Abstract
We hypothesize that decreased temporal variability of emotional network connectivities, corresponding to a continual state of hyperactivity, may play a role in mediating symptoms in schizophrenia. Resting-state magnetic resonance data were collected from 64 subjects, including 21 positive symptom profile schizophrenia patients (PSZ group), 19 negative symptom profile schizophrenia patients (NSZ group), and 24 healthy controls. The emotional brain network was defined based on the coordinates obtained from multi-level kernel density analysis. The temporal variability of intra-network functional connectivities (FCs) was calculated by constructing networks from blood oxygen level-dependent signals at successive, non-overlapping time windows, and was compared between groups. The results showed that the mean FC-variability of the whole emotional network (P = 0.021), and the FC-variabilities in the bilateral anterior insula (both, P < 0.001) were significantly decreased in the PSZ group compared with the control and NSZ groups. Abnormally enhanced negative coupling between variability and FC strength (V-S coupling) was observed in the PSZ group (P = 0.027). In summary, this study found a relation between the positive symptoms of schizophrenia and decreased variability of emotional network connectivities. These findings may help us better understand the neurobiological effect of the time-varying properties of the brain network in schizophrenia patients, and the underlying relation to the generation of psychosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysfunction of emotional processing is regarded as a core mechanism of schizophrenia (SZ) (Vai et al. 2015). It includes deficits in emotional expression (Blanchard and Cohen 2006), increased sensitivity for negative affect (Horan et al. 2008), dysfunction of emotional recognition (Horan et al. 2008), and social cognition and psychosocial functioning disability (Hooker and Park 2002). There is evidence that suggests disability of emotional function is closely related to the clinical phenotype of schizophrenia (Kohler et al. 2000; Vai et al. 2015). However, the underlying neurobiological process of emotional dysfunction in schizophrenia, and its role in mediating psychotic symptoms, is still under investigation.
A series of neuroimaging studies have investigated brain abnormalities in patients with schizophrenia using functional connectivity probes (Li et al. 2017; Zhang et al. 2015a, b), and have focused on the association between clinical symptoms and emotion-related brain activities. However, the results of the studies have been inconsistent (Barkhof et al. 2015; Hall et al. 2004; Kohler et al. 2010; Weniger et al. 2004). Some studies support a relation between emotional processing and the severity of negative symptoms (Barkhof et al. 2015; Kohler et al. 2010), while others have found a relation to positive symptoms (Hall et al. 2004; Weniger et al. 2004). Similarly, studies based on connectivity analyses of brain regions of the emotional network, or connectivities with other functional regions, have not produced consistent results (Du et al. 2017; Salvador et al. 2010; Zhang et al. 2015a, b).
In recent years, there has been a large interest in the time-varying properties of brain connectivity. Specifically, it has been demonstrated by resting-state functional magnetic resonance imaging (rs-fMRI) that brain functional connectivity (FC) patterns can be time-varying across a short time-window (e.g., 30–60 s), a phenomenon referred to as dynamic FC (Calhoun et al. 2014; Chen et al. 2019; Hutchison et al. 2013; Zalesky et al. 2014). In contrast, traditional resting-state FC (RSFC), obtained by averaging brain activity over several minutes (used in most previous studies of schizophrenia) may only capture the “static” features of brain connectivity, and therefore do not address the time-varying nature of brain activity and connectivity (Calhoun et al. 2014). Thus, observation of dynamic FC may be helpful uncover non-stationary connectivity abnormalities (Deng et al. 2019; Du et al. 2017; Zhang et al. 2016).
For decades, the predominant hypothesis of the neurobiological basis for the generation of psychotic symptoms in patients with schizophrenia has been dysfunction of the dopamine system. Previous studies have indicated that the dopamine system is driven into an overly responsive state due to dysfunction of inhibitory interneurons, which leads to neural hyperactivity in the brain (Grace 2012; Joo et al. 2018). In this situation, constant neural hyperactivity in schizophrenia may be consistent with decreased temporal variability of dynamic FCs (Liao et al. 2015).
