Abstract
Early-onset Parkinson’s disease (EOPD) has a clinical course and characteristics distinct from middle-late-onset Parkinson’s disease (M-LOPD). Although many studies have investigated these differences, the neural mechanisms of these characteristics remain unclear. This study aimed to investigate the morphological differences, and their related clinical significance, between EOPD and M-LOPD patients. We recruited two groups of patients, 28 EOPD patients and 37 M-LOPD patients, and two age- and sex-matched control groups (23 controls in each group). The voxel-based morphometry (VBM) technique was used to examine changes in gray matter (GM) density between patients and their corresponding controls. Compared with controls, EOPD patients had lower GM density in the left putamen, inferior frontal gyrus and insula, and higher GM density in the right occipital lobe and bilateral cerebellum posterior lobes. M-LOPD patients had lower GM density in the left cerebellum posterior lobe, occipital lobe and right supplementary motor area (SMA), and higher GM density in the left middle temporal gyrus. Correlation analyses showed that GM density values in the right cerebellum posterior lobe positively correlated with the Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores and the Hoehn–Yahr stages in EOPD patients. Our results reveal different patterns of structural changes in EOPD and M-LOPD patients. A probable compensatory effect of the cerebellum was observed and may partly explain the slower decline of motor function in EOPD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a chronic neurodegenerative disorder with onset usually between 50 and 75 years of age, with an average age of 60 years (Tsai et al. 2002; Quinn et al. 1987; Tanner and Aston 2000). However, there is a subgroup of PD, early-onset Parkinson’s disease (EOPD), in which patients present with a parkinsonian syndrome before the age of 50 years (Quinn et al. 1987; Schrag and Schott 2006). The clinical evolution and characteristics of EOPD are somewhat different from those of middle-late-onset Parkinson’s disease (M-LOPD). For example, compared with M-LOPD, EOPD patients tend to have a slower disease progression (particularly with regard to falls, freezing and gait disturbances) and later development of cognitive impairment, but often have greater incidences of motor complications (such as dyskinesias, dystonia and motor fluctuations) (Schrag and Schott 2006; Alves et al. 2005; Mehanna et al. 2014). Moreover, despite the slower disease progression, EOPD patients often suffer from a poorer quality of life, with significantly higher depression scores than those of M-LOPD patients with similar disease severity (Mehanna et al. 2014; Knipe et al. 2011).
A number of studies have found that onset-of-age related brain changes exist in EOPD patients, and are possibly related to the distinct clinical characteristics of these patients. A pathological study showed a greater degree of nigral-cell loss in EOPD patients compared with M-LOPD patients, although no differences in basic Lewy body pathology were observed (Gibb and Lees 1988). Molecular studies have found greater dopamine density loss in EOPD patients, along with a slower rate of loss, which might partly explain the slower disease progression in these patients (Shih et al. 2007; de la Fuente-Fernandez et al. 2011). Additionally, a probable differential iron deposition pattern, relative to M-LOPD patients, was detected in EOPD patients in a magnetic resonance imaging (MRI) study (Bartzokis et al. 1999). Moreover, recent functional MRI studies revealed that different disease severities correlated with brain functional alterations in EOPD and LOPD patients (Sheng et al. 2016; Hou et al. 2016). Overall, this suggests that brain metabolism and function in EOPD might be different to that in M-LOPD patients, but little is known about the different structural changes in the brains of these two groups.
Therefore, the purpose of this study was to investigate, using the voxel-based morphometry (VBM) technique, whether there are morphological differences in various brain areas in EOPD and M-LOPD patients. This may provide some clues to help explain the different clinical manifestations of each of these forms of PD.
Materials and methods
Participants
Sixty-five right-handed patients with PD, according to the UK Parkinson’s Disease Brain Bank criteria, were recruited from the Department of Neurology, Second Affiliated Hospital of Zhejiang University. To study the effect of age at onset, PD patients were categorized into a M-LOPD group (onset >50 years, 37 patients) or an EOPD group (onset ≤50 years, 28 patients). In addition, 23 right-handed healthy controls were recruited from the community as a “Young” control group (YC) (age- and sex-matched to EOPD), and another 23 right-handed controls were recruited to an “Old” control group (OC) (age and sex-matched to M-LOPD). All participants with evidence of other neuropsychiatric diseases or brain trauma were excluded. The study was approved by the Medical Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine, and all participants provided informed consent at the beginning of the study.
