Abstract
MRI is a powerful tool to evaluate brain anatomy and function in normal children and its use in research applications has steadily increased. As imaging technology improves, and sensitivity to brain pathology increases, unanticipated (and potentially clinically important) findings on research neuroimaging studies will also increase. We evaluated the prevalence and type of unanticipated and potentially clinically significant imaging findings in a group of 114 normal children enrolled in an ongoing MRI imaging study of normal brain development for the Pediatric Functional Neuroimaging Research Network. Brain imaging findings were classified using standardized scales developed for the Network and findings were reported to participants and their primary healthcare provider according to a standard reporting pathway. Classification scales, reporting processes, and illustrated examples of findings are included and discussed. Unanticipated imaging findings were identified in approximately 12.5 % of children participating in this study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
MRI is a powerful tool to evaluate brain anatomy and function in children and has resulted in improved understanding of many disease states as well as normal patterns of brain development. Estimates for the number of neuroimaging research subjects per year are in the tens of thousands, with this number expected to continue to increase. (Illes et al. 2006). Neuroimaging research studies commonly enroll healthy participants as controls (which are compared to participants with known neurologic disorders) or to study normal brain development. Motives for joining research studies are variable and likely differ between individuals and socioeconomic groups. These include financial compensation, individual curiosity, and the desire to further scientific research (Kirschen et al. 2006). Parents enrolling pediatric participants in MRI research studies likely have additional motives for enrollment, including concern for a health problem in their child, or personal interest in their child’s brain development.
As imaging technology improves, and sensitivity to brain pathology increases, unanticipated (and potentially clinically important) findings on research neuroimaging studies may also increase. There is continuous development of imaging techniques at higher field strengths (3 T and 7 T) with use of expanded imaging sequences that are increasingly sensitive to potential brain abnormalities. Yet few studies have evaluated the prevalence and type of unexpected findings in neuroimaging research (Weber and Knopf 2006; Potchen et al. 2013; Katzman et al. 1999), and even fewer have explored the rate of such findings in a neurologically normal pediatric population (Kim et al. 2002). Previous estimates are that approximately 8 % of neurologically normal participants may have potentially significant findings on neuroimaging research studies (Kim et al. 2002). As pointed out by Brown and Hasso (Brown and Hasso 2008) “The question for the neuroimaging researcher is not whether asymptomatic pathologies exist in their study population, but rather how many will be found.” With such a high prevalence of potentially abnormal findings, concerns have arisen as to how to effectively handle unanticipated findings, and how to communicate findings to research participants. Certainly, the rate of unanticipated findings concerns future neuroimagers who desire to characterize normal pediatric brain anatomy. The qualifications of those evaluating pediatric neuroimaging obtained for research purposes should also be considered. In one study, 84 % of participants in MRI research studies surveyed indicated that they did not expect research imaging to be reviewed by a radiologist (Illes et al. 2004a, b). However, the majority of the same participants indicated that if a brain abnormality was detected they would expect the finding to be communicated. (Illes et al. 2004a, b).
Effective methods for detecting and disclosing unexpected imaging findings have been discussed previously in an ethics panel conference at the National Institutes of Health and recommendations for neuroimaging investigators were established (Illes et al. 2006). Neuroimaging researchers should anticipate unexpected findings, arrange for qualified review of images, and communicate findings to participants in a timely way. Finally, the NIH panel recommended that IRB protocols that include neuroimaging should address incidental findings and explain a transparent method for reporting these findings. Although progressive, this conference failed to yield a consensus on a uniform method to disclose unanticipated findings, nor did it make any recommendations on the required experience level or training of those performing the imaging review.
The current study examines the prevalence and spectrum of unexpected findings in an MRI research study of normal brain development in children, and proposes a useful classification system for these findings as well as a robust pathway for participant notification and clinical follow-up. The roles of the scientists and physicians involved in disclosing unexpected findings in neuroimaging research is reviewed. Ethical issues relating to parent/legal guardian permission and participant assent as well as expectations for disclosure in this vulnerable population are examined. Questions to be answered include: What is the significance of findings that cannot be correlated with clinical symptoms, especially to parents of developing children? Who should assume the responsibility for interpreting research imaging and communicating unanticipated findings?