Therefore, we hypothesized that decreased temporal variability of emotional network connectivities, corresponding to a continual state of neural hyperactivity, is possibly related to symptoms of schizophrenia. Furthermore, this variability change may underlie a relation between the generation of symptoms and inhibitory dysfunction of the emotional network in patients with schizophrenia.
Methods
Subjects
All procedures involving human participants were performed in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Institutional Ethics Committee. Each subject was verbally informed of the purpose of the study, and provided written informed consent. Diagnosis of schizophrenia was based on a structured clinical interview for DSM-IV Axis I Disorders (SCID) by 2 experienced clinical psychiatrists. Detailed inclusion and exclusion criteria were described in Supplementary Appendix 1.In addition, the Positive and Negative Syndrome Scale (PANSS) (Kay et al. 1987) was administered to each patient.
Negative symptom profile schizophrenia (NSZ) was defined as a moderate rating on more than 3 items on the negative syndrome scale, and a rating of moderate on less than 3 items on the positive syndrome scale. Positive symptom profile schizophrenia (PSZ) was defined as a moderate rating on more than 3 items on the positive syndrome subscale, and a rating of moderate on less than 3 items on the negative syndrome subscale. After excluding subjects who moved excessively during the MR scan, 64 subjects were included in the study: 21 in the PSZ group (13 females, 8 males; mean age, 26 years), 19 in the NSZ group (12 females, 7 males; mean age, 27 years), and 24 healthy controls (CON group: 10 females, 14 males; mean age, 27 years). Of the 40 schizophrenia patients, 19 were recruited from the outpatient department, and 21 were recruited while they were inpatients. The 24 healthy control subjects were recruited through advertisements. Demographic and clinical data of the subjects are summarized in Table 1.
Image acquisition and preprocessing
All participants underwent MRI with a 3 T GE Signa HDXT scanner. Detailed parameters for scanning and preprocessing were described in Supplementary Appendix 2. Notably, due to the uncertainty of the biological significance, the mean global signal was not regressed during preprocessing (Scholvinck et al. 2010; Wong et al. 2013).
Construction of the emotional network
The brain regions of the emotional network were defined based on the coordinates from a prior study that used multi-level kernel density analysis (MKDA) (Adolfi et al. 2017). A spherical region of interest (ROI) with a 5 mm radius centered at each peak coordinate was created as a node of the emotional network. A total of 49 ROIs were created, and used as seeds to calculate the inter-regional FCs (Fig. 1a, Supplementary Table 1). The FC was calculated as the Pearson’s correlation coefficient between the blood oxygen level-dependent (BOLD) time course of each pair of emotional ROIs.
Flowchart of the calculation of temporal variability of functional connectivities (FCs) of the emotional network. a Regions of interest (ROIs) of the emotional network with a total number of m. b The signal of each ROI was segmented into n successive, non-overlapping time windows. c Graph of the emotional network constructed based on the inter-ROI FC at each time window. The column in the red box represents the connectivity profile of ROI k at each time window
Calculation of the FC-variability, FC strength, and variability-strength coupling
The calculation of temporal variability of FCs followed an approach previously described by (Zhang et al. 2016), as shown in Fig. 1. To further clarify if the FC-variability change was accompanied with increased or decreased connectivity, we calculated and compared the FC strength (FCS) corresponding to the variability measurement(s) with between-group variability differences. Here, both the FC-variability and FCS were previously established metric with the detailed method description given in Supplementary Appendix 3. Furthermore, for each subject, the coupling between variability and strength of the FCs (V-S coupling) was calculated as the correlation coefficient between FCS and temporal variability of FCs across all the ROIs. Fisher-z-transformation was then performed on the resultant r values to increase the data normality for subsequent statistical comparisons (Liu et al. 2018).