Thirty-four patients in our study had already received medical treatment for PD (13 EOPD group, and 21 in M-LOPD group), and the anti-parkinsonian medicine was terminated at least 12 h prior to the clinical evaluation and imaging, to make the patients in the OFF-state. Scores obtained from the Unified Parkinson’s Disease Rating Scale (UPDRS) and Hoehn and Yahr (H&Y) Stage were assessed for all patients prior to scanning. And the Daily Levodopa Equivalents Does (DLED) of the medicated patients were also calculated (Tomlinson et al. 2010). Table 1 shows the clinical details of all participants.
Image acquisition and preprocessing
All data were acquired using a 3.0 T MRI scanner (GE Medical Systems, Signa EXCITE). Earplugs and foam pads were used to reduce noise and head motion. Three-dimensional axial Fast Spoiled Gradient Recalled (3D–FSGPR) images were collected with the following parameters: whole brain; TR/TE = 5.1 ms/1.2 ms; FOV = 24× 24 cm2; matrix = 256× 256; slices = 124; thickness = 1.2 mm; and space = 0 mm.
Image preprocessing was conducted using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm) implemented in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). The images were segmented into GM, white matter (WM) and cerebrospinal fluid (CSF). Then a high-dimensional DARTEL normalization protocol was applied to the GM maps, following John Ashburner’s method (Ashburner 2007). A Montreal Neurological Institute (MNI) space transformation was included using a Gaussian smoothing kernel of 6-mm full width at half-maximum.
Statistical analysis
Statistical analysis was carried out using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA). To test the clinical differences of between the EOPD and M-LOPD groups, two-sample t tests were performed for age, disease duration, DLED, UPDRS motor scores and H&Y stages. A Chi-square test was used to compare differences in sex between the two groups. Two-sample t test and Chi-square test were also conducted to test the age and sex differences, respectively, between patients and their corresponding controls. The significance threshold was set at p < 0.05.
GM maps were compared between groups of patients and their corresponding controls using a two-sample t test (p < 0.001 and cluster size >100 voxels, uncorrected) in SPM8. Age and sex were used as the main covariates. Regions showing significant differences were further extracted and the mean GM density values were calculated by using the Marsbar toolbox (http://marsbar.sourceforge.net/). Partial correlation analyses were then performed between the GM density values from each of the significant clusters and the UPDRS motor scores, H&Y stages in patient groups, controlling for age and sex. Here, as a preliminary and exploratory study, we did not perform multiple correction in correlation analyses.
Results
Clinical characteristics
No significant differences were observed in age (EOPD vs. YC: 49.6 ± 5.9 years vs. 51.3 ± 6.3 years, P = 0.318; M-LOPD vs. OC: 62.4 ± 5.9 years vs. 64.2 ± 6.7 years, P = 0.282) or sex (EOPD vs. YC: 15/13 vs. 12/11, P = 0.922; M-LOPD vs. OC: 18/19 vs. 12/11, P = 0.792) between patients and their respective control groups. Between the EOPD and M-LOPD groups, no significant differences were found in sex (15/13 vs. 18/19, P = 0.696), disease duration (4.5 ± 3.6 years vs. 4.1 ± 3.1 years, P = 0.620), DLED (203.8 ± 265.9 mg vs. 296.7 ± 332.1 mg, P = 0.229), UPDRS motor scores (24.0 ± 15.5 vs. 29.3 ± 14.6, P = 0.161). H&Y stage was lower in the EOPD group (2.1 ± 0.7 vs. 2.5 ± 0.6, P = 0.004; Table 1).
