Abstract
Indexes derived from diffusion tensor imaging (DTI) are sensitive to changes of both T2-hyperintense and normal-appearing brain white matter (WM) in elderly subjects with variable cognitive status. We investigated correlations between global cognitive performance and DTI-derived indexes along the WM tracts in the brain of patients with vascular mild cognitive impairment (MCI) and small vessel disease (SVD). Seventy-six patients with vascular MCI and SVD were assessed through Montreal Cognitive Assessment (MoCA) and Mini Mental State Examination (MMSE) test and underwent DTI examination on a 1.5 T MR scanner. We used Tract Based Spatial Statistics (TBSS) to assess voxel-wise in the entire brain the spatial distribution of the correlation between values of fractional anisotropy, mean, axial/radial diffusivity and global cognitive performance as assessed with MoCA and MMSE tests. All correlations were statistically tested with a significant p-value <0.05 using a family-wise error correction for multiple comparisons. The MoCA score significantly correlated with fractional anisotropy (positive correlation) and mean, axial and radial diffusivity (negative correlations) in WM tracts of cerebral hemispheres and corpus callosum, as well as in the intra-thalamic WM tracts and the superior cerebellar peduncle decussation in the midbrain. No significant correlations were observed for MMSE score. Global cognitive performance, as measured by the MoCA score, in patients with vascular MCI and SVD is associated with microstructural changes in WM tracts underlying intra- and inter-hemispheric cerebral, thalamo-cortical and cerebello-thalamic connections.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hyperintensity of the cerebral white matter (WM) in magnetic resonance T2-weighted images (T2WI), also termed leukoaraiosis, is a common condition in the elderly (Fazekas et al. 1987; Pantoni et al. 2005). It is associated with small vessel disease (SVD), motor disability and variable cognitive deficits, the latter implying a role of T2WI hyperintensity of the WM in vascular mild cognitive impairment (MCI) and dementia (Lamar et al. 2008; Roman et al. 2002).
When employed judiciously (Jones et al. 2013), diffusion-weighted imaging and diffusion tensor imaging (DTI) provide powerful tools for quantitative investigation of WM properties. Accordingly, these techniques have been applied to characterize WM areas exhibiting both increased and normal T2 signal in elderly subjects with SVD (Mascalchi et al. 2002; O’Sullivan et al. 2001). In particular, using region-of-interest (ROI) or histogram analysis of the whole WM or of segmented T2WI hyperintensities, the apparent diffusion coefficient (ADC)/mean diffusivity (MD) were seen to correlate negatively and fractional anisotropy (FA) to correlate positively with global and selective cognitive performance in patients with SVD and vascular MCI (Della Nave et al. 2007; O’Sullivan et al. 2004a; Vernooij et al. 2009; Xu et al. 2010; Pasi et al. 2015).
Tract-based spatial statistics (TBSS) is a voxel-wise method to regionally analyze DTI-derived indexes across the whole brain WM without any a priori hypotheses (Smith et al. 2006), that has been applied to the investigation of brain T2WI hyperintensities and SVD in elderly subjects with or without MCI (Mascalchi et al. 2014; Otsuka et al. 2012; Tuladhar et al. 2015) and in young or middle-aged subjects with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) (Mascalchi et al. 2017). The latter is the most frequent inherited brain SVD and is considered a model of SVD associated with cerebral T2 hyperintensities (Chabriat et al. 2009).
Recently, the median value of MD and FA in the whole cerebral WM was reported to significantly correlate (negative and positive correlation, respectively) with MoCA scores but not with MMSE scores in patients with vascular MCI and SVD (Pasi et al., 2015). In this study, we hypothesize that we could observe a significant correlation between DTI-derived indexes in some specific WM tracts serving distinct brain circuitries, including those of the brainstem and cerebellum, and measurements of cognitive deficits in patients with vascular MCI and SVD. For such an aim, we performed a voxel-wise whole brain TBSS analysis of DTI-derived indexes. In addition, we evaluated the spatial relationship between the WM tracts showing microstructural changes as outlined by TBSS analysis of DTI-derived indexes and the areas appearing hyperintense on T2-FLAIR images.
Materials and methods
Subjects
In this study, we assessed 76 subjects with evidence of MCI and brain WM T2WI hyperintensity of moderate or severe extension and SVD who were enrolled in the vascular MCI (VMCI) Tuscany study (Poggesi et al. 2012).
The VMCI-Tuscany Study is a longitudinal study designed to estimate the effect of a large set of clinical, neuroimaging, and biological markers of SVD in predicting the transition from VMCI to dementia (Poggesi et al. 2012). To be included, patients have to be classified as affected by vascular MCI with SVD according to the following criteria: 1) MCI defined according to the Winblad et al. criteria (Winblad et al. 2004) and operationalized according to Salvadori et al. (Salvadori et al. 2016); 2) visible evidence on FLAIR-T2 weighted images of moderate-to-severe degrees of WM T2WI hyperintensity, according to the modified version of the Fazekas scale (Pantoni et al. 2005).
Patients with other causes of WM lesions (e.g., multiple sclerosis, history of brain irradiation or other causes), as well as patients incapable to provide an informed consent were excluded from the VMCI-Tuscany study. In addition, for the present investigation, we also excluded subjects with non-lacunar infarcts in the cerebral cortex, cerebellum or brainstem. This choice was motivated by the possibility that such infarcts entail Wallerian degeneration phenomena which may act as confounders in the interpretation of DTI-derived indexes of the WM (Goveas et al. 2015).