Statistical analysis
Differences in age, temporal variability of FCs, FCS, and V-S coupling between the 3 groups (PSZ, NSZ, CON) were evaluated using 1-way ANOVA, or Kruskal-Wallis H test for non-normally distributed data. Repeated measures ANOVA was used for assessing group-level differences in the ROI-wise measurements, in addition to the ANOVA analyses on the averaged values. Differences based on sex were examined using the χ2 test. Comparisons of disease duration, antipsychotic drug dosage (counted as chlorpromazine equivalents), and PANSS scores between the 2 schizophrenia groups were performed using the 2-sample t-test, or Mann-Whitney U test for non-normally distributed data. A statistical significance level was set at P < 0.05, and Bonferroni correction was used for ROI-level statistical comparisons (i.e., P < 0.05/49 = 0.001). For network measurements with significant between-group differences, we analyzed their correlation to the clinical symptoms of the schizophrenia patients (including the scores of the PANSS, and PANSS subscales). Partial correlation analysis was performed by including age, sex, antipsychotic drug dosage, and mean frame-wise displacement (FD) as covariates. A statistical significance level of P < 0.05 was adopted, with Bonferroni correction for the times of correlation analyses.
Validation analysis
First, to test the possible influence of antipsychotic medications on the analysis results, we performed correlation analyses between antipsychotic drug dosages using chlorpromazine equivalents and the imaging measurements that exhibited significant between group differences. Second, considering that the effect of head motion on FC has been emphasized in recent studies (Power et al. 2014; Zeng et al. 2014), we compared the mean FD between groups, and assessed its correlations to all the network measurements that exhibited significant between-group differences. Third, statistical comparisons of all the network measurements were performed after adjusting for the effects of age, sex, and mean FD to ensure that the observed results were independent of these confounding factors.
Results
Participant demographic and clinical data
Age (P = 0.548) and sex (P = 0.268) were similar among the 3 groups. There was no significant difference of disease duration (P = 0.335) and antipsychotic drug dosage (P = 0.700) between the PSZ and NSZ groups. The positive and negative PANSS scores were higher, respectively, in PSZ and NSZ groups (both, P < 0.001). No significant differences were found in the total score and general PANSS scores between the 2 groups. A summary of the group comparisons is presented in Table 1.
Temporal variability of emotional network Connectivities
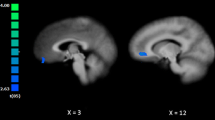
Group comparisons and post hoc analyses showed that the mean FC-variability of the emotional network in the PSZ group was significantly decreased (F = 4.139, P = 0.021) compared to that of the NSZ and CON groups, while no significant difference was found between the NSZ and CON group (Table 2, Fig. 2a). Similarly, repeated measures showed significant between-group differences in FC-variability across all ROIs (F = 4.139, P = 0.021, Fig. 2d). Moreover, significant interaction effect between the two factors of ROI and group was found (F = 1.720, P = 0.012), suggesting that the between-group difference of FC-variability was heterogeneous among different ROIs. With respect to regional comparisons, the FC-variabilities of the bilateral anterior insula in the PSZ group were significantly decreased compared with those of the NSZ and CON groups (both, P < 0.001). No significant differences were found between the NSZ and CON groups in these 2 regions, as determined by post hoc analyses (Table 2, Fig. 2b, c).
Variability measurements with significant differences between positive symptom profile schizophrenia patients (PSZ), negative symptom profile schizophrenia patients (NSZ), and healthy controls (CON). Pair-wise comparisons with significant differences in post hoc analyses are indicated with an asterisk “*”
To further clarify if the observed variability decrease was accompanied with increased or decreased connectivity, we compared the whole-network FCS and the regional FCS in the bilateral anterior insula between the 3 groups. Compared with the NSZ and CON groups, a tendency of higher FCS in the right anterior insula was noted in the PSZ group (χ2 = 7.377, P = 0.025). However, significance was not present after adjustment for confounding factors. Comparisons of the whole-network FCS and the regional FCS in the left insula found no significant differences in any of the 3 groups (Table 2).
