimaging markers and underlying mechanisms related to cognitive impairment in PD.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aarsland, D., Larsen, J. P., Tandberg, E., & Laake, K. (2000). Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. Journal Of The American Geriatrics Society, 48(8), 938–942. doi:https://doi.org/10.1111/j.1532-5415.2000.tb06891.x
Abe, Y., Kachi, T., Kato, T., Arahata, Y., Yamada, T., Washimi, Y., & Sobue, G. (2003). Occipital hypoperfusion in Parkinson’s disease without dementia: correlation to impaired cortical visual processing. Journal Of Neurology, Neurosurgery And Psychiatry, 74(4), 419–422. doi:https://doi.org/10.1136/jnnp.74.4.419
Achard, S., Salvador, R., Whitcher, B., Suckling, J., & Bullmore, E. (2006). A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. Journal Of Neuroscience, 26(1), 63–72. doi:https://doi.org/10.1523/jneurosci.3874-05.2006
Amboni, M., Tessitore, A., Esposito, F., Santangelo, G., Picillo, M., Vitale, C., & Barone, P. (2015). Resting-state functional connectivity associated with mild cognitive impairment in Parkinson’s disease. J Neurol, 262(2), 425–434. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25428532. doi:https://doi.org/10.1007/s00415-014-7591-5
Baggio, H. C., Segura, B., Sala-Llonch, R., Marti, M. J., Valldeoriola, F., Compta, Y., & Junque, C. (2015). Cognitive impairment and resting-state network connectivity in Parkinson’s disease. Hum Brain Mapp, 36(1), 199–212. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25164875. doi:https://doi.org/10.1002/hbm.22622
Borghammer, P., Chakravarty, M., Jonsdottir, K. Y., Sato, N., Matsuda, H., Ito, K., & Gjedde, A. (2010). Cortical hypometabolism and hypoperfusion in Parkinson’s disease is extensive: probably even at early disease stages. Brain Struct Funct, 214(4), 303–317. doi:https://doi.org/10.1007/s00429-010-0246-0
Braak, H., Del Tredici, K., Rüb, U., de Vos, R. A., Steur, J., E. N., & Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology Of Aging, 24(2), 197–211. doi:https://doi.org/10.1016/s0197-4580(02)00065-9
Braak, H., Rüb, U., Steur, J., Tredici, E. N. D., K., & de Vos, R. A. (2005). Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology, 64(8), 1404–1410. doi:https://doi.org/10.1212/01.Wnl.0000158422.41380.82
Broen, M. P., Moonen, A. J., Kuijf, M. L., Dujardin, K., Marsh, L., Richard, I. H., & Leentjens, A. F. (2015). Factor analysis of the Hamilton Depression Rating Scale in Parkinson’s disease. Parkinsonism & Related Disorders, 21(2), 142–146. doi:https://doi.org/10.1016/j.parkreldis.2014.11.016
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals Of The New York Academy Of Sciences, 1124, 1–38. doi:https://doi.org/10.1196/annals.1440.011
Burton, E. J., McKeith, I. G., Burn, D. J., Williams, E. D., & O’Brien, J. T. (2004). Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain, 127(Pt 4), 791–800. doi:https://doi.org/10.1093/brain/awh088
Bušková, J., Peřinová, P., Miletínová, E., Dušek, P., Růžička, E., Šonka, K., & Kemlink, D. (2019). Validation of the REM sleep behavior disorder screening questionnaire in the Czech population. Bmc Neurology, 19(1), 110. doi:https://doi.org/10.1186/s12883-019-1340-4
Chen, C. H., Lee, B. C., & Lin, C. H. (2020). Integrated Plasma and Neuroimaging Biomarkers Associated with Motor and Cognition Severity in Parkinson’s Disease. J Parkinsons Dis, 10(1), 77–88. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31868681. doi:https://doi.org/10.3233/JPD-191766
Chen, S., Chan, P., Sun, S., Chen, H., Zhang, B., Le, W., & Xiao, Q. (2016). The recommendations of Chinese Parkinson’s disease and movement disorder society consensus on therapeutic management of Parkinson’s disease. Transl Neurodegener, 5, 12. doi:https://doi.org/10.1186/s40035-016-0059-z