Recruited patients underwent a thorough clinical, neuropsychological and laboratory assessment (Poggesi et al. 2012; Salvadori et al. 2015) along with a dedicated MRI study. The research was conducted in accordance with the Helsinki Declaration. The study was approved by the local ethics committees and a written informed consent was obtained from all individual participants included in the study.
A control group composed of 24 healthy control subjects (12 men and 12 women, mean age 72.5 ± 4.7 years, mean ± standard deviation) was included in the study. No significant difference in age (p = 0.09 at t-test) and in gender proportion (p = 0.65 at χ2 test) was found between the patient and control group. Healthy controls had no familial or personal history of neurologic or psychiatric disorders and underwent a neurologic examination that showed no abnormalities. The presence of cognitive impairment in controls has been excluded through the Mini Mental State Examination (MMSE) (mean score corrected for age and education level 28.9 ± 1.2, range 26.2–30, in the normal range for the Italian population (Measso et al. 1993)). Moreover, their MRI showed no (in 16 subjects) or only mild (in 8 subjects) brain WM hyperintensities on FLAIR-T2WI (according to the modified version of the Fazekas scale) and no lacunes. Patients and controls underwent the same imaging protocol on the same MR scanner.
MRI examination
The subjects were examined on a clinical 1.5 T scanner (Intera, Philips Medical System, Best, The Netherlands) with 33 mT/m maximum gradients strength and 6-channel radiofrequency head coil.
The MR examination protocol included a sagittal 3D T1-weighted turbo gradient echo sequence (TR = 8.1 ms, TE = 3.7 ms, flip angle = 8°, TI = 764 ms, FOV = 256 mm × 256 mm, matrix size = 256 × 256, 160 contiguous slices, slice thickness = 1 mm, NEX =1) and an axial FLAIR sequence (TR = 11,000 ms, TE = 140 ms, TI = 2800 ms, flip angle = 90°, FOV = 250 mm × 250 mm, matrix size = 280 × 202, 40 contiguous slices, slice thickness = 3 mm, interslice gap = 0.6 mm). For DTI, axial diffusion-weighed images were obtained with single-shot echo planar imaging sequence [TR = 9394 ms, TE = 89 ms, FOV = 256 mm × 256 mm, matrix size = 128 × 128, 50 slices, slice thickness = 3 mm, no inter-slice gap, NEX = 3, SENSE acceleration factor = 2; diffusion sensitizing gradients applied along 15 non-collinear directions using b-value of 0 (b0 image) and 1000 s/mm2].
Neuropsychological assessment
All patients underwent assessment through comprehensive neuropsychological test battery (Salvadori et al. 2015). In the present investigation we selected for the TBSS correlation analysis the subject’s score on the Montreal Cognitive Assessment (MoCA) test as well as MMSE scores. MoCA is a simple, stand-alone cognitive scale with good sensitivity and specificity in detecting MCI and, differently from MMSE that is the most widely used brief screening instrument for dementia, includes attentional, psychomotor speed, and executive items (Nasreddine et al. 2005; Folstein et al. 1975). For this reason it has been applied and recommended as a screening tool in vascular MCI (Pendlebury et al. 2010; Salvadori et al. 2015; Xu et al. 2014; Hachinski et al. 2006). MoCA and MMSE scores range from 0 to 30, with higher scores indicating a better cognitive performance, and correction for the effects of age and education was carried out using norms validated in the healthy Italian population (Conti et al. 2015; Measso et al. 1993).
Processing and data analyses
Conventional MRI
An observer with 10 years of experience (#1) visually evaluated the extent of WM T2WI hyperintensities on FLAIR images according to the modified Fazekas scale (Pantoni et al. 2005). Accordingly, patients were subdivided into four groups: grade 0 (no changes); grade 1 (mild changes): single lesions <10 mm and/or areas of “grouped” lesions <20 mm in any diameter; grade 2 (moderate changes): single hyperintense lesions of 10–20 mm, and hyperintense areas linked by no more than “connecting bridges” >20 mm in any diameter; and grade 3 (severe changes): both single and confluent hyperintense areas of ≥20 mm in any diameter).
Moreover, the same observer evaluated the presence of lacunar infarcts, which were defined as hypointense lesions on T1-weighted imaging with corresponding totally or partially hyperintense lesion on FLAIR images with a diameter < 20 mm. Lacunar infarcts were classified as absent, 1–3, and > 3 (Pasi et al. 2015).
Intra-rater agreement for observer #1 was assessed on 40 randomly selected MRI examinations scored twice, and turned out to be almost perfect for Fazekas score (linear weighted Cohen’s kappa κ = 0.91) and lacunar infarcts classification (κ = 0.82).
To assess inter-rater visual agreement, a second observer (#2) with 8 years of experience, independently, performed the same assessment in all patients and control subjects.
Inter-rater agreement was substantial for both Fazekas score (κ = 0.70) and lacunar infarcts classification (κ = 0.66). In the following analysis, we employed consensus rating between two observers and, in case of discordant rating, we employed the rating by the most experienced rater (#1).
Diffusion-weighted imaging
Diffusion-weighted images were corrected for head motion and eddy current distortions using FMRIB’s Diffusion Toolbox 2.0, part of FSL 4.1.9 (Smith et al. 2004) after which brain tissue was segmented using BET (Smith 2002). The b-matrix was reoriented by applying the rotational part of the affine transformation employed in the eddy-correction step (Leemans and Jones 2009). A tensor model was fitted to the raw data using a constrained nonlinear least squares procedure implemented in the software package CAMINO (Cook et al. 2006), and residual non-positive definite tensors (in isolated regions where the nonlinear algorithm failed to converge, mainly located at the edge of the brain) were removed by tensor interpolation in the log-euclidean domain (Arsigny et al. 2006). The following diffusion tensor invariants were then computed from the estimated tensor field using the software package DTI-TK (Zhang et al. 2007): MD, FA, axial diffusivity (AD) and radial diffusivity (RD).