Variability-strength coupling of FCs
The CON (r = −0.507, P < 0.001), PSZ (r = −0.790, P < 0.001), and NSZ (r = −0.708, P < 0.001) groups exhibited significant negative V-S coupling across the group-averaged ROI values of the emotional network (Fig. 3a–c). Comparison of individual level V-S coupling between the 3 groups showed significantly enhanced negative coupling in the PSZ group compared with the CON group (P = 0.027). Post hoc analyses showed no significant difference between the NSZ and CON group, or between the NSZ and PSZ group (Table 2, Fig. 3d).
Negative associations between strength and variability of functional connectivities (FCs) across all the regions of interest (ROIs) of the emotional network in healthy controls (CON group, a, positive symptom profile schizophrenia patients (PSZ group, b, negative symptom profile schizophrenia patients (NSZ group, c, and the between-group comparisons of variability-strength coupling (V-S coupling, d. To show the group-level associations between strength and variability of FCs, each dot in 3A-C represents the average functional connectivity strength of the corresponding group for a single ROI, from all 49 ROIs. Pair-wise comparisons of V-S coupling with significant differences in post hoc analyses are indicated with an asterisk “*” in 3D
Correlation analyses
In the PSZ and NSZ groups, the correlations of clinical symptoms to imaging measurements with group-level significance were examined. No significant correlation was found between any network measurement of interest and the PANSS and PANSS subscale scores in the PSZ or the NSZ group.
Validation analysis
No significant correlations of antipsychotic medication dosage in chlorpromazine equivalents and FD to any imaging parameters which exhibited significant between-group differences were identified in the PSZ or NSZ group (all, P > 0.1). In addition, the between-group FD difference was not significant (χ2 = 1.719, P = 0.423). After adjustment for age, sex, and mean FD, the significant between-group differences in whole network FC-variability of the emotional network, FC-variabilities in the bilateral insula, and V-S coupling all remained (Table 2).
Discussion
The results of this study provide evidence that positive symptoms in schizophrenia are related to decreased temporal variability of emotional network connectivities. This finding is consistent with prior studies that have shown that the emotional network in schizophrenia is characterized by increased activity and connectivity (Du et al. 2017; Salvador et al. 2010; Vai et al. 2015). This is because the brain component maintaining a continual, high level of activity or information transmission is expected to demonstrate low temporal variability, i.e., a negative association between FC-variability and BOLD activity and degree of FC (Liao et al. 2015; Zhang et al. 2016). This notion is also supported by our finding that a tendency of FCS increase was accompanied with variability decrease in the right insula, as well as the finding of enhanced negative V-S coupling in PSZ group. Jointly, these findings may indicate that a continual state of neural hyperactivity of the emotional brain network (especially the right insular region) relates to the positive symptoms in schizophrenia. It is worth noting that neither the whole-network FCS nor the FCSs of insular regions showed significance in PSZ patients. This result supports the finding of a prior study that dynamic FC measurement can be more sensitive for detecting the brain abnormalities in psychiatric disorders compared with static FC measurement (Jin et al. 2017).
The relations between insula abnormalities and behavioral dysregulation in terms of emotional dysfunction and psychotic symptoms are well-documented (Lee et al. 2016; Pang et al. 2017). The insula and its associated neural circuits are considered to be involved in the emotional response, and mediating impulsivity in schizophrenia (Blair 2016; Gu et al. 2013); thus, are closely related to the generation of excitement symptoms in schizophrenia (Joo et al. 2018). A prior dynamic FC study demonstrated that high temporal FC-variability of a brain region is compatible with receiving transmodule information from diverse functional communities of a network, and thus is reflective of flexibility in multimodule information interaction, as well as the ability to integrate more complex cognitive activities (Zhang et al. 2016). From this perspective, the observed FC-variability decrease in the anterior insula in schizophrenia patients in this study may imply decreased flexibility of the insula in interacting with other components of the emotional network. Taken together, these findings may suggest that change of FC-variability in the anterior insula in schizophrenia may be reflective of an altered intra-network interaction pattern of this region in the emotional network, and perhaps play a role in mediating positive symptoms of schizophrenia.
