Abstract
Previous studies have suggested that white matter disruption plays an important role in disorders of consciousness (DOC) after severe brain injury. Nevertheless, the integrity of white matter architecture supporting consciousness and its relations with clinical severity in patients with DOC remain to be established. In this study, diffusion tensor imaging (DTI) data was collected from 14 DOC patients and 15 healthy control subjects. We combined tract-based spatial statistics (TBSS) with region of interest (ROI) analysis to examine differences of DTI metrics on white matter skeletons between DOC patients and healthy controls, and the association between white matter integrity and patients’ residual consciousness assessed by Coma Recovery Scale-Revised (CRS-R). We found that: (1) patients with DOC had widespread white matter integrity disruptions, especially in the fornix; (2) the alteration of white matter microstructure was mainly attributed to the increase in radial diffusivity, possibly reflecting demyelination; (3) the behavioral CRS-R assessment score was positively correlated with white matter integrity in the fornix, uncinate fasciculus, pontine crossing tract, and posterior limb of internal capsule. Our results suggest that despite the widespread abnormalities of white matter following severe brain injury, the impairment of consciousness is likely to result from disruptions of key pathways that link brain regions in distributed networks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients in disorders of consciousness (DOC), involving vegetative state (VS) and minimally conscious state (MCS), are characterized by no or minimal awareness of themselves and the environment following an acquired severe brain injury (Giacino et al. 2002; Laureys et al. 2004). To date, clinical diagnosis of patients with DOC remains a critical challenge, due to that their behavioral responses may be quite limited or inconsistent. Recent advances in neuroimaging techniques provide exciting new opportunities to accurately assess levels of consciousness and residual functional capacity for patients with DOC (Giacino et al. 2014; Owen 2014).
A theoretical framework based on information theory suggests that consciousness depends on the brain’s capacity for information integration (Tononi 2008, 2012). Disruption of white matter tracts after severe brain injury, which results in the inability to transfer information between brain regions, has been described in both animal models (Gennarelli et al. 1982) and human studies (Adams et al. 2000). Diffusion tensor imaging (DTI) is a powerful non-invasive MRI technique that enables investigations of white matter microstructures and integrity in vivo (Basser and Pierpaoli 1996). With this technique, metrics such as fractional anisotropy (FA) and mean diffusivity (MD) can be derived to quantify the degree of white matter disruption (Le Bihan et al. 2001). Moreover, changes in axial and radial diffusivities are shown to be primarily related to axonal damage and demyelination, respectively (Song et al. 2003). Thus, DTI provides information not only on lesion localization but also on the pathophysiologic mechanisms of white matter damage in patients with DOC.
A number of DTI studies have been carried out to identify white matter alterations in patients with DOC. These studies have found significant impairment of white matter tracts connecting striatum, thalamus, frontal cortex, and parietal/occipital/temporal cortex (Lant et al. 2016; Newcombe et al. 2010; van der Eerden et al. 2014; Weng et al. 2017). Other studies have attempted to distinguish different consciousness levels (e.g., VS and MCS) based on diffusion characteristics (Fernández-Espejo et al. 2012; Fernandez-Espejo et al. 2011; Zheng et al. 2017), or to correlate DTI results with injury severity and clinical outcome (Newcombe et al. 2011; Perlbarg et al. 2009; Rutgers et al. 2008). The findings suggest that DTI could provide valuable biomarkers for DOC and shed light on the neural correlates of consciousness (Cavaliere et al. 2014). However, the region of interest (ROI) approach was typically employed for data analysis of previous work. As consciousness is likely to depend on distributed network function, a prior knowledge about structural causes of impaired consciousness is not readily available. Besides, the relationship between white matter integrity and residual consciousness in patients with DOC should be further elucidated.