TBSS
Voxel-wise statistical analysis of FA, MD, AD and RD data was carried out using TBSS (Smith et al. 2006), also part of FSL, which involves: (a) nonlinear registration of every FA image to every other one; (b) identification of the “most representative image”, as the one which requires the least warping to align every other image to it; (c) affine alignment of this target image into MNI152 space; (d) transformation of every other image into MNI space by combining the single nonlinear transforms into the target image with the affine transform into MNI space; (e) creation of a mean FA image, and (f) thinning to create a mean FA “skeleton” that represents the centers of all tracts common to the group. Each subject’s aligned FA data was then projected onto this skeleton, and previously computed warps were successively applied to FA, MD, AD and RD, thereby transforming all images into MNI space for subsequent skeletonization as above. Resulting data was then fed into voxel-wise cross-subject statistics. The latter included full correction for multiple comparisons across space using permutation-based nonparametric inference within the general linear model (GLM) framework (using 5000 permutations).
Preliminarily, we performed a between-group comparison of DTI-derived indexes between patients with MCI and SVD and healthy control subjects through a GLM. In addition, a within-group analysis in the patients with MCI and SVD group has been performed through a second GLM: all patients were considered as a single population in order to test for significant correlation of the MoCA and MMSE scores with voxel-wise skeletonized FA, MD, AD and RD (Winkler et al. 2014). The design matrix included age, gender and years of education as nuisance covariates. GLM contrasts for investigating both positive and negative correlation were set. P values were calculated and corrected for multiple comparisons using the “2D” parameter settings with threshold-free cluster enhancement, thereby avoiding the use of an arbitrary threshold for the initial cluster-formation (Smith and Nichols 2009). A p-value <0.05 corrected for multiple comparisons across space, was considered statistically significant (Winkler et al. 2014).
The WM tracts in which significant correlations were detected were labeled using the Balboni et al. anatomy textbook (Balboni et al. 2000) and the Wycoco et al. practical guide for radiologists (Wycoco et al. 2013).
White matter lesion analysis
In order to evaluate the degree of overlap between TBSS significance maps and the areas of visible T2 WM hyperintensity (WM lesions), a single observer outlined the latter in all vascular MCI patients on FLAIR images using a semiautomated segmentation technique based on user-supervised local thresholding (Jim 5.0, Xinapse System, Leicester, UK; www.xinapse.com/Manual/). A mean FLAIR-lesion map of the vascular MCI population was generated using FSL tools and a three-step procedure. First, a study-specific T1 template was created after registering all native 3D T1-weighted images on the high-resolution (1 mm3) MNI152 standard brain by using a linear transformation (FLIRT, FMRIB Linear Image Registration Tool) (Jenkinson and Smith 2001) and a subsequent nonlinear transformation (FNIRT, FMRIB Nonlinear Image Registration Tool) (Andersson et al. 2007); then all registered images were merged and averaged. Second, the lesion mask of each patient was nonlinearly registered on the T1 template by the combination of the following registrations: native FLAIR image on native T1-weighted image through FLIRT (“boundary-based registration” cost function) and native T1-weighted image on the T1 template through FNIRT. Finally, a group FLAIR-lesion map was generated first merging and then averaging all the FLAIR-lesion masks previously registered on the T1 template. The group FLAIR-lesion map thus provides a voxel-wise representation of the percentage of patients who have WM lesions in a determinate location. The group FLAIR-lesion map was overlaid with the significance maps resulting from between- and within-group analyses of each DTI-derived index.
Results
Demographic, clinical and conventional MRI features of the 76 patients are reported in Table 1.
As compared to the group of healthy controls, the patient group with vascular MCI and SVD showed extensive areas of increased MD and RD and of decreased FA involving cerebral WM, corpus callosum, thalami, brainstem, superior and middle cerebellar peduncles and cerebellum (Supplementary Figs. 1-3). Areas of increased AD were also present in the cerebral WM, corpus callosum and thalami (Supplementary Fig. 4). No areas of significantly decreased MD or RD, of increased FA or decreased AD were observed.
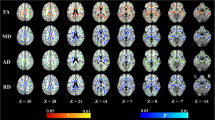
In patients with vascular MCI and SVD, MoCA score was correlated significantly with FA (positive correlation) as well as MD, AD and RD (negative correlation) in the WM tracts of almost the entire cerebral hemispheres, in an almost symmetrical fashion (Fig. 1 and Supplementary Figs. 5-7). WM tracts involved included superior and inferior longitudinal fasciculus, inferior frontal-occipital fasciculus, uncinate fasciculus, cingulum, internal capsule, forceps major and minor, corticospinal tracts and optic and thalamic radiations. MoCA score was significantly correlated with FA (positive correlation), MD and RD (negative correlation) in the WM fibers of the rostrum, body and splenium of the corpus callosum (Fig. 2).
MoCA score was also significantly correlated with FA (positive correlation) as well as MD, AD and RD (negative correlation) in the intra-thalamic WM tracts (Fig. 1 and Supplementary Figs. 5-7).