GM density differences
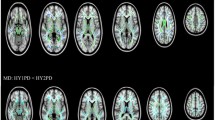
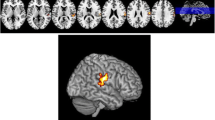
Compared with YC, EOPD patients had reduced GM density in the left putamen, inferior frontal gyrus and insula, and increased GM density in the right occipital lobe and bilateral cerebellum posterior lobes (Fig. 1; Table 2). M-LOPD patients, when compared with OC, had a different pattern with reduced GM density in the left cerebellum posterior lobe, left occipital lobe and right supplementary motor area (SMA), and increased GM density in the left middle temporal gyrus (Fig. 2; Table 2).
Correlation analysis
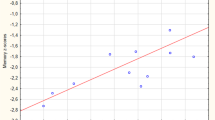
Partial correlation analyses showed that the GM density of the right cerebellum posterior lobe had significantly increased GM density values in EOPD patients, and this positively correlated with UPDRS motor scores (r = 0.431, p = 0.028) and H&Y stage (r = 0.453, p = 0.020; Fig. 3).
No correlations were found between clinical parameters and GM density values in other structurally altered areas in either EOPD or M-LOPD patients.
Discussion
Different patterns of structural changes were observed in the EOPD and M-LOPD patients in our study. The M-LOPD patients had GM loss in the cerebellar, occipital and SMA regions, and higher GM density in the temporal regions. The EOPD patients had lower GM density in the putamen, inferior frontal gyrus and insula, and higher GM density in the cerebellar and occipital regions. In addition, the GM density values of the right cerebellum posterior lobe positively correlated with UPDRS motor scores and H&Y stage in EOPD patients.
In EOPD patients, we observed GM loss in the putamen, inferior frontal gyrus and insula. The reduced GM density in the putamen fits with the pathophysiological basis of PD (Owen et al. 1998), and maybe attributed to reduced dopaminergic innervation in the nucleus (Lee et al. 2014). Recent studies have indicated that there was more prominent impairment in the putamen in EOPD relative to LOPD patients (Sheng et al. 2016; Liu et al. 2015). This may explain why GM loss in the putamen was found in EOPD but not in LOPD patients in our study. It has been reported that GM loss in the inferior frontal gyrus and insula is related to depression in PD patients (Reijnders et al. 2010; Kostic et al. 2010). As EOPD patients have a higher incidence of depression (Mehanna et al. 2014; Knipe et al. 2011), we speculate that the GM loss in these regions might be partly responsible for this.
The cerebellum has been reported to influence motor functions in PD patients (Wu and Hallett 2013). Our finding of cerebellar GM loss in M-LOPD patients is in line with previous morphological studies (Borghammer et al. 2010; Benninger et al. 2009; Camicioli et al. 2009), which probably implicates the cerebellum in the pathology of PD. However, increasing evidence suggests that the cerebellum may play a compensatory role in PD patients (Wu and Hallett 2013; Yu et al. 2007; Wu et al. 2010; Lewis et al. 2007). For example, functional neuroimaging studies have demonstrated that patients with PD have increased activation in the cerebellum during motor learning and execution processes, maintaining their performance at a near normal level (Yu et al. 2007; Catalan et al. 1999; Wu and Hallett 2008; Mentis et al. 2003). A recent morphometric study detected a cerebellar GM increase with behavioral improvements after balance training in PD patients (Sehm et al. 2014), which seemed to further prompt the cerebellar compensatory effect for the motor impairment. The EOPD patients have a relatively slower progression of the disease severity (Schrag and Schott 2006), which was also suggested by our data that with similar durations, the EOPD patients had a lower H&Y stage than the M-LOPD patients. In the present study, the GM density in the cerebellum was increased as the motor function declined (represented by UPDRS motor scores and H&Y stage) in EOPD patients. We speculated that the increased cerebellar GM density may be a result of continuous compensatory efforts for the decline of the motor function (Hufner et al. 2011) in EOPD patients, and thus, making the motor impairment progressed more slowly in this cohort of patients. In addition, The cerebellum is recognized as being important for the coordination of voluntary movement, gait, posture and motor function (Wu and Hallett 2013). This may explain why EOPD patients, with higher cerebellar GM density, have a slower progression of motor symptoms particularly like falls, freezing and gait disturbances (Schrag and Schott 2006).