In our present work, we combined tract-based spatial statistics (TBSS) with ROI-wise analysis to study the relationships between distributed white matter damage and impaired consciousness in patients with severe brain injury. As a fully automated whole-brain analysis approach, TBSS involves “skeletonization” of the white matter and focuses on the centers of the tracts (Smith et al. 2006), which can improve the sensitivity, objectivity, and interpretability of analysis of multi-subject diffusion imaging, and limit the impact of brain atrophy-related partial volume effects after prolonged brain injury. Recently, a few WM atlases (e.g., the ICBM-DTI-81 WM labels atlas) have been proposed (Mori et al. 2008). These WM atlases in the standard space parcellate the entire WM into multiple labeled ROIs, allowing for evaluation of the correlations between diffusion changes in specific tracts and patients’ residual consciousness assessed by Coma Recovery Scale-Revised (CRS-R) (Giacino et al. 2004). Our hypothesis was that increased disruption in specific white matter tracts will be associated with greater impairment of consciousness.
Materials and methods
Participants
The overall research protocol was approved by the Institutional Review Board at the PLA Army General Hospital. Informed consent was obtained from the patients’ legal guardians and normal control subjects. Forty-one patients with DOC and 20 normal controls (NC) were enrolled in the study. All the patients were recruited at least two months post-insult (average 4.1 months). Subjects in the control group had no history of neurological or systemic illness, head injury, and drug or alcohol abuse (intake). Subjects who had excessive head motion during imaging or were unable to complete the MRI scan were excluded from the analysis. Patients with serious brain atrophy or deformation were also excluded from this study to minimize the registration errors to the standard brain space. As a result, 14 patients (6 men and 8 women; mean age, 43.6 ± 13.4 years) and 15 healthy control subjects (4 men and 11 women; mean age, 34.4 ± 11.8 years) were eligible for analysis. The two groups were not significantly different in terms of age (t = 3.92, p = 0.06) or sex (χ2 = 0.36, p = 0.45).
Detailed information about the demographic and clinical characteristics of the patients is shown in Table 1. The etiologies of DOC included hypoxic-ischemic encephalopathy (HIE) in six patients, intracranial hemorrhage (ICH) in two patients, and traumatic brain injury (TBI) in six patients. The diagnoses included eleven cases of vegetative state (VS) and three cases of minimally conscious state (MCS). Coma Recovery Scale-Revised (CRS-R) was used to evaluate the patients’ level of consciousness on the day of MRI scanning (Giacino et al. 2004). The diagnosis in this study was made by experienced physicians according to the CRS-R scale. The examiner was experienced and blind to the imaging data. The assessment explored various sensory and cognitive aspects, including auditory, visual, verbal and motor functions, as well as communication and arousal levels, with the total score ranging from ‘0’ (worst) to ‘23’ (best).
MRI acquisition protocol
All the subjects underwent the same brain MR imaging protocol, performed on a GE 3.0 T MR scanner (General Electric Medical System, Milwaukee, WI) using an 8-channel head coil. DTI images were collected by using a single-shot, diffusion-weighted echo-planar imaging sequence with the following scan parameters: repetition time (TR) = 9000 ms; echo time (TE) = 80 ms; field of view (FOV) = 256 mm × 256 mm; slice thickness = 2 mm; flip angle (FA) = 90°; matrix size = 128 × 128; 75 axial slices; 64 diffusion weighted directions with a b value of 1000s/mm2, and 3 images with a b value of 0 s/mm2. High-resolution T1-weighted anatomical images were acquired using a 3D fast spoiled gradient-echo (FSPGR) sequence with the following parameters: TR = 8.16 ms; TE = 3.18 ms; FOV = 256 mm × 256 mm; FA = 7°; voxel size = 1 mm × 1 mm × 1 mm, 188 sagittal slices.