An interesting finding of this study is enhanced negative coupling between temporal variability and connectivity strength of the emotional network in PSZ patients. As previously discussed, to maintain high efficiency of information transmission some brain regions need to have greater BOLD activity most of the time to allow for continual high-level functional interactions with other regions. Continual high-level functional interaction is compatible with a high FCS. In contrast, the temporal variability of these regions is expected to be low, since these regions maintain a high level of information transmission over time. This type of negative coupling is considered a balanced organization pattern of a healthy brain, which helps promote stability of network architecture (Liao et al. 2015; Zhang et al. 2016). In our study, enhanced negative V-S coupling of the emotional network was noted in PSZ patients. This enhancement in PSZ patients might be interpreted as a tendency of the emotional network to reconfigure itself, or an adaptive response that increases the stability of the brain network in PSZ patients, in order to protect against more severe psychosis. In contrast, enhancement of V-S coupling was not significant in NSZ patients, and this loss of compensation in NSZ patients is perhaps in line with the fact that negative symptoms in patients with schizophrenia are related to weaker functional ability and poorer prognosis (Milev et al. 2005), compared with positive symptoms.
Several limitations of this study should be noted. First, the sample size was relatively small which may limit the statistical power and consequently lead to insignificant findings of the correlation analyses. Second, although the results of validation testing do not support direct relations between network measurements and antipsychotic medication usage, the influence of medications cannot be completely ruled out. Thus, future investigations with medication naïve subjects are still necessary. Third, we only evaluated symptom severity with the PANSS. Behavioral tests relating to emotional status (e.g., the Positive and Negative Affect Scale) may be better suited for elucidating the role of the brain emotional network in schizophrenia. Finally, this study was the first to propose V-S coupling as a measurement for evaluation; however, its biological significance remains to be investigated. Thus, the finding regarding V-S coupling should be interpreted with caution.
Conclusion
The results of this study provide evidence that the positive symptoms of schizophrenia are related to decreased FC-variability of the emotional network. This variability decrease was noted to be accompanied with an abnormally enhanced negative coupling between variability and FC strength of the emotional network in the patients. These findings may help us better understand the neurobiological effects of the time-varying properties of the brain network, and the relation to the genesis of psychosis in schizophrenia patients.
References
Adolfi, F., Couto, B., Richter, F., Decety, J., Lopez, J., Sigman, M., et al. (2017). Convergence of interoception, emotion, and social cognition: a twofold fMRI meta-analysis and lesion approach. Cortex, 88124–88142.
Barkhof, E., de Sonneville, L. M., Meijer, C. J., & de Haan, L. (2015). Processing of facial and nonsocial information is differentially associated with severity of symptoms in patients with multiepisode schizophrenia. The Journal of Nervous and Mental Disease, 203(2), 112–119.
Blair, R. J. (2016). The neurobiology of impulsive aggression. Journal of Child and Adolescent Psychopharmacology, 26(1), 4–9.
Blanchard, J. J., & Cohen, A. S. (2006). The structure of negative symptoms within schizophrenia: implications for assessment. Schizophrenia Bulletin, 32(2), 238–245.
Calhoun, V. D., Miller, R., Pearlson, G., & Adali, T. (2014). The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron, 84(2), 262–274.
Chen, H., Liu, K., Zhang, B., Zhang, J., Xue, X., Lin, Y., et al. (2019). More optimal but less regulated dorsal and ventral visual networks in patients with major depressive disorder. Journal of Psychiatric Research, 110, 172–178.
Deng, Y., Liu, K., Cheng, D., Zhang, J., Chen, H., Chen, B., et al. (2019). Ventral and dorsal visual pathways exhibit abnormalities of static and dynamic connectivities, respectively, in patients with schizophrenia. Schizophrenia Research, 206103–206110.