The occipital lobe has been reported to play a role in the visual process (DeYoe et al. 1996), and is associated with visual hallucinations in PD patients (Ramirez-Ruiz et al. 2007). Lee et al. revealed that occipital areas are associated with the performance of “frontal lobe” functional tasks, indicating that the occipital lobe may compensate for primary fronto-striatal pathology in PD patients (Lee et al. 2013). Correspondingly, increased GM density in this area in EOPD patients might also indicate an compensatory effect. On the contrary, we observed decreased GM density in M-LOPD patients, and the GM loss found in the occipital lobe could contribute to cognitive impairment in PD patients (Hwang et al. 2013; Zhang et al. 2015; Garcia-Diaz et al. 2014). It has been reported that EOPD patients often experience a later development of cognitive impairment relative to M-LOPD patients (Schrag and Schott 2006). Taken together, the different changes within the occipital lobes in these two PD groups probably contribute to the distinct differences in the development of cognitive changes.
In M-LOPD patients, we also observed that GM loss occurs in the SMA. The SMA plays a key role in the generation of self-initiated, multi-segmental voluntary movements (Nachev et al. 2008). It was reported that the reduced activity of SMA in PD might be due to the decreased positive efferent feedback arising from the basal ganglia–thalamocortical motor loop, and some PD symptoms, such as bradykinesia, might be related to abnormalities in SMA (Eggers et al. 2015; Hamada et al. 2009). A structural MR study found a relationship between SMA GM loss and impaired working memory in PD patients (Biundo et al. 2011). Therefore, based on the early development of cognitive impairment and faster motor decline in M-LOPD patients (Schrag and Schott 2006; Alves et al. 2005; Mehanna et al. 2014), GM loss in the SMA might contribute to this pattern, which was not seen in EOPD patients. In addition, we did find increased GM density in the middle temporal gyrus in M-LOPD patients. This area is involved in visual space processing and the observed atrophy is likely to correlate with cognitive impairment in PD patients (Tam et al. 2005; Song et al. 2011). However, increased GM density in the middle temporal gyrus has not been reported previously, and the role of this should be further investigated in the future.
There was a limitation in our study, in that the EOPD group had a lower H&Y stage than the M-LOPD patients. EOPD patients tend to have a slower disease progression (Alves et al. 2005), probably relating to a lower H&Y stage relative to M-LOPD of a similar duration. This is a clinical characteristic of EOPD, but it may also influence the differences in GM density changes between the EOPD and M-LOPD groups.
In summary, we found different patterns of structural changes in EOPD and M-LOPD patients. Remarkably, the increased cerebellar GM density in EOPD patients probably highlighted a compensatory role of the cerebellum in slowing the motor decline in this cohort of patients.
References
Alves, G., Wentzel-Larsen, T., Aarsland, D., & Larsen, J. P. (2005). Progression of motor impairment and disability in Parkinson disease: A population-based study. Neurology, 65(9), 1436–1441. doi:10.1212/01.wnl.0000183359.50822.f2.
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. doi:10.1016/j.neuroimage.2007.07.007.
Bartzokis, G., Cummings, J. L., Markham, C. H., Marmarelis, P. Z., Treciokas, L. J., Tishler, T. A., Marder, S. R., & Mintz, J. (1999). MRI evaluation of brain iron in earlier- and later-onset Parkinson’s disease and normal subjects. Magnetic Resonance Imaging, 17(2), 213–222.
Benninger, D. H., Thees, S., Kollias, S. S., Bassetti, C. L., & Waldvogel, D. (2009). Morphological differences in Parkinson's disease with and without rest tremor. Journal of Neurology, 256(2), 256–263. doi:10.1007/s00415-009-0092-2.
Biundo, R., Formento-Dojot, P., Facchini, S., Vallelunga, A., Ghezzo, L., Foscolo, L., Meneghello, F., & Antonini, A. (2011). Brain volume changes in Parkinson's disease and their relationship with cognitive and behavioural abnormalities. Journal of the Neurological Sciences, 310(1–2), 64–69. doi:10.1016/j.jns.2011.08.001.