DTI image preprocessing
The preprocessing of DTI data was carried out using the FMRIB Software Library (FSL 5.0.8, http://www.fmrib.ox.ac.uk/fsl). The diffusion-weighted images were coregistered to the b0 image using an affine transformation for the correction of head motion and eddy current induced image distortion. The gradient table information was corrected for rotations (Leemans and Jones 2009). Non-brain regions were removed from the original b0 images using the Brain Extraction Tool (BET). Diffusion tensors were then reconstructed by fitting a diffusion tensor model at each voxel of the diffusion images, and the diffusion tensor metrics were generated for each subject, including FA, MD, axial diffusivity (λ1), and radial diffusivity (λ23). Because field maps were not acquired in this study, a 2-step process was used to reduce the inherent susceptibility-induced spatial distortions in EPI-based acquisitions. First, the b0 images were linearly coregistered into the same geometric space as the FLAIR images by using the FMRIB Linear Image Registration Tool (FLIRT). The images were then nonlinearly coregistered by using the Advanced Normalization Tools package (ANTs). These two transformations were then applied to each of the DTI outputs so that all the scalar measurements were resampled into the native FLAIR geometric space.
Voxel-wise TBSS analysis
Whole-brain voxel-wise differences between the DOC subjects and healthy controls were studied using TBSS (Smith et al. 2006). First, FA data of all the subjects was transformed into the FMRIB58_FA standard-space image (1 × 1 × 1 mm MNI152 space) by means of nonlinear registration. Then, the transformed FA images were averaged to generate a mean FA image which was subsequently skeletonized, representing tracts common to all of the subjects. In order to prevent the inclusion of non-skeleton voxels, each subject’s aligned FA map was mapped onto the “mean FA skeleton” using a lower threshold of FA of 0.2 in order to exclude gray matter voxels. The approach of carefully tuned non-linear registration, followed by a creation of a mean FA skeleton intends to face the cross-subject spatial variability effect. The nonlinear warps and skeleton projection stages were then repeated using data for MD, λ1, and λ23.
In this study, we used two-sample nonparametric t-tests to obtain group differences between the DOC and control groups, with age and sex as covariates. The voxel-wise nonparametric testing was performed using Randomise in FSL (with 5000 permutations). All statistical maps were family-wise error (FWE) corrected using p < 0.05, based on the threshold-free cluster enhancement (TFCE) statistic image (Smith and Nichols 2009).
ROI-wise statistical analysis
To investigate the diffusion changes in specific tracts, the ICBM-DTI-81 WM labels atlas was used to parcel the entire WM into 48 regions of interest (ROIs), which were used for the ROI-wise statistical analysis (Mori et al. 2008). The regional FA data was calculated by averaging the values within each region of the WM atlas. Two-sample t-tests were performed to compare the resultant ROI-based data between the DOC and control groups. Moreover, in the DOC group, linear regression analysis was used to evaluate the correlation between the ROI-based FA values and CRS-R scores. Age and sex were treated as covariates in the statistical analysis. This analysis was applied to all 48 atlas-based ROIs respectively, and a false discovery rate (FDR) procedure was performed at a q value of 0.05 to correct for multiple comparisons.
The patients experienced different types of etiology. We further conducted an exploratory mediation analysis (Baron and Kenny 1986) to examine whether etiology might influence the relationships between the ROI-based FA values and CRS-R scores. Let x, y, and z denote FA, etiology type, and CRS-R total score, respectively. The etiology type was modeled as 0–1 covariates (0: TBI; 1: non-TBI). To test for mediation, the following regression models were estimated: first, regressing the etiology type on the FA value (y = β1x + e, where e denotes the random error); second, regressing the CRS-R score on the FA value (z = β2x + e); and third, regressing the CRS-R score on both the FA value and on the etiology type (z = β3x + β4y + e).