de Lau, L. M. L., & Breteler, M. M. B. (2006). Epidemiology of Parkinson’s disease. The Lancet Neurology, 5(6), 525–535. doi:https://doi.org/10.1016/s1474-4422(06)70471-9
Dong, W., Qiu, C., Jiang, X., Shen, B., Zhang, L., Liu, W., & Chen, J. (2020). Can the Executive Control Network be Used to Diagnose Parkinson’s Disease and as an Efficacy Indicator of Deep Brain Stimulation? Parkinsons Dis, 2020, 6348102. doi:https://doi.org/10.1155/2020/6348102
Emre, M., Aarsland, D., Brown, R., Burn, D. J., Duyckaerts, C., Mizuno, Y., & Dubois, B. (2007). Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement Disorders, 22(12), 1689–1707. doi:https://doi.org/10.1002/mds.21507. quiz 1837. Retrieved fromhttps://www.ncbi.nlm.nih.gov/pubmed/17542011
Ferrer, I. (2009). Early involvement of the cerebral cortex in Parkinson’s disease: convergence of multiple metabolic defects. Progress In Neurobiology, 88(2), 89–103. doi:https://doi.org/10.1016/j.pneurobio.2009.02.004
Gao, Y., Nie, K., Mei, M., Guo, M., Huang, Z., Wang, L., & Wang, L. (2018). Changes in Cortical Thickness in Patients With Early Parkinson’s Disease at Different Hoehn and Yahr Stages. Front Hum Neurosci, 12, 469. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30542273. doi:https://doi.org/10.3389/fnhum.2018.00469
Goetz, C. G. (2010). [Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): a new scale for the evaluation of Parkinson’s disease]. Rev Neurol (Paris), 166(1), 1–4. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19910010. doi:https://doi.org/10.1016/j.neurol.2009.09.001
Goetz, C. G., Poewe, W., Rascol, O., Sampaio, C., Stebbins, G. T., & Counsell, C. (2004)… . Movement Disorder Society Task Force on Rating Scales for Parkinson’s, D. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord, 19(9), 1020–1028. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15372591. doi:https://doi.org/10.1002/mds.20213
Goldman, J. G., Williams-Gray, C., Barker, R. A., Duda, J. E., & Galvin, J. E. (2014). The spectrum of cognitive impairment in Lewy body diseases. Movement Disorders, 29(5), 608–621. doi:https://doi.org/10.1002/mds.25866
González-Redondo, R., García-García, D., Clavero, P., Gasca-Salas, C., García-Eulate, R., Zubieta, J. L., & Rodríguez-Oroz, M. C. (2014). Grey matter hypometabolism and atrophy in Parkinson’s disease with cognitive impairment: a two-step process. Brain, 137(Pt 8), 2356–2367. doi:https://doi.org/10.1093/brain/awu159
Gratwicke, J., Jahanshahi, M., & Foltynie, T. (2015). Parkinson’s disease dementia: a neural networks perspective. Brain, 138(Pt 6), 1454–1476. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25888551. doi:https://doi.org/10.1093/brain/awv104
Hagmann, P., Cammoun, L., Gigandet, X., Meuli, R., Honey, C. J., Wedeen, V. J., & Sporns, O. (2008). Mapping the structural core of human cerebral cortex. Plos Biology, 6(7), e159. doi:https://doi.org/10.1371/journal.pbio.0060159
Hodgson, T., Chamberlain, M., Parris, B., James, M., Gutowski, N., Husain, M., & Kennard, C. (2007). The role of the ventrolateral frontal cortex in inhibitory oculomotor control. Brain, 130(Pt 6), 1525–1537. doi:https://doi.org/10.1093/brain/awm064
Hwang, K. S., Beyer, M. K., Green, A. E., Chung, C., Thompson, P. M., Janvin, C., & Apostolova, L. G. (2013). Mapping cortical atrophy in Parkinson’s disease patients with dementia. J Parkinsons Dis, 3(1), 69–76. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23938313. doi:https://doi.org/10.3233/JPD-120151
Ibarretxe-Bilbao, N., Junque, C., Marti, M. J., & Tolosa, E. (2011). Brain structural MRI correlates of cognitive dysfunctions in Parkinson’s disease. Journal Of The Neurological Sciences, 310(1–2), 70–74. doi:https://doi.org/10.1016/j.jns.2011.07.054