Du, Y., Pearlson, G. D., Lin, D., Sui, J., Chen, J., Salman, M., et al. (2017). Identifying dynamic functional connectivity biomarkers using GIG-ICA: application to schizophrenia, schizoaffective disorder, and psychotic bipolar disorder. Human Brain Mapping, 38(5), 2683–2708.
Grace, A. A. (2012). Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology, 62(3), 1342–1348.
Gu, X., Hof, P. R., Friston, K. J., & Fan, J. (2013). Anterior insular cortex and emotional awareness. The Journal of Comparative Neurology, 521(15), 3371–3388.
Hall, J., Harris, J. M., Sprengelmeyer, R., Sprengelmeyer, A., Young, A. W., Santos, I. M., et al. (2004). Social cognition and face processing in schizophrenia. The British Journal of Psychiatry, 185169–185170.
Hooker, C., & Park, S. (2002). Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Research, 112(1), 41–50.
Horan, W. P., Blanchard, J. J., Clark, L. A., & Green, M. F. (2008). Affective traits in schizophrenia and schizotypy. Schizophrenia Bulletin, 34(5), 856–874.
Hutchison, R. M., Womelsdorf, T., Gati, J. S., Everling, S., & Menon, R. S. (2013). Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Human Brain Mapping, 34(9), 2154–2177.
Jin, C., Jia, H., Lanka, P., Rangaprakash, D., Li, L., Liu, T., Hu, X., & Deshpande, G. (2017). Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Human Brain Mapping, 38(9), 4479–4496.
Joo, Y. H., Kim, J. H., Son, Y. D., Kim, H. K., Shin, Y. J., Lee, S. Y., & Kim, J. H. (2018). The relationship between excitement symptom severity and extrastriatal dopamine D2/3 receptor availability in patients with schizophrenia: a high-resolution PET study with [(18)F]fallypride. European Archives of Psychiatry and Clinical Neuroscience, 268(6), 529–540.
Kay, S. R., Fiszbein, A., & Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276.
Kohler, C. G., Bilker, W., Hagendoorn, M., Gur, R. E., & Gur, R. C. (2000). Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biological Psychiatry, 48(2), 127–136.
Kohler, C. G., Walker, J. B., Martin, E. A., Healey, K. M., & Moberg, P. J. (2010). Facial emotion perception in schizophrenia: a meta-analytic review. Schizophrenia Bulletin, 36(5), 1009–1019.
Lee, S. H., Niznikiewicz, M., Asami, T., Otsuka, T., Salisbury, D. F., Shenton, M. E., et al. (2016). Initial and progressive gray matter abnormalities in insular Gyrus and temporal pole in first-episode schizophrenia contrasted with first-episode affective psychosis. Schizophrenia Bulletin, 42(3), 790–801.
Li, T., Wang, Q., Zhang, J., Rolls, E. T., Yang, W., Palaniyappan, L., Zhang, L., Cheng, W., Yao, Y., Liu, Z., Gong, X., Luo, Q., Tang, Y., Crow, T. J., Broome, M. R., Xu, K., Li, C., Wang, J., Liu, Z., Lu, G., Wang, F., & Feng, J. (2017). Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophrenia Bulletin, 43(2), 436–448. https://doi.org/10.1093/schbul/sbw099.
Liao, X., Yuan, L., Zhao, T., Dai, Z., Shu, N., Xia, M., et al. (2015). Spontaneous functional network dynamics and associated structural substrates in the human brain. Frontiers in Human Neuroscience, 9, 478.
Liu, K., Zhao, X., Lu, X., Zhu, X., Chen, H., Wang, M., et al. (2018). Effect of selective serotonin reuptake inhibitor on prefrontal-striatal connectivity is dependent on the level of TNF-alpha in patients with major depressive disorder. Psychological Medicine, 1–9.
Milev, P., Ho, B. C., Arndt, S., & Andreasen, N. C. (2005). Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. The American Journal of Psychiatry, 162(3), 495–506.