Results
Widespread whiter matter disruption in DOC
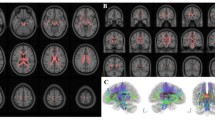
Voxel-wise TBSS analysis results of FA, MD, λ1 and λ23 differences between the DOC patients and healthy controls are shown in Fig. 1. Significantly decreased FA was observed in widespread WM tracts in DOC patients compared with control subjects. Meanwhile, the same contrast also showed higher MD and λ23 for the patients in almost the whole WM skeleton. The between-group differences for λ1 were less extensive but still marked. Elevated λ1 in the patient group was seen in several tracts including the anterior, superior, and posterior corona radiate, posterior thalamic radiation, external capsule, retrolenticular part of internal capsule, and superior longitudinal fasciculus. There were no WM regions that showed higher FA, or lower MD, λ1 or λ23 in the patient group.
Voxel-wise TBSS analysis results of FA, MD, λ1 and λ23 differences between the DOC and control groups. Green represents the mean white matter skeleton of all subjects. Blue-light blue (thickened for better visibility) represents areas with decreased FA, whereas red-yellow (thickened for better visibility) represents areas with increased MD, λ1 and λ23 in the DOC group
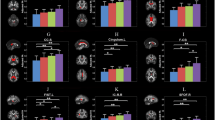
ROI-wise analysis further showed that the DOC patients had significantly lower FA in all atlas-based tract ROIs (p < 0.05, FDR corrected). Among these ROIs, the fornix exhibited the most severe impairment in the DOC patients (t = -20.7, Fig. 2). MD was significantly increased in the fornix in patients compared with controls (t = 8.8). A significant increase in λ1 and λ23 was also observed in this area. However, the increase in λ1 (t = 5.1) was smaller than the increase in λ23 (t = 10.6).
Correlations between FA and clinical measures
The ROI-wise regression analysis results between FA values and clinical measures in the patient group are shown in Fig. 3. Significant positive correlations (p < 0.05, FDR corrected) between FA and CRS-R scores were found in the pontine crossing tract (R = 0.627), fornix (R = 0.687), and left uncinate fasciculus (R = 0.668). The FA value in the right posterior limb of internal capsule also showed a trend for a similar positive correlation (R = 0.539, p < 0.05, uncorrected) with the CRS-R score. The results suggest that the more integrity of these white matter structures, the better preservation of residual consciousness in patients with DOC.
Furthermore, we examined whether the etiology type (TBI vs. non-TBI) might serve as a potential mediator of the link between the FA values and CRS-R scores. The mediation models for the associations between the FA value, etiology type, and CRS-R score are shown in Fig. 4. For step 1, FA was not significantly correlated with the etiology type in these ROIs (p > 0.60). For step 2, FA was significantly correlated with the CRS-R scores (p < 0.05). For step 3, the etiology type was not significantly correlated with the CRS-R scores (p > 0.70). Moreover, the positive correlations between FA and the CRS-R scores were still significant after controlling for etiology type. The results provided evidence that the relationship between FA and the CRS-R scores was not likely to be mediated by different types of brain injury.
Discussion
In this study, we found that patients with DOC had widespread white matter integrity disruptions, especially in the fornix. Moreover, FA within the fornix, uncinate fasciculus, pontine crossing tract, and posterior limb of internal capsule were shown to correlate with behavioral CRS-R assessment scores. Although widespread abnormalities of white matter were identified in patients with DOC, the correlations between clinical scores and specific white matter tracts suggest that consciousness is likely to depend on key pathways that link regions in distributed brain networks.