Ibarretxe-Bilbao, N., Junque, C., Segura, B., Baggio, H. C., Marti, M. J., Valldeoriola, F., & Tolosa, E. (2012). Progression of cortical thinning in early Parkinson’s disease. Movement Disorders, 27(14), 1746–1753. doi:https://doi.org/10.1002/mds.25240
Ibarretxe-Bilbao, N., Tolosa, E., Junque, C., & Marti, M. J. (2009). MRI and cognitive impairment in Parkinson’s disease. Movement Disorders, 24(Suppl 2), S748–753. doi:https://doi.org/10.1002/mds.22670
Kummer, A., Cardoso, F., & Teixeira, A. L. (2010). Generalized anxiety disorder and the Hamilton Anxiety Rating Scale in Parkinson’s disease. Arquivos De Neuro-Psiquiatria, 68(4), 495–501. doi:https://doi.org/10.1590/s0004-282x2010000400005
Laird, A. R., Fox, P. M., Eickhoff, S. B., Turner, J. A., Ray, K. L., McKay, D. R., & Fox, P. T. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal Of Cognitive Neuroscience, 23(12), 4022–4037. doi:https://doi.org/10.1162/jocn_a_00077
Lee, J. E., Cho, K. H., Song, S. K., Kim, H. J., Lee, H. S., Sohn, Y. H., & Lee, P. H. (2014). Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiatry, 85(1), 7–16. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23828835. doi:https://doi.org/10.1136/jnnp-2013-305062
Levy, G., Tang, M. X., Louis, E. D., Côté, L. J., Alfaro, B., Mejia, H., & Marder, K. (2002). The association of incident dementia with mortality in PD. Neurology, 59(11), 1708–1713. doi:https://doi.org/10.1212/01.wnl.0000036610.36834.e0
Wang, L. A., Pang, A. L., Zhang, L. M., & Ren, H. (2014). Application of Montreal Cognitive Assessment Scale and Mini-Mental State Scale in the screening of mild cognitive impairment in Parkinson’s disease. International Journal of Neurology and Neurosurgery, 41(01), 16–19. doi:https://doi.org/10.16636/j.cnki.jinn.2014.01.023
Li, K., Su, W., Li, S. H., Jin, Y., & Chen, H. B. (2018). Resting State fMRI: A Valuable Tool for Studying Cognitive Dysfunction in PD. Parkinsons Dis, 2018, 6278649. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29850015. doi:https://doi.org/10.1155/2018/6278649
Litvan, I., Goldman, J. G., Troster, A. I., Schmand, B. A., Weintraub, D., Petersen, R. C., & Emre, M. (2012). Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord, 27(3), 349–356. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22275317. doi:https://doi.org/10.1002/mds.24893
Mak, E., Su, L., Williams, G. B., Firbank, M. J., Lawson, R. A., Yarnall, A. J., & O’Brien, J. T. (2015). Baseline and longitudinal grey matter changes in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain, 138(Pt 10), 2974–2986. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26173861. doi:https://doi.org/10.1093/brain/awv211
Middleton, F. A., & Strick, P. L. (2001). Cerebellar projections to the prefrontal cortex of the primate. Journal Of Neuroscience, 21(2), 700–712. doi:https://doi.org/10.1523/jneurosci.21-02-00700.2001
Morrison, J. H., & Hof, P. R. (1997). Life and death of neurons in the aging brain. Science, 278(5337), 412–419. doi:https://doi.org/10.1126/science.278.5337.412
Oades, R. D., & Halliday, G. M. (1987). Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Research, 434(2), 117–165. doi:https://doi.org/10.1016/0165-0173(87)90011-7
Pagonabarraga, J., Corcuera-Solano, I., Vives-Gilabert, Y., Llebaria, G., Garcia-Sanchez, C., Pascual-Sedano, B., & Gomez-Anson, B. (2013). Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson’s disease. PLoS One, 8(1), e54980. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23359616. doi:https://doi.org/10.1371/journal.pone.0054980
Pellicano, C., Assogna, F., Piras, F., Caltagirone, C., Pontieri, F. E., & Spalletta, G. (2012). Regional cortical thickness and cognitive functions in non-demented Parkinson’s disease patients: a pilot study. Eur J Neurol, 19(1), 172–175. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21771199. doi:https://doi.org/10.1111/j.1468-1331.2011.03465.x