Borghammer, P., Ostergaard, K., Cumming, P., Gjedde, A., Rodell, A., Hall, N., & Chakravarty, M. M. (2010). A deformation-based morphometry study of patients with early-stage Parkinson's disease. European Journal of Neurology, 17(2), 314–320. doi:10.1111/j.1468-1331.2009.02807.x.
Camicioli, R., Gee, M., Bouchard, T. P., Fisher, N. J., Hanstock, C. C., Emery, D. J., & Martin, W. R. (2009). Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism & Related Disorders, 15(3), 187–195. doi:10.1016/j.parkreldis.2008.05.002.
Catalan, M. J., Ishii, K., Honda, M., Samii, A., & Hallett, M. (1999). A PET study of sequential finger movements of varying length in patients with Parkinson's disease. Brain : A Journal of Neurology, 122(Pt 3), 483–495.
DeYoe, E. A., Carman, G. J., Bandettini, P., Glickman, S., Wieser, J., Cox, R., Miller, D., & Neitz, J. (1996). Mapping striate and extrastriate visual areas in human cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America, 93(6), 2382–2386.
Eggers, C., Gunther, M., Rothwell, J., Timmermann, L., & Ruge, D. (2015). Theta burst stimulation over the supplementary motor area in Parkinson's disease. Journal of Neurology, 262(2), 357–364. doi:10.1007/s00415-014-7572-8.
de la Fuente-Fernandez, R., Schulzer, M., Kuramoto, L., Cragg, J., Ramachandiran, N., Au, W. L., Mak, E., McKenzie, J., McCormick, S., Sossi, V., Ruth, T. J., Lee, C. S., Calne, D. B., & Stoessl, A. J. (2011). Age-specific progression of nigrostriatal dysfunction in Parkinson's disease. Annals of Neurology, 69(5), 803–810. doi:10.1002/ana.22284.
Garcia-Diaz, A. I., Segura, B., Baggio, H. C., Marti, M. J., Valldeoriola, F., Compta, Y., Vendrell, P., Bargallo, N., Tolosa, E., & Junque, C. (2014). Structural MRI correlates of the MMSE and pentagon copying test in Parkinson's disease. Parkinsonism & Related Disorders, 20(12), 1405–1410. doi:10.1016/j.parkreldis.2014.10.014.
Gibb, W. R., & Lees, A. J. (1988). A comparison of clinical and pathological features of young- and old-onset Parkinson's disease. Neurology, 38(9), 1402–1406.
Hamada, M., Ugawa, Y., & Tsuji, S. (2009). High-frequency rTMS over the supplementary motor area improves bradykinesia in Parkinson's disease: Subanalysis of double-blind sham-controlled study. Journal of the Neurological Sciences, 287(1–2), 143–146. doi:10.1016/j.jns.2009.08.007.
Hou, Y., Yang, J., Luo, C., Ou, R., Song, W., Liu, W., Gong, Q., & Shang, H. (2016). Patterns of striatal functional connectivity differ in early and late onset Parkinson's disease. Journal of Neurology. doi:10.1007/s00415-016-8211-3.
Hufner, K., Stephan, T., Flanagin, V. L., Deutschlander, A., Dera, T., Karch, C., Linn, J., Glasauer, S., Dieterich, M., Strupp, M., & Brandt, T. (2011). Cerebellar and visual gray matter brain volume increases in congenital nystagmus. Frontiers in Neurology, 2, 60. doi:10.3389/fneur.2011.00060.
Hwang, K. S., Beyer, M. K., Green, A. E., Chung, C., Thompson, P. M., Janvin, C., Larsen, J. P., Aarsland, D., & Apostolova, L. G. (2013). Mapping cortical atrophy in Parkinson's disease patients with dementia. Journal of Parkinson’s disease, 3(1), 69–76. doi:10.3233/JPD-120151.
Knipe, M. D., Wickremaratchi, M. M., Wyatt-Haines, E., Morris, H. R., & Ben-Shlomo, Y. (2011). Quality of life in young- compared with late-onset Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society, 26(11), 2011–2018. doi:10.1002/mds.23763.
Kostic, V. S., Agosta, F., Petrovic, I., Galantucci, S., Spica, V., Jecmenica-Lukic, M., & Filippi, M. (2010). Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology, 75(10), 857–863. doi:10.1212/WNL.0b013e3181f11c1d.