FA and MD are prominent DTI-derived quantitative measures of the white matter microstructure, which describe the degree of diffusion anisotropy and the average amount of diffusion in a voxel, respectively (Assaf and Pasternak 2008; Le Bihan et al. 2001). Based on the voxel-wise TBSS analyses, we found decreased FA and increased MD in almost the whole white matter skeleton in patients with DOC, which is consistent with previous brain injury studies (Kinnunen et al. 2011; van der Eerden et al. 2014). Accordingly, the alteration of FA and MD was mainly attributed to the increase in radial diffusivity, suggesting that demyelination may be an important contributing factor to white matter abnormalities in DOC (Song et al. 2003). Our results also show a remarkable increase in axial diffusivity in patients with DOC. Previous work has tended to show that brain injury produces early reductions in axial diffusivity, possibly reflecting axonal damage (van der Eerden et al. 2014). The patients in our study were in the subacute stage (4.1 ± 2.0 months). It has been shown that brain injury-induced reductions in axial diffusivity gradually reached normal or supranormal levels over time (Sidaros et al. 2008). Interestingly, our observation is also consistent with a previous animal study that demonstrated an elevation in both axial and radial diffusivities in the subacute stage of brain injury (Mac Donald et al. 2007). Previous studies suggested that the increased axial diffusivity in patients with prolonged brain injury may reflect adaptive axonal recovery (Kinnunen et al. 2011). It is also possible that the overall increase of diffusivity metrics corresponded to significant macrophage infiltration, demyelination, and edema, which make diffusion easier in all directions for lack of micro-organisation (Mac Donald et al. 2007).
Although widespread white matter abnormality was present, we found specific white matter tracts that were significantly associated with the diagnostic assessment of patients with DOC. Specifically, the fornix exhibited the most severe impairment, as well as the most significant correlation with clinical CRS-R scores. As the major pathway connecting the hippocampus to the mammillary bodies, the fornix plays a vital role in the transfer of episodic memory in the human brain (Douet and Chang 2014; Tsivilis et al. 2008). Previous studies have consistently shown white matter abnormalities in the fornix in TBI patients, which were correlated with deficits of episodic memory function (Adnan et al. 2013; Kinnunen et al. 2011; Wang et al. 2008). Consciousness invariably accompanies the retrieval of the detailed information that underlies recollection. Previous studies have proposed that autonoetic consciousness, the rudimentary state of affective, homeostatic, and sensory-perceptual mental experiences, is a defining feature of episodic memory (Moscovitch et al. 2016; Vandekerckhove et al. 2013). Our results further highlight a tight association between fornix integrity and residual consciousness in patients with severe brain injury. However, the direct relationship between episodic memory function and consciousness is not clear and needs to be explored in depth.
The other white matter structures that are associated with the diagnostic assessment of patients with DOC include the pontine crossing tract, posterior limb of internal capsule and uncinate fasciculus. The pontine crossing tract is a key component of the ascending reticular activating system from pontine reticular formation to the thalamus (Yeo et al. 2013). The posterior limb of internal capsule is mainly constituted by the superior thalamic radiation and long corticofugal pathways, and the uncinate fasciculus connects the frontal lobe and the anterior temporal lobe (Mori et al. 2008). Interestingly, these white matter tracts are in line with the anterior forebrain mesocircuit mode, which posits that different levels of consciousness depend on the basic connectivity patterns of the entire cortical-thalamo-cortical system (Giacino et al. 2014; Schiff 2010). White matter abnormalities in these tracts can thus produce broad reductions in cerebral activity, resulting in diminished response to environment stimuli and the failure to initiate goal-directed behaviors in patients with DOC.
It should be noted that the patients in the study had a variety of underlying neurological conditions. TBI and non-TBI (e.g., anoxic injuries) etiologies exhibit different pathophysiologic features, even though they may lead to similar clinical manifestations (van der Eerden et al. 2014). However, previous studies have also shown that patients in MCS have a much better preservation of large-scale brain networks than patients in VS, independent of the etiology (Demertzi et al. 2015). We further performed an exploratory mediation analysis in white matter structures that were related to patients’ residual consciousness (Fig. 4). We observed that the etiology type was correlated with neither FA nor the CRS-R scores. Moreover, the positive correlations between FA and the CRS-R scores were still significant after controlling for etiology type. Our preliminary results suggest that DOC may share some common neural mechanisms that are independent of the causes of brain injury.