Pereira, J. B., Ibarretxe-Bilbao, N., Marti, M. J., Compta, Y., Junqué, C., Bargallo, N., & Tolosa, E. (2012). Assessment of cortical degeneration in patients with Parkinson’s disease by voxel-based morphometry, cortical folding, and cortical thickness. Human Brain Mapping, 33(11), 2521–2534. doi:https://doi.org/10.1002/hbm.21378
Pereira, J. B., Svenningsson, P., Weintraub, D., Bronnick, K., Lebedev, A., Westman, E., & Aarsland, D. (2014). Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology, 82(22), 2017–2025. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24808018. doi:https://doi.org/10.1212/WNL.0000000000000483
Pievani, M., de Haan, W., Wu, T., Seeley, W. W., & Frisoni, G. B. (2011). Functional network disruption in the degenerative dementias. Lancet Neurology, 10(9), 829–843. doi:https://doi.org/10.1016/s1474-4422(11)70158-2
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., & Deuschl, G. (2015). MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord, 30(12), 1591–1601. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26474316. doi:https://doi.org/10.1002/mds.26424
Rektorova, I., Krajcovicova, L., Marecek, R., & Mikl, M. (2012). Default mode network and extrastriate visual resting state network in patients with Parkinson’s disease dementia. Neurodegener Dis, 10(1–4), 232–237. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22269223. doi:https://doi.org/10.1159/000334765
Schindlbeck, K. A., & Eidelberg, D. (2018). Network imaging biomarkers: insights and clinical applications in Parkinson’s disease. The Lancet Neurology, 17(7), 629–640. doi:https://doi.org/10.1016/s1474-4422(18)30169-8
Suzuki, W. A., & Amaral, D. G. (1994). Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. Journal Of Neuroscience, 14(3 Pt 2), 1856–1877. doi:https://doi.org/10.1523/jneurosci.14-03-01856.1994
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., & Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Movement Disorders, 25(15), 2649–2653. doi:https://doi.org/10.1002/mds.23429
Tononi, G., Edelman, G. M., & Sporns, O. (1998). Complexity and coherency: integrating information in the brain. Trends In Cognitive Sciences, 2(12), 474–484. doi:https://doi.org/10.1016/s1364-6613(98)01259-5
Trufanov, A. G., Odinak, M. M., Litvinenko, I. V., Rezvantsev, M. V., & Voronkov, L. V. (2013). [Early diagnosis of dementia with the help of MR-morphometry in patients with Parkinson’s disease]. Voenno-Meditsinskii Zhurnal, 334(9), 29–34
van den Heuvel, M. P., & Hulshoff Pol, H. E. (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity. European Neuropsychopharmacology, 20(8), 519–534. doi:https://doi.org/10.1016/j.euroneuro.2010.03.008
Williams-Gray, C. H., Evans, J. R., Goris, A., Foltynie, T., Ban, M., Robbins, T. W., & Barker, R. A. (2009). The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain, 132(Pt 11), 2958–2969. doi:https://doi.org/10.1093/brain/awp245
Wolters, A. F., van de Weijer, S. C. F., Leentjens, A. F. G., Duits, A. A., Jacobs, H. I. L., & Kuijf, M. L. (2019). Resting-state fMRI in Parkinson’s disease patients with cognitive impairment: A meta-analysis. Parkinsonism Relat Disord, 62, 16–27. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30580907. doi:https://doi.org/10.1016/j.parkreldis.2018.12.016
Wu, T., & Hallett, M. (2013). The cerebellum in Parkinson’s disease. Brain, 136(Pt 3), 696–709. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23404337. doi:https://doi.org/10.1093/brain/aws360