Within-group regression TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute standard space) in patients with vascular MCI and SVD. Yellow identifies the WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) negative correlation of MD with MoCA scores. They include WM tracts in the cerebral hemispheres, corpus callosum, thalami and decussation of superior cerebellar peduncles in the midbrain. The spatial distribution of these WM tracts is almost symmetrical. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations
Finally, MoCA score was significantly correlated with FA (positive correlation) as well as MD and RD (negative correlation) in the decussation of the superior cerebellar peduncles in the midbrain corresponding to the dentate-rubro-thalamic or dentate-thalamic tracts (Figs. 1 and 2 and Supplementary Figs. 5-7).
Within-group regression TBSS analysis of the midline structures in patients with vascular MCI and SVD. TBSS reveals that the values of DTI-derived indexes are correlated with individual MoCA scores. In particular, FA (in red) values are significantly (p value <0.05 corrected, threshold-free cluster enhancement) correlated (positive correlation) with MoCA score, whereas MD (in yellow) and RD (in green) values are significantly correlated (negative correlation) with MoCA score. In the MD and RD maps, note the spot in the upper midbrain corresponding to the decussation of the superior cerebellar peduncles. See text for abbreviations
The significance maps of all DTI-derived indexes showed an intermediate degree of overlay with the group FLAIR-lesion map (Fig. 1 and Supplementary Figs. 1-7).
No significant correlations between MMSE score and DTI-derived indexes were observed.
Discussion
The preliminary between-group TBSS analysis of DTI-derived indexes showed that patients with vascular MCI and SVD have extensive areas of microstructural changes in the cerebrum, brainstem and cerebellum when compared to healthy elderly subjects without cognitive impairment and absent or midbrain WM hyperintensities on FLAIR/T2-weighted images. Such a spatial distribution of changes in DTI-derived indexes is very similar to that observed using TBSS in patients with CADASIL (Mascalchi et al. 2017).
Visually rated extension of cerebral T2WI hyperintensity and atrophy of the corpus callosum have been previously reported to correlate with cognitive deficits in elderly patients with T2WI hyperintensity and SVD (Gouw et al. 2006; Jokinen et al. 2007). Moreover, O’Sullivan et al. (O’Sullivan et al. 2004a) reported that MD measured in several ROIs in the normal-appearing cerebral WM correlated with full scale IQ and tests of executive functions in a sample of 36 patients with ischemic T2WI hyperintensities. The same authors found a similar correlation between Trail Making Test B-A score, a measure of attentional set shifting and executive function, and MD values in frontal WM in healthy elderly subjects (O’Sullivan et al. 2001). In a previous VMCI-Tuscany study on the same patients of the current study, the median value of MD and FA in the whole cerebral WM correlated with MoCA scores but not with MMSE (Pasi et al. 2015).
To our knowledge, two previous studies used TBSS in order to assess possible correlations between cognitive performance and DTI-derived indexes in the WM of patients with T2WI and SVD (Otsuka et al. 2012; Tuladhar et al. 2015). Otsuka et al. (Otsuka et al. 2012) examined 24 patients with extensive T2WI hyperintensity and SVD and variable clinical features including dementia, and reported a significant positive correlation between MMSE and FA (i.e., lower FA was associated with worse cognitive performance) in cerebral hemispheric deep WM and in the corpus callosum. Tuladhar et al. (Tuladhar et al. 2015) examined 440 elderly, non-demented subjects with SVD and variable extension of T2WI hyperintensity and severity of cognitive deficit (of which only 2.9% fulfilled the criteria for MCI). They found that FA and MD of specific WM tracts correlated (FA, negative correlation; MD, positive correlation) with the degree of cognitive disturbances. In particular, FA, MD and RD in the genu and splenium of the corpus callosum showed the strongest significant correlation with global cognitive function and executive functions, while FA and MD in the cingulum bundle correlated significantly with verbal memory performance. MMSE did not show any significant correlation with DTI-derived indexes.
Our investigation differs from the above two studies (Otsuka et al. 2012; Tuladhar et al. 2015) for the following two aspects.
First, we evaluated only patients with evidence of vascular MCI and SVD.
Second, we explored the correlation between DTI-derived indexes and two cognitive scales, using MoCA test, a screening test specifically developed, and MMSE. In particular, the MoCA test is a screening tool suggested from the NINDS-CSN harmonization standards for the evaluation of vascular cognitive impairment due to the presence of several items assessing executive functions, attention and concentration (Hachinski et al. 2006). Furthermore, studies on content validity confirm that the MoCA covers most of the domains that represent cognitive impairment in cerebrovascular disease, and is sensitive to the milder forms of cognitive impairment that often accompany cerebrovascular disease (Koski 2013). The choice of a screening tool was due to its feasibility, usefulness and availability in clinical practice, where a second level and comprehensive neuropsychological assessment (e.g. Trail Making test, Stroop test, etc.) is not always available. Thus we believe that the result of a significant correlation between DTI-derived indexes and the MoCA scores further support the usefulness of a simple cognitive test as an early marker of brain damage leading to cognitive dysfunction.
We found significant correlations in the WM belonging to four systems, namely the cerebral hemisphere WM underlying intra-hemispheric cerebral connections, the corpus callosum underlying inter-hemispheric cerebral connections, the intra-thalamic WM underlying thalamo-cortical connections, and the dentate-thalamic or dentate-rubro-thalamic WM underlying cerebellar-cerebral connections.