Methods
Neuroimaging data was obtained from an ongoing MRI imaging study of normal brain development led by the Pediatric Functional Imaging Research Network. This project, supported by a contract from NICHD (HHSN275200900018C) has the goal of constructing a database containing neuroimaging and neurobehavioral data from 200 normally developing, healthy children ranging in age from birth to 18 years. For inclusion in the study, subjects and first degree relatives had a negative history for neurologic or psychiatric disease, were term infants (37 weeks < Gestational Age < 42 weeks), had body weights in normal range (10 % < Body Weight < 90 %), and had a normal neurologic exam. Informed permission of a parent or guardian of children between 0 and 17 years of age was obtained. Assent of children between 5 and 10 was verbally confirmed. Assent of participants aged 11 and up was obtained in writing.
MR Imaging was performed on a 3 Tesla MRI scanner (Philips Achieva; Best, The Netherlands). Study imaging protocol included Arterial Spin Labeling (ASL) and Blood Oxygen Level Dependent (BOLD) fMRI datasets using active and passive language tasks as well as resting state paradigms. Diffusion Tensor Imaging (DTI) and High Angular Resolution Diffusion Imaging (HARDI) datasets were also created. Anatomic imaging included 3D datasets with 1 mm isotropic resolution using both T1-MPRAGE and T2-FLAIR sequences. Multiplanar reformatted images were reviewed (Merge PACS, Chicago, IL, U.S.A.). All anatomic imaging was reviewed by a board certified neuroradiologist (JLL) with 20 years’ experience interpreting MRI (7 years primarily pediatric practice) who was a member of the study protocol, and supported by the study NIH contract. Imaging data was stored in a secure searchable online database for data monitoring, and eventual use by authorized study researchers. Imaging and behavioral data from this study are now available to scientific users through an NIH monitored registration process at: https://research.cchmc.org/c-mind/.
To standardize the radiologic review of the anatomical images, we developed a study classification system. The classification system is designed to: 1. Identify pathology that could interfere with analyses that require strictly normative data, and 2. Provide a clear framework for identifying those subjects that may have medically important abnormalities. We defined three image classifiers: A. Imaging classification – a parameter that classifies potential abnormalities on a scale of 0–4, described below; B. Anatomical Distortion - a binary (0/1) scale that indicates whether the pathology detected in the image causes any significant distortion of the geometry within the brain (e.g. displacement by a mass, or enlarged ventricles); and C. Follow-Up Required - a binary (0/1) scale that indicates whether any clinical follow-up is needed (0 – no, 1 – yes). Detailed descriptions for the numerical scales are as follows:
-
A. Imaging classification (0–4):
-
0 - Normal: No abnormalities or anatomic variations detected
-
1 - Normal anatomic variants: Anatomic variations that have no clinical significance.
-
2 - Potentially significant abnormality: Imaging findings out of the range of normal or normal anatomic variation, that require correlation with clinical findings to determine their true significance for the health of the subject (if any).
-
3 - Likely clinically significant abnormality: Imaging findings out of the range of normal or normal anatomic variation that have a high likelihood of clinical significance requiring clinical and/or imaging follow-up.
-
4 - Imaging markedly degraded by artifact: No interpretation possible. Imaging is so limited that no confident evaluation as to clinically significant abnormalities is possible.
-
-
B. Anatomic distortion (0/1):
-
0 - No: No significant distortion of normal anatomy on the T1 weighted images.
-
1 - Yes: Possibly significant anatomic distortion on the T1 weighted images.
-
-
C. Follow-up Required (0/1):
-
0 - No: Imaging findings do not warrant follow up with family or primary care provider.
-
1 - Yes: Clinical correlation with symptoms and/or potential imaging follow-up is appropriate given the possible significance of the initial findings on the research scans.
-
All imaging findings were classified by the study neuroradiologist using these scales as formatted in Table 1.