Yin, K., Zhou, C., Yin, L., Zhu, Y., Yin, W., Lu, Y., & Yang, X. (2021). Resting-state functional magnetic resonance imaging of the cerebellar vermis in patients with Parkinson’s disease and visuospatial disorder. Neurosci Lett, 760, 136082. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34171405. doi:https://doi.org/10.1016/j.neulet.2021.136082
Zarei, M., Ibarretxe-Bilbao, N., Compta, Y., Hough, M., Junque, C., Bargallo, N., & Marti, M. J. (2013). Cortical thinning is associated with disease stages and dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry, 84(8), 875–881. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23463873. doi:https://doi.org/10.1136/jnnp-2012-304126
Zhan, Z. W., Lin, L. Z., Yu, E. H., Xin, J. W., Lin, L., Lin, H. L., & Pan, X. D. (2018). Abnormal resting-state functional connectivity in posterior cingulate cortex of Parkinson’s disease with mild cognitive impairment and dementia. CNS Neurosci Ther, 24(10), 897–905. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29500931. doi:https://doi.org/10.1111/cns.12838
Acknowledgements
We thank the patients with Parkinson’s disease and the healthy control group for their participation in our study.
Funding
This work was supported by the Yunnan Province Clinical Research Center for Neurological Diseases (Xinglong Yang, 202002AA100204), Clinical Research Center for Geriatric Diseases of Yunnan Province - diagnosis and treatment of geriatric comorbidity and clinical translational research (Xinglong Yang, 202102AA310069), National Natural Science Foundation of China (Xinglong Yang, 81960242), Chengdu Medical College - Chengdu Seventh People’s Hospital Joint Scientific Research Fund (Baiyuan Yang, 2020LHJYZD-02), Yunnan Applied Basic Research Project (Xinglong Yang, 2019FE001-048, 202001AT070001), Special clinical research project for young and middle-aged Doctors in Lingnan Neurology Department (Xinglong Yang,).修改为:This work was supported by National Natural Science Foundation of China (Xinglong Yang, 81960242), Chengdu Medical College - Chengdu Seventh People’s Hospital Joint Scientific Research Fund (Baiyuan Yang, 2020LHJYZD-02), Yunnan Applied Basic Research Project (Xinglong Yang, 2019FE001-048, 202001AT070001), Special clinical research project for young and middle-aged Doctors in Lingnan Neurology Department (Xinglong Yang) and the Major Science and Technology Special Project of Yunnan Province (Xinglong Yang, 202102AA100061, 202103AA100069).
Author information
Authors and Affiliations
Contributions
Author contributions included conception and study design (Yongyun Zhu, Baiyuan Yang, Chuanbin Zhou, Ailan Pang and Xinglong Yang), data collection or acquisition (Chao Gao, Yanfei Hu, Wei Fang Yin, Kangfu Yin, Yangfan Zhu, Guoliang Jiang and Hui Ren), statistical analysis (Yongyun Zhu, Baiyuan Yang and Chuanbin Zhou), interpretation of results (Yongyun Zhu, Baiyuan Yang, Chuanbin Zhou, Ailan Pang and Xinglong Yang), drafting the manuscript work or revising it critically for important intellectual content (Yongyun Zhu, Baiyuan Yang, Chuanbin Zhou, Ailan Pang and Xinglong Yang) and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (All authors).
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University.
Consent to participate
All participants provided written informed consent for their anonymized clinical data to be analyzed and published for research purposes.
Consent to publish
If the manuscript is accepted, we agree to publish it.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Y., Yang, B., Zhou, C. et al. Cortical atrophy is associated with cognitive impairment in Parkinson’s disease: a combined analysis of cortical thickness and functional connectivity. Brain Imaging and Behavior 16, 2586–2600 (2022). https://doi.org/10.1007/s11682-022-00714-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-022-00714-w