The two former correlations are consistent with available data (Otsuka et al. 2012; Tuladhar et al. 2015). Moreover, DTI-derived indexes of thalamus correlate with cognition in patients with CADASIL without dementia (O’Sullivan et al. 2004b). On the other hand, so far, no study has reported link between cognitive performances as measured by MoCA scores and DTI-derived indexes in intra-thalamic WM and in the dentate-rubro-thalamic WM in patients with vascular MCI and SVD.
The thalamus is traditionally considered a gray matter structure. However, it contains WM tracts which subdivide it into anatomically and functionally distinct nuclei (Balboni et al. 2000). We submit the following hypothesis to explain the correlation we observed. Modifications of the DTI-derived indexes of intra-thalamic WM tracts may reflect changes in the WM fibers afferent to the thalamic nuclei, including those from the reticular formation in the brainstem and the cerebellar dentate (see below). Additionally, these correlations may reflect changes in efferent WM fibers to associative cerebral cortex from thalamic nuclei that are not part of the sensitive ascending pathways, including medial, lateral and intralaminar nuclei. Overall, the correlation between DTI-derived indexes in the intra-thalamic WM and MoCA scores may suggest compromised thalamic function in integration of higher functions in patients with vascular MCI and SVD.
The dentate-rubro-thalamic and dentate-thalamic pathways represent the main links between the cerebellar and cerebral cortex. These pathways are involved in motor control but may also connect the cognitive (posterior) cerebellum, including lobules VI, VII, VIIB and Crus I, which is involved in language, verbal memory, spatial tasks and executive functions, with the associative cerebral cortex (Schmahmann 1996; Stoodley and Schmahmann 2009). Accordingly, in a study on Friedreich ataxia (Akhlaghi et al. 2014), it was recently demonstrated that microstructural changes in dentatorubral tract (as detected with DTI-derived indexes) might be responsible for measurable cognitive deficits.
Moreover, microstructural changes of the cerebral-cerebellar pathways may underlie the pathophysiological changes in the cerebellum associated with cognitive deficits in patients with MCI and SVD that are revealed by resting-state fMRI (Diciotti et al. 2017).
We found an intermediate degree of overlay between the significance maps of all DTI-derived indexes and the FLAIR-lesion map in the between-group and within-group analyses. This implies that both WM hyperintense lesions and normal-appearing WM contribute to the significance maps and ultimately to cognitive impairment as assessed with MoCA. In particular, the importance of the contribute of the normal-appearing WM is in line with previous DTI studies which used a ROI approach and several cognitive tests (Jokinen et al. 2013; O’Sullivan et al. 2001; Yuan et al. 2017), suggesting that the cognitive deficits are consistently sustained also by the microstructural damage of the normal-appearing WM revealed by DTI.
In our opinion, the present results justify future studies aimed to get further insights into the role of WM lesions and normal-appearing WM in cognitive dysfunction in MCI and SVD, by using DTI-derived indexes and the potential of machine learning techniques. In fact, machine learning techniques can be applied on a single-patient basis in predicting a group label or a continuous value. Importantly, the predictive nature of machine learning strategies is more in line with the aim of clinical diagnosis and prognosis in the single patient as compared to conventional group-based analysis.
Conclusion
Our study suggests that impaired cognitive performance in patients with vascular MCI and SVD (as reflected by MoCA but not MMSE scores) is associated with microstructural changes in several WM tracts, possibly implying impairment of intra- and inter-hemispheric cerebral, thalamo-cortical and cerebello-cerebral connections.
References
Akhlaghi, H., Yu, J., Corben, L., Georgiou-Karistianis, N., Bradshaw, J. L., Storey, E., et al. (2014). Cognitive deficits in Friedreich ataxia correlate with micro-structural changes in dentatorubral tract. Cerebellum, 13(2), 187–198. https://doi.org/10.1007/s12311-013-0525-4.
Andersson, J. L. R., Jenkinson, M., & Smith, S. M. (2007). Non-linear registration, aka spatial normalisation. FMRIB technical report (pp. TR07JA02).
Arsigny, V., Fillard, P., Pennec, X., & Ayache, N. (2006). Log-Euclidean metrics for fast and simple calculus on diffusion tensors. Magnetic Resonance in Medicine, 56(2), 411–421. https://doi.org/10.1002/mrm.20965.
Balboni, G. C., Bastianini, A., Brizzi, E., Castorina, S., Comparini, L., Donato, R. F., et al. (2000). Anatomia Umana (Vol 2). In Ermes.
Chabriat, H., Joutel, A., Dichgans, M., Tournier-Lasserve, E., & Bousser, M. G. (2009). Cadasil. Lancet Neurology, 8(7), 643–653. https://doi.org/10.1016/S1474-4422(09)70127-9.
Conti, S., Bonazzi, S., Laiacona, M., Masina, M., & Coralli, M. V. (2015). Montreal cognitive assessment (MoCA)-Italian version: Regression based norms and equivalent scores. Neurological Sciences, 36(2), 209–214. https://doi.org/10.1007/s10072-014-1921-3.
Cook, P. A., Bai, Y., Nedjati-Gilani, S., Seunarine, K. K., Hall, M. G., Parker, G. J., et al. Camino: Open-source diffusion-MRI reconstruction and processing. In 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine, Seattle, WA, USA, 2006 (pp. 2759).
Della Nave, R., Foresti, S., Pratesi, A., Ginestroni, A., Inzitari, M., Salvadori, E., et al. (2007). Whole-brain histogram and voxel-based analyses of diffusion tensor imaging in patients with leukoaraiosis: Correlation with motor and cognitive impairment. AJNR. American Journal of Neuroradiology, 28(7), 1313–1319. https://doi.org/10.3174/ajnr.A0555.