Examples of normal anatomic variants [Class 1] might include prominent cisterna magna, cavum septum pellucidum, cerebellar tonsillar ectopia <5 mm without CSF effacement, or slight ventricular asymmetry. Possibly clinically significant findings [Class 2] might include small regions of non-specific white matter signal, cerebellar tonsillar ectopia >5 mm or with CSF effacement, callosal anomalies, or significant paranasal sinus opacification. Likely clinically significant findings [Class 3] might include mass lesions, encephalomalacia, hydrocephalus, or aneurysm. This classification scheme identifies participants with completely normal images and those with clinically insignificant anatomic variants in order to allow researchers the option of including each group in subsequent data analysis (Tables 2 and 3).
Images were also classified with a binary scale according to the presence [1] or absence [0] of Anatomic Distortion (defined as an imaging finding producing pathologic distortion of normal anatomy). Examples of findings that produce anatomical distortion might include: Callosal hypogenesis, visible malformation of cortical development, heterotopic gray matter, significant or localized ventricular enlargement or asymmetry, significant localized volume loss, encephalomalacia, or significant white matter signal changes visible on T1 or with associated ventricular or gyral changes. Cases were classified separately regarding anatomic distortion because cases classified as normal variants on the Imaging Classification scale could, in theory, be unsuitable for inclusion depending upon the goal of the planned research. Additionally, cases with significant extracranial findings could still be useable for research purposes (no anatomic brain distortion) depending upon the goal of the planned research. For example, Class 1 or 2 images that exhibit small areas of FLAIR signal abnormality not producing anatomic distortion or clear abnormality on review of T1-weighted images, or with normal variants in posterior fossa CSF spaces could be rated as [0] on the Anatomical Distortion scale and might be included in subsequent analysis as appropriate to the study aims.
Finally, images classified by the study radiologist as [1] on the Follow-Up Required scale require that the PI will follow the specified referral pathway to begin the follow-up process. By definition, a study with an imaging classification of 2 or 3 requires PI notification.
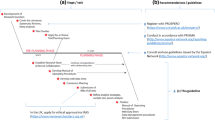
In addition to the standardized image review process for research brain images, a process was established for reporting the findings to the participants through their primary healthcare provider and confirming the follow-up with research participants. The likelihood of an incidental finding and associated follow-up procedures are discussed during the informed consent process, supported by an IRB-approved protocol and permission/assent forms that lay out the plan for handling unanticipated findings transparently. Key steps in this process are as follows:
-
1)
Radiologist reviews all anatomic brain imaging scans in a timely fashion after notification by a study coordinator. All studies (including planned repeat and longitudinal studies) are reviewed and reported.
-
2)
If a potentially clinically significant abnormality is detected (Class 2 or 3), the radiologist notifies the principle investigator (PI) and/or study coordinator of the findings with a written summary and recommendations. (Fig. 1) If an urgent finding is identified, additional direct contact of the study coordinator / PI is made.
-
3)
PI or delegate contacts family primary healthcare provider (PCP) and explains subject’s participation and findings.
-
4)
The radiologist discusses interpretation with the PCP if desired.
-
5)
PCP will follow up with family regarding findings and any referrals needed in follow-up.
-
6)
PI will follow up with family and PCP in writing, 1–2 weeks later to insure that the above chain did not break at any point.
Image review datasheet used in this study for PI and PCP notification of abnormal findings. Case is the same as in Fig. 2a
Results
The methods and scales defined above have now been used for 3 years as part of the Pediatric Functional Neuroimaging Research Network protocols. Here we report the results of findings identified using these processes in a normally developing, healthy pediatric cohort of 114 subjects, ranging in age from 2 months to 18 years (mean 8.3 years); 67 female, 47 male. 114 subjects had 3D T1 images and 83/114 had 3D T2-FLAIR images acquired as part of the study. 98 subjects had completely normal [0] imaging or normal anatomic variants [1] not requiring any further evaluation (Fig. 2). 14 subjects (12.5 % of interpretable imaging) had either possibly clinically significant [2] (12) or likely clinically significant [3] (2) abnormalities that warranted reporting to the patient and primary care provider. The most common Class 2 and 3 findings were related to white matter (WM) abnormalities (6), congenital anomalies (4), and paranasal sinus findings (2).
Examples of normal variants identified on research MRI examinations of normal children. a. Sagittal T