Several potential limitations of the present study should be considered. First, the sample size was relatively small and was carefully selected to exclude patients with large focal lesions, which may preclude the generalization of the results. Second, in this study, the patients underwent the CRS-R assessments on the day of MRI scanning. Recent studies have suggested repeated examinations using the CRS-R within a short time interval, in order to avoid misdiagnosis due to fluctuation of arousal etc (Wannez et al. 2017). Third, our patients were in the subacute stage, and some patients may still have been in an active state of functional reorganization. Longitudinal studies would be required to explore the evolution of structural brain changes after brain injury and its relationship with functional improvements. Finally, additional neuroimaging evidence, such as resting-state functional connectivity other than structural abnormality, is needed as a synthesized biomarker for more reliable clinical diagnosis of this complex disorder.
In conclusion, we found widespread white matter microstructure disruptions in patients with DOC, which are likely caused by demyelination. Moreover, patients’ residual consciousness was related to the integrity of white matter tracts supporting episodic memory and anterior forebrain functions. Our findings suggest that consciousness is likely to depend on key pathways that link regions in distributed brain networks.
References
Adams, J. H., Graham, D. I., & Jennett, B. (2000). The neuropathology of the vegetative state after an acute brain insult. Brain, 123 (Pt 7), 1327–1338.
Adnan, A., Crawley, A., Mikulis, D., Moscovitch, M., Colella, B., & Green, R. (2013). Moderate-severe traumatic brain injury causes delayed loss of white matter integrity: evidence of fornix deterioration in the chronic stage of injury. Brain Injury, 27(12), 1415–1422. https://doi.org/10.3109/02699052.2013.823659.
Assaf, Y., & Pasternak, O. (2008). Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. Journal of Molecular Neuroscience, 34(1), 51–61. https://doi.org/10.1007/s12031-007-0029-0.
Baron, R. M., & Kenny, D. A. (1986). The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51(6), 1173–1182.
Basser, P. J., & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B, 111(3), 209–219.
Cavaliere, C., Aiello, M., Di Perri, C., Fernandez-Espejo, D., Owen, A. M., & Soddu, A. (2014). Diffusion tensor imaging and white matter abnormalities in patients with disorders of consciousness. Frontiers in Human Neuroscience, 8, 1028. https://doi.org/10.3389/fnhum.2014.01028.
Demertzi, A., Antonopoulos, G., Heine, L., Voss, H. U., Crone, J. S., de Los Angeles, C., et al. (2015). Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain, 138(Pt 9), 2619–2631. https://doi.org/10.1093/brain/awv169.
Douet, V., & Chang, L. (2014). Fornix as an imaging marker for episodic memory deficits in healthy aging and in various neurological disorders. Frontiers in Aging Neuroscience, 6, 343. https://doi.org/10.3389/fnagi.2014.00343.
Fernandez-Espejo, D., Bekinschtein, T., Monti, M. M., Pickard, J. D., Junque, C., Coleman, M. R., et al. (2011). Diffusion weighted imaging distinguishes the vegetative state from the minimally conscious state. Neuroimage, 54(1), 103–112. https://doi.org/10.1016/j.neuroimage.2010.08.035.
Fernández-Espejo, D., Soddu, A., Cruse, D., Palacios, E. M., Junque, C., Vanhaudenhuyse, A., et al. (2012). A role for the default mode network in the bases of disorders of consciousness. Annals of Neurology, 72(3), 335–343. https://doi.org/10.1002/ana.23635.
Gennarelli, T. A., Thibault, L. E., Adams, J. H., Graham, D. I., Thompson, C. J., & Marcincin, R. P. (1982). Diffuse axonal injury and traumatic coma in the primate. Annals of Neurology, 12(6), 564–574. https://doi.org/10.1002/ana.410120611.
Giacino, J. T., Ashwal, S., Childs, N., Cranford, R., Jennett, B., Katz, D. I., et al. (2002). The minimally conscious state: definition and diagnostic criteria. Neurology, 58(3), 349–353.