Diciotti, S., Orsolini, S., Salvadori, E., Giorgio, A., Toschi, N., Ciulli, S., Ginestroni, A., Poggesi, A., de Stefano, N., Pantoni, L., Inzitari, D., Mascalchi, M., & VMCI Tuscany investigators. (2017). Resting state fMRI regional homogeneity correlates with cognition measures in subcortical vascular cognitive impairment. Journal of the Neurological Sciences, 373, 1–6. https://doi.org/10.1016/j.jns.2016.12.003.
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., & Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR. American Journal of Roentgenology, 149(2), 351–356. https://doi.org/10.2214/ajr.149.2.351.
Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). "mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198.
Gouw, A. A., Van der Flier, W. M., van Straaten, E. C., Barkhof, F., Ferro, J. M., Baezner, H., et al. (2006). Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: The LADIS study. Journal of Neurology, 253(9), 1189–1196. https://doi.org/10.1007/s00415-006-0193-5.
Goveas, J., O'Dwyer, L., Mascalchi, M., Cosottini, M., Diciotti, S., De Santis, S., et al. (2015). Diffusion-MRI in neurodegenerative disorders. Magnetic Resonance Imaging, 33(7), 853–876. https://doi.org/10.1016/j.mri.2015.04.006.
Hachinski, V., Iadecola, C., Petersen, R. C., Breteler, M. M., Nyenhuis, D. L., Black, S. E., Powers, W. J., DeCarli, C., Merino, J. G., Kalaria, R. N., Vinters, H. V., Holtzman, D. M., Rosenberg, G. A., Wallin, A., Dichgans, M., Marler, J. R., & Leblanc, G. G. (2006). National Institute of Neurological Disorders and Stroke-Canadian stroke network vascular cognitive impairment harmonization standards. Stroke, 37(9), 2220–2241. https://doi.org/10.1161/01.Str.0000237236.88823.47.
Jenkinson, M., & Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156.
Jokinen, H., Ryberg, C., Kalska, H., Ylikoski, R., Rostrup, E., Stegmann, M. B., Waldemar, G., Madureira, S., Ferro, J. M., van Straaten, E. C. W., Scheltens, P., Barkhof, F., Fazekas, F., Schmidt, R., Carlucci, G., Pantoni, L., Inzitari, D., Erkinjuntti, T., & on behalf of the LADIS group. (2007). Corpus callosum atrophy is associated with mental slowing and executive deficits in subjects with age-related white matter hyperintensities: The LADIS study. Journal of Neurology, Neurosurgery, and Psychiatry, 78(5), 491–496. https://doi.org/10.1136/jnnp.2006.096792.
Jokinen, H., Schmidt, R., Ropele, S., Fazekas, F., Gouw, A. A., Barkhof, F., Scheltens, P., Madureira, S., Verdelho, A., Ferro, J. M., Wallin, A., Poggesi, A., Inzitari, D., Pantoni, L., Erkinjuntti, T., & LADIS Study Group. (2013). Diffusion changes predict cognitive and functional outcome: The LADIS study. Annals of Neurology, 73(5), 576–583. https://doi.org/10.1002/ana.23802.
Jones, D. K., Knosche, T. R., & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. NeuroImage, 73, 239–254. https://doi.org/10.1016/j.neuroimage.2012.06.081.
Koski, L. (2013). Validity and applications of the Montreal cognitive assessment for the assessment of vascular cognitive impairment. Cerebrovascular Diseases, 36(1), 6–18. https://doi.org/10.1159/000352051.
Lamar, M., Catani, M., Price, C. C., Heilman, K. M., & Libon, D. J. (2008). The impact of region-specific leukoaraiosis on working memory deficits in dementia. Neuropsychologia, 46(10), 2597–2601. https://doi.org/10.1016/j.neuropsychologia.2008.04.007.
Leemans, A., & Jones, D. K. (2009). The B-matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine, 61(6), 1336–1349. https://doi.org/10.1002/mrm.21890.
Mascalchi, M., Ginestroni, A., Toschi, N., Poggesi, A., Cecchi, P., Salvadori, E., Tessa, C., Cosottini, M., de Stefano, N., Pracucci, G., Pantoni, L., Inzitari, D., Diciotti, S., & VMCI Tuscany investigators. (2014). The burden of microstructural damage modulates cortical activation in elderly subjects with MCI and leuko-araiosis. A DTI and fMRI study. Human Brain Mapping, 35(3), 819–830. https://doi.org/10.1002/hbm.22216.
Mascalchi, M., Pantoni, L., Giannelli, M., Valenti, R., Bianchi, A., Pracucci, G., Orsolini, S., Ciulli, S., Tessa, C., Poggesi, A., Pescini, F., Inzitari, D., & Diciotti, S. (2017). Diffusion tensor imaging to map brain microstructural changes in CADASIL. Journal of Neuroimaging, 27(1), 85–91. https://doi.org/10.1111/jon.12374.
Mascalchi, M., Tessa, C., Moretti, M., Della Nave, R., Boddi, V., Martini, S., Inzitari, D., & Villari, N. (2002). Whole brain apparent diffusion coefficient histogram: A new tool for evaluation of leukoaraiosis. Journal of Magnetic Resonance Imaging, 15(2), 144–148.
Measso, G., Cavarzeran, F., Zappalà, G., Lebowitz, B. D., Crook, T. H., Pirozzolo, F. J., Amaducci, L. A., Massari, D., & Grigoletto, F. (1993). The mini-mental state examination: Normative study of an Italian random sample. Developmental Neuropsychology, 9(2), 77–85. https://doi.org/10.1080/87565649109540545.