Lee, E. Y., Sen, S., Eslinger, P. J., Wagner, D., Shaffer, M. L., Kong, L., Lewis, M. M., Du, G., & Huang, X. (2013). Early cortical gray matter loss and cognitive correlates in non-demented Parkinson's patients. Parkinsonism & Related Disorders, 19(12), 1088–1093. doi:10.1016/j.parkreldis.2013.07.018.
Lee, H. M., Kwon, K. Y., Kim, M. J., Jang, J. W., Suh, S. I., Koh, S. B., & Kim, J. H. (2014). Subcortical grey matter changes in untreated, early stage Parkinson's disease without dementia. Parkinsonism & Related Disorders, 20(6), 622–626.
Lewis, M. M., Slagle, C. G., Smith, A. B., Truong, Y., Bai, P., McKeown, M. J., Mailman, R. B., Belger, A., & Huang, X. (2007). Task specific influences of Parkinson's disease on the striato-thalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience, 147(1), 224–235. doi:10.1016/j.neuroscience.2007.04.006.
Liu, S. Y., Wu, J. J., Zhao, J., Huang, S. F., Wang, Y. X., Ge, J. J., Wu, P., Zuo, C. T., Ding, Z. T., & Wang, J. (2015). Onset-related subtypes of Parkinson's disease differ in the patterns of striatal dopaminergic dysfunction: A positron emission tomography study. Parkinsonism & Related Disorders, 21(12), 1448–1453. doi:10.1016/j.parkreldis.2015.10.017.
Mehanna, R., Moore, S., Hou, J. G., Sarwar, A. I., & Lai, E. C. (2014). Comparing clinical features of young onset, middle onset and late onset Parkinson's disease. Parkinsonism & Related Disorders, 20(5), 530–534. doi:10.1016/j.parkreldis.2014.02.013.
Mentis, M. J., Dhawan, V., Nakamura, T., Ghilardi, M. F., Feigin, A., Edwards, C., Ghez, C., & Eidelberg, D. (2003). Enhancement of brain activation during trial-and-error sequence learning in early PD. Neurology, 60(4), 612–619.
Nachev, P., Kennard, C., & Husain, M. (2008). Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience, 9(11), 856–869. doi:10.1038/nrn2478.
Owen, A. M., Doyon, J., Dagher, A., Sadikot, A., & Evans, A. C. (1998). Abnormal basal ganglia outflow in Parkinson's disease identified with PET. Implications for higher cortical functions. Brain, 121(Pt 5), 949–965.
Quinn, N., Critchley, P., & Marsden, C. D. (1987). Young onset Parkinson's disease. Movement Disorders : Official Journal of the Movement Disorder Society, 2(2), 73–91. doi:10.1002/mds.870020201.
Ramirez-Ruiz, B., Marti, M. J., Tolosa, E., Gimenez, M., Bargallo, N., Valldeoriola, F., & Junque, C. (2007). Cerebral atrophy in Parkinson's disease patients with visual hallucinations. European Journal of Neurology, 14(7), 750–756. doi:10.1111/j.1468-1331.2007.01768.x.
Reijnders, J. S., Scholtissen, B., Weber, W. E., Aalten, P., Verhey, F. R., & Leentjens, A. F. (2010). Neuroanatomical correlates of apathy in Parkinson's disease: A magnetic resonance imaging study using voxel-based morphometry. Movement Disorders : Official Journal of the Movement Disorder Society, 25(14), 2318–2325. doi:10.1002/mds.23268.
Schrag, A., & Schott, J. M. (2006). Epidemiological, clinical, and genetic characteristics of early-onset parkinsonism. The Lancet Neurology, 5(4), 355–363. doi:10.1016/S1474-4422(06)70411-2.
Sehm, B., Taubert, M., Conde, V., Weise, D., Classen, J., Dukart, J., Draganski, B., Villringer, A., & Ragert, P. (2014). Structural brain plasticity in Parkinson's disease induced by balance training. Neurobiology of Aging, 35(1), 232–239. doi:10.1016/j.neurobiolaging.2013.06.021.