Giacino, J. T., Fins, J. J., Laureys, S., & Schiff, N. D. (2014). Disorders of consciousness after acquired brain injury: the state of the science. Nature Reviews. Neurology, 10(2), 99–114. https://doi.org/10.1038/nrneurol.2013.279.
Giacino, J. T., Kalmar, K., & Whyte, J. (2004). The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Archives of Physical Medicine and Rehabilitation, 85(12), 2020–2029. https://doi.org/10.1016/j.apmr.2004.02.033.
Kinnunen, K. M., Greenwood, R., Powell, J. H., Leech, R., Hawkins, P. C., Bonnelle, V., et al. (2011). White matter damage and cognitive impairment after traumatic brain injury. Brain, 134(Pt 2), 449–463. https://doi.org/10.1093/brain/awq347.
Lant, N. D., Gonzalez-Lara, L. E., Owen, A. M., & Fernandez-Espejo, D. (2016). Relationship between the anterior forebrain mesocircuit and the default mode network in the structural bases of disorders of consciousness. Neuroimage Clinic, 10, 27–35. https://doi.org/10.1016/j.nicl.2015.11.004.
Laureys, S., Owen, A. M., & Schiff, N. D. (2004). Brain function in coma, vegetative state, and related disorders. The Lancet Neurology, 3(9), 537–546. https://doi.org/10.1016/s1474-4422(04)00852-x.
Le Bihan, D., Mangin, J. F., Poupon, C., Clark, C. A., Pappata, S., Molko, N., et al. (2001). Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging, 13(4), 534–546.
Leemans, A., & Jones, D. K. (2009). The B-matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine, 61(6), 1336–1349. https://doi.org/10.1002/mrm.21890.
Mac Donald, C. L., Dikranian, K., Bayly, P., Holtzman, D., & Brody, D. (2007). Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. The Journal of Neuroscience, 27(44), 11869–11876. https://doi.org/10.1523/JNEUROSCI.3647-07.2007.
Mori, S., Oishi, K., Jiang, H., Jiang, L., Li, X., Akhter, K., et al. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage, 40(2), 570–582. https://doi.org/10.1016/j.neuroimage.2007.12.035.
Moscovitch, M., Cabeza, R., Winocur, G., & Nadel, L. (2016). Episodic memory and beyond: the hippocampus and neocortex in transformation. Annual Review of Psychology, 67(1), 105–134. https://doi.org/10.1146/annurev-psych-113011-143733. doi.
Newcombe, V., Chatfield, D., Outtrim, J., Vowler, S., Manktelow, A., Cross, J., et al. (2011). Mapping traumatic axonal injury using diffusion tensor imaging: correlations with functional outcome. PLoS ONE, 6(5), e19214. https://doi.org/10.1371/journal.pone.0019214.
Newcombe, V. F. J., Williams, G. B., Scoffings, D., Cross, J., Carpenter, T. A., Pickard, J. D., et al. (2010). Aetiological differences in neuroanatomy of the vegetative state: insights from diffusion tensor imaging and functional implications. Journal of Neurology, Neurosurgery, and Psychiatry, 81(5), 552–561. https://doi.org/10.1136/jnnp.2009.196246.
Owen, A. M. (2014). Disorders of consciousness: Diagnostic accuracy of brain imaging in the vegetative state. Nature Reviews. Neurology, 10(7), 370–371. https://doi.org/10.1038/nrneurol.2014.102.
Perlbarg, V., Puybasset, L., Tollard, E., Lehericy, S., Benali, H., & Galanaud, D. (2009). Relation between brain lesion location and clinical outcome in patients with severe traumatic brain injury: a diffusion tensor imaging study using voxel-based approaches. Human Brain Mapping, 30(12), 3924–3933. https://doi.org/10.1002/hbm.20817.
Rutgers, D. R., Fillard, P., Paradot, G., Tadié, M., Lasjaunias, P., & Ducreux, D. (2008). Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. American Journal of Neuroradiology, 29(9), 1730–1735. https://doi.org/10.3174/ajnr.A1213.