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
O'Sullivan, M., Morris, R. G., Huckstep, B., Jones, D. K., Williams, S. C., & Markus, H. S. (2004a). Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. Journal of Neurology, Neurosurgery, and Psychiatry, 75(3), 441–447.
O'Sullivan, M., Singhal, S., Charlton, R., & Markus, H. S. (2004b). Diffusion tensor imaging of thalamus correlates with cognition in CADASIL without dementia. Neurology, 62(5), 702–707.
O'Sullivan, M., Summers, P. E., Jones, D. K., Jarosz, J. M., Williams, S. C., & Markus, H. S. (2001). Normal-appearing white matter in ischemic leukoaraiosis: A diffusion tensor MRI study. Neurology, 57(12), 2307–2310.
Otsuka, Y., Yamauchi, H., Sawamoto, N., Iseki, K., Tomimoto, H., & Fukuyama, H. (2012). Diffuse tract damage in the hemispheric deep white matter may correlate with global cognitive impairment and callosal atrophy in patients with extensive leukoaraiosis. AJNR. American Journal of Neuroradiology, 33(4), 726–732. https://doi.org/10.3174/ajnr.A2853.
Pantoni, L., Basile, A. M., Pracucci, G., Asplund, K., Bogousslavsky, J., Chabriat, H., Erkinjuntti, T., Fazekas, F., Ferro, J. M., Hennerici, M., O’Brien, J., Scheltens, P., Visser, M. C., Wahlund, L. O., Waldemar, G., Wallin, A., & Inzitari, D. (2005). Impact of age-related cerebral white matter changes on the transition to disability -- the LADIS study: Rationale, design and methodology. Neuroepidemiology, 24(1–2), 51–62. https://doi.org/10.1159/000081050.
Pasi, M., Salvadori, E., Poggesi, A., Ciolli, L., Del Bene, A., Marini, S., et al. (2015). White matter microstructural damage in small vessel disease is associated with Montreal cognitive assessment but not with mini mental state examination performances: Vascular mild cognitive impairment Tuscany study. Stroke, 46(1), 262–264. https://doi.org/10.1161/STROKEAHA.114.007553.
Pendlebury, S. T., Cuthbertson, F. C., Welch, S. J. V., Mehta, Z., & Rothwell, P. M. (2010). Underestimation of cognitive impairment by mini-mental state examination versus the Montreal cognitive assessment in patients with transient ischemic attack and stroke a population-based study. Stroke, 41(6), 1290–1293. https://doi.org/10.1161/Strokeaha.110.579888.
Poggesi, A., Salvadori, E., Pantoni, L., Pracucci, G., Cesari, F., Chiti, A., et al. (2012). Risk and Determinants of Dementia in Patients with Mild Cognitive Impairment and Brain Subcortical Vascular Changes: A Study of Clinical, Neuroimaging, and Biological Markers-The VMCI-Tuscany Study: Rationale, Design, and Methodology. Int J Alzheimers Dis, 2012, 608013, doi:10.1155/2012/608013.
Roman, G. C., Erkinjuntti, T., Wallin, A., Pantoni, L., & Chui, H. C. (2002). Subcortical ischaemic vascular dementia. Lancet Neurology, 1(7), 426–436.
Salvadori, E., Poggesi, A., Pracucci, G., Inzitari, D., & Pantoni, L. (2015). Development and psychometric properties of a neuropsychological battery for mild cognitive impairment with small vessel disease: The VMCI-Tuscany study. Journal of Alzheimer's Disease, 43(4), 1313–1323. https://doi.org/10.3233/JAD-141449.
Salvadori, E., Poggesi, A., Valenti, R., Pracucci, G., Pescini, F., Pasi, M., Nannucci, S., Marini, S., Del Bene, A., Ciolli, L., Ginestroni, A., Diciotti, S., Orlandi, G., Di Donato, I., De Stefano, N., Cosottini, M., Chiti, A., Federico, A., Dotti, M.T., Bonuccelli, U., Inzitari, D., Pantoni, L.; VMCI-Tuscany Study Group. (2016). Operationalizing mild cognitive impairment criteria in small vessel disease: The VMCI-Tuscany study. Alzheimers Dement, 12(4), 407–418. https://doi.org/10.1016/j.jalz.2015.02.010.
Schmahmann, J. D. (1996). From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping, 4(3), 174–198, doi:10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2–0. https://doi.org/10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0.
Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. https://doi.org/10.1002/hbm.10062.
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., Watkins, K. E., Ciccarelli, O., Cader, M. Z., Matthews, P. M., & Behrens, T. E. J. (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024.
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. https://doi.org/10.1016/j.neuroimage.2004.07.051.
Smith, S. M., & Nichols, T. E. (2009). Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. https://doi.org/10.1016/j.neuroimage.2008.03.061.
Stoodley, C. J., & Schmahmann, J. D. (2009). Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage, 44(2), 489–501. https://doi.org/10.1016/j.neuroimage.2008.08.039.
Tuladhar, A. M., van Norden, A. G., de Laat, K. F., Zwiers, M. P., van Dijk, E. J., Norris, D. G., et al. (2015). White matter integrity in small vessel disease is related to cognition. Neuroimage Clin, 7, 518–524. https://doi.org/10.1016/j.nicl.2015.02.003.