Pang, L., Kennedy, D., Wei, Q., Lv, L., Gao, J., Li, H., et al. (2017). Decreased functional connectivity of insular cortex in drug naive first episode schizophrenia: In relation to symptom severity. PLoS One, 12(1), e167242.
Power, J. D., Mitra, A., Laumann, T. O., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 84320–84341.
Salvador, R., Sarro, S., Gomar, J. J., Ortiz-Gil, J., Vila, F., Capdevila, A., et al. (2010). Overall brain connectivity maps show cortico-subcortical abnormalities in schizophrenia. Human Brain Mapping, 31(12), 2003–2014.
Scholvinck, M. L., Maier, A., Ye, F. Q., Duyn, J. H., & Leopold, D. A. (2010). Neural basis of global resting-state fMRI activity. Proceedings of the National Academy of Sciences of the United States of America, 107(22), 10238–10243.
Vai, B., Sferrazza, P. G., Poletti, S., Radaelli, D., Donnici, E., Bollettini, I., et al. (2015). Abnormal cortico-limbic connectivity during emotional processing correlates with symptom severity in schizophrenia. European Psychiatry, 30(5), 590–597.
Weniger, G., Lange, C., Ruther, E., & Irle, E. (2004). Differential impairments of facial affect recognition in schizophrenia subtypes and major depression. Psychiatry Research, 128(2), 135–146.
Wong, C. W., Olafsson, V., Tal, O., & Liu, T. T. (2013). The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage, 83, 983–990.
Zalesky, A., Fornito, A., Cocchi, L., Gollo, L. L., & Breakspear, M. (2014). Time-resolved resting-state brain networks. Proceedings of the National Academy of Sciences of the United States of America, 111(28), 10341–10346.
Zeng, L. L., Wang, D., Fox, M. D., Sabuncu, M., Hu, D., Ge, M., et al. (2014). Neurobiological basis of head motion in brain imaging. Proceedings of the National Academy of Sciences of the United States of America, 111(16), 6058–6062.
Zhang, J., Kendrick, K. M., Lu, G., & Feng, J. (2015a). The fault lies on the other side: altered brain functional connectivity in psychiatric disorders is mainly caused by counterpart regions in the opposite hemisphere. Cerebral Cortex, 25(10), 3475–3486. https://doi.org/10.1093/cercor/bhu173.
Zhang, R., Wei, Q., Kang, Z., Zalesky, A., Li, M., Xu, Y., et al. (2015b). Disrupted brain anatomical connectivity in medication-naive patients with first-episode schizophrenia. Brain Structure & Function, 220(2), 1145–1159. https://doi.org/10.1093/cercor/bhu173.
Zhang, J., Cheng, W., Liu, Z., Zhang, K., Lei, X., Yao, Y., et al. (2016). Neural, electrophysiological and anatomical basis of brain-network variability and its characteristic changes in mental disorders. Brain, 139(Pt 8), 2307–2321.
Funding
The study was supported by the grants from the National Natural Science Foundation of China (No. 81801682, No. 81701674, and No. 31640028), and also supported by the grant from Guangdong Natural Science Foundation (No. 2018030310296).
Author information
Authors and Affiliations
Contributions
Conception and study design (Yanjia Deng, Kai Liu, and Ge Wen). Data collection and acquisition (Dongliang Chen, Ge Wen, and Yingjia Li). Imaging data and statistical analyses (Kai Liu, Shuguang Han, and Youyong Kong). Assessment of the clinical data (Yong Lin and Bin Zhang). Drafting the manuscript (Yanjia Deng and Kai Liu). Revising the manuscript (Shuguang Han, Hui Li, and Bin Zhang). Approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (All authors).
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethical committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Deng, Y., Han, S., Cheng, D. et al. Simultaneously decreased temporal variability and enhanced variability-strength coupling of emotional network connectivities are related to positive symptoms in patients with schizophrenia. Brain Imaging and Behavior 15, 76–84 (2021). https://doi.org/10.1007/s11682-019-00234-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-019-00234-0