Sheng, K., Fang, W., Zhu, Y., Shuai, G., Zou, D., Su, M., Han, Y., & Cheng, O. (2016). Different alterations of cerebral regional homogeneity in early-onset and late-onset Parkinson's disease. Frontiers in Aging Neuroscience, 8, 165. doi:10.3389/fnagi.2016.00165.
Shih, M. C., Franco de Andrade, L. A., Amaro Jr., E., Felicio, A. C., Ferraz, H. B., Wagner, J., Hoexter, M. Q., Lin, L. F., Fu, Y. K., Mari, J. J., Tufik, S., & Bressan, R. A. (2007). Higher nigrostriatal dopamine neuron loss in early than late onset Parkinson's disease?--a [99mTc]-TRODAT-1 SPECT study. Movement Disorders : Official Journal of the Movement Disorder Society, 22(6), 863–866. doi:10.1002/mds.21315.
Song, S. K., Lee, J. E., Park, H. J., Sohn, Y. H., Lee, J. D., & Lee, P. H. (2011). The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Movement Disorders : Official Journal of the Movement Disorder Society, 26(2), 289–296. doi:10.1002/mds.23477.
Tam, C. W., Burton, E. J., McKeith, I. G., Burn, D. J., & O'Brien, J. T. (2005). Temporal lobe atrophy on MRI in Parkinson disease with dementia: A comparison with Alzheimer disease and dementia with Lewy bodies. Neurology, 64(5), 861–865. doi:10.1212/01.WNL.0000153070.82309.D4.
Tanner, C. M., & Aston, D. A. (2000). Epidemiology of Parkinson's disease and akinetic syndromes. Current Opinion in Neurology, 13(4), 427–430.
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., & Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Movement Disorders : Official journal of the Movement Disorder Society, 25(15), 2649–2653. doi:10.1002/mds.23429.
Tsai, C. H., Lo, S. K., See, L. C., Chen, H. Z., Chen, R. S., Weng, Y. H., Chang, F. C., & Lu, C. S. (2002). Environmental risk factors of young onset Parkinson's disease: A case-control study. Clinical Neurology and Neurosurgery, 104(4), 328–333.
Wu, T., & Hallett, M. (2008). Neural correlates of dual task performance in patients with Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 79(7), 760–766. doi:10.1136/jnnp.2007.126599.
Wu, T., & Hallett, M. (2013). The cerebellum in Parkinson's disease. Brain : A Journal of Neurology, 136(Pt 3), 696–709. doi:10.1093/brain/aws360.
Wu, T., Wang, L., Hallett, M., Li, K., & Chan, P. (2010). Neural correlates of bimanual anti-phase and in-phase movements in Parkinson's disease. Brain : A Journal of Neurology, 133(Pt 8), 2394–2409. doi:10.1093/brain/awq151.
Yu, H., Sternad, D., Corcos, D. M., & Vaillancourt, D. E. (2007). Role of hyperactive cerebellum and motor cortex in Parkinson's disease. NeuroImage, 35(1), 222–233. doi:10.1016/j.neuroimage.2006.11.047.
Zhang, J., Zhang, Y. T., Hu, W. D., Li, L., Liu, G. Y., & Bai, Y. P. (2015). Gray matter atrophy in patients with Parkinson's disease and those with mild cognitive impairment: A voxel-based morphometry study. International Journal of Clinical and Experimental Medicine, 8(9), 15383–15392.
Acknowledgements
We wish to thank all the Parkinson’s disease patients and normal controls who participated in our research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest to report.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
This study was funded by the 12th Five-year Plan for National Science and Technology Supporting Program of China (Grant No. 2012BAI10B04) and the National Natural Science Foundation of China (Grant Nos. 81571654, 81371519 and 81301190).
Rights and permissions
About this article
Cite this article
Xuan, M., Guan, X., Huang, P. et al. Different patterns of gray matter density in early- and middle-late-onset Parkinson’s disease: a voxel-based morphometry study. Brain Imaging and Behavior 13, 172–179 (2019). https://doi.org/10.1007/s11682-017-9745-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9745-4