Vernooij, M. W., Ikram, M. A., Vrooman, H. A., Wielopolski, P. A., Krestin, G. P., Hofman, A., Niessen, W. J., van der Lugt, A., & Breteler, M. M. B. (2009). White matter microstructural integrity and cognitive function in a general elderly population. Archives of General Psychiatry, 66(5), 545–553. https://doi.org/10.1001/archgenpsychiatry.2009.5.
Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L. O., Nordberg, A., Backman, L., Albert, M., Almkvist, O., Arai, H., Basun, H., Blennow, K., de Leon, M., DeCarli, C., Erkinjuntti, T., Giacobini, E., Graff, C., Hardy, J., Jack, C., Jorm, A., Ritchie, K., van Duijn, C., Visser, P., & Petersen, R. C. (2004). Mild cognitive impairment - beyond controversies, towards a consensus: Report of the international working group on mild cognitive impairment. Journal of Internal Medicine, 256(3), 240–246. https://doi.org/10.1111/j.1365-2796.2004.01380.x.
Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M., & Nichols, T. E. (2014). Permutation inference for the general linear model. NeuroImage, 92, 381–397. https://doi.org/10.1016/j.neuroimage.2014.01.060.
Wycoco, V., Shroff, M., Sudhakar, S., & Lee, W. (2013). White matter anatomy. what the radiologist needs to know. Neuroimaging Clin N Am, 23(2), 197–216. https://doi.org/10.1016/j.nic.2012.12.002.
Xu, Q., Cao, W. W., Mi, J. H., Yu, L., Lin, Y., & Li, Y. S. (2014). Brief screening for mild cognitive impairment in subcortical ischemic vascular disease: A comparison study of the Montreal cognitive assessment with the mini-mental state examination. European Neurology, 71(3–4), 106–114. https://doi.org/10.1159/000353988.
Xu, Q., Zhou, Y., Li, Y. S., Cao, W. W., Lin, Y., Pan, Y. M., et al. (2010). Diffusion tensor imaging changes correlate with cognition better than conventional MRI findings in patients with subcortical ischemic vascular disease. Dementia and Geriatric Cognitive Disorders, 30(4), 317–326. https://doi.org/10.1159/000320491.
Yuan, J. L., Wang, S. K., Guo, X. J., Teng, L. L., Jiang, H., Gu, H., & Hu, W. L. (2017). Disconnections of Cortico-subcortical pathways related to cognitive impairment in patients with Leukoaraiosis: A preliminary diffusion tensor imaging study. European Neurology, 78(1–2), 41–47. https://doi.org/10.1159/000477899.
Zhang, H., Avants, B. B., Yushkevich, P. A., Woo, J. H., Wang, S., McCluskey, L. F., et al. (2007). High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: An example study using amyotrophic lateral sclerosis. IEEE Transactions on Medical Imaging, 26(11), 1585–1597. https://doi.org/10.1109/TMI.2007.906784.
Funding
The VMCI-Tuscany was funded by Tuscany Region Health Programme in the framework of the “Bando Regione Salute 2009”.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Supplementary Fig. 1
Between-group TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute standard space) identifies in yellow WM tracts showing a significant (p-value <0.05 corrected, threshold-free cluster enhancement) increase of MD in patients with vascular MCI and SVD as compared to healthy controls. They include WM tracts in the cerebral hemispheres, corpus callosum, thalami, midbrain, pons, middle cerebellar peduncles and left cerebellar hemisphere. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 213 kb)
Supplementary Fig. 2
Between-group TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) identifies in red WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) decrease of FA in patients with vascular MCI and SVD as compared to healthy controls. They include WM tracts in the cerebral hemispheres, corpus callosum, thalami, midbrain, pons, superior and middle cerebellar peduncles and cerebellar hemispheres. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 218 kb)
Supplementary Fig. 3
Between-group TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) identifies in green WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) increase of RD in patients with vascular MCI and SVD as compared to healthy controls. They include WM tracts in the cerebral hemispheres, corpus callosum, thalami, midbrain, pons, superior and middle cerebellar peduncles and left cerebellar hemisphere. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 213 kb)
Supplementary Fig. 4
Between group TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) identifies in blu/light-blu WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) increase of AD in patients with vascular MCI and SVD as compared to healthy controls. They include WM tracts in the cerebral hemispheres, corpus callosum and thalami. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 206 kb)
Supplementary Fig. 5
Within-group regression TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) in patients with vascular MCI and SVD. Red identifies WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) positive correlation of FA with MoCA scores. They include WM tracts in the cerebral hemispheres, corpus callosum, and thalami. The spatial distribution of these WM tracts is bilateral but not symmetrical. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 159 kb)
Supplementary Fig. 6
Within-group regression TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) in patients with vascular MCI and SVD. Blue identifies the WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) negative correlation of axial diffusivity with MoCA scores. They include WM tracts in the cerebral hemispheres, corpus callosum, and left thalamus. The spatial distribution of these WM tracts is bilateral but not symmetrical. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 150 kb)
Supplementary Fig. 7
Within-group regression TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) in patients with vascular MCI and SVD. Green identifies the WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) negative correlation of RD with MoCA scores. They include WM tracts in the cerebral hemispheres, corpus callosum, thalami and decussation of superior cerebellar peduncles in the midbrain. The spatial distribution of these WM tracts is bilateral but not symmetrical. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 157 kb)
Rights and permissions
About this article
Cite this article
Mascalchi, M., Salvadori, E., Toschi, N. et al. DTI-derived indexes of brain WM correlate with cognitive performance in vascular MCI and small-vessel disease. A TBSS study. Brain Imaging and Behavior 13, 594–602 (2019). https://doi.org/10.1007/s11682-018-9873-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9873-5