Schiff, N. D. (2010). Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends in Neurosciences, 33(1), 1–9. https://doi.org/10.1016/j.tins.2009.11.002.
Sidaros, A., Engberg, A. W., Sidaros, K., Liptrot, M. G., Herning, M., Petersen, P., et al. (2008). Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain, 131(Pt 2), 559–572. https://doi.org/10.1093/brain/awm294.
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024.
Smith, S. M., & Nichols, T. E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage, 44(1), 83–98. https://doi.org/10.1016/j.neuroimage.2008.03.061.
Song, S. K., Sun, S. W., Ju, W. K., Lin, S. J., Cross, A. H., & Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage, 20(3), 1714–1722.
Tononi, G. (2008). Consciousness as integrated information: a provisional manifesto. The Biological Bulletin, 215(3), 216–242.
Tononi, G. (2012). Integrated information theory of consciousness: an updated account. Archives Italiennes de Biologie, 150(2–3), 56–90. https://doi.org/10.4449/aib.v149i5.1388.
Tsivilis, D., Vann, S. D., Denby, C., Roberts, N., Mayes, A. R., Montaldi, D., et al. (2008). A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nature Neuroscience, 11(7), 834–842. https://doi.org/10.1038/nn.2149.
van der Eerden, A. W., Khalilzadeh, O., Perlbarg, V., Dinkel, J., Sanchez, P., Vos, P. E., et al. (2014). White matter changes in comatose survivors of anoxic ischemic encephalopathy and traumatic brain injury: comparative diffusion-tensor imaging study. Radiology, 270(2), 506–516. https://doi.org/10.1148/radiol.13122720.
Vandekerckhove, M., Bulnes, L. C., & Panksepp, J. (2013). The emergence of primary anoetic consciousness in episodic memory. Frontiers in Behavioral Neuroscience, 7, 210. https://doi.org/10.3389/fnbeh.2013.00210.
Wang, J. Y., Bakhadirov, K., Devous, M. D., Abdi, S., McColl, H., Moore, R. C., et al (2008). Diffusion tensor tractography of traumatic diffuse axonal injury. Archives of Neurology, 65(5), 619–626. https://doi.org/10.1001/archneur.65.5.619.
Wannez, S., Heine, L., Thonnard, M., Gosseries, O., & Laureys, S. & Coma Science Group, c. (2017). The repetition of behavioral assessments in diagnosis of disorders of consciousness. Annals of Neurology, 81(6), 883–889, https://doi.org/10.1002/ana.24962.
Weng, L., Xie, Q., Zhao, L., Zhang, R., Ma, Q., Wang, J., et al. (2017). Abnormal structural connectivity between the basal ganglia, thalamus, and frontal cortex in patients with disorders of consciousness. Cortex, 90, 71–87. https://doi.org/10.1016/j.cortex.2017.02.011.
Yeo, S. S., Chang, P. H., & Jang, S. H. (2013). The ascending reticular activating system from pontine reticular formation to the thalamus in the human brain. Frontiers in Human Neuroscience, 7, 416. https://doi.org/10.3389/fnhum.2013.00416.
Zheng, Z. S., Reggente, N., Lutkenhoff, E., Owen, A. M., & Monti, M. M. (2017). Disentangling disorders of consciousness: Insights from diffusion tensor imaging and machine learning. Human Brain Mapping, 38(1), 431–443. https://doi.org/10.1002/hbm.23370.
Funding
This work was supported by the National Natural Science Foundation of China (61673391, 61473221, 81600919), and the Beijing Natural Science Foundation (7164302).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patients’ legal guardians and the normal control subjects in the study.
Rights and permissions
About this article
Cite this article
Wang, L., Yang, Y., Chen, S. et al. White matter integrity correlates with residual consciousness in patients with severe brain injury. Brain Imaging and Behavior 12, 1669–1677 (2018). https://doi.org/10.1007/s11682-018-9832-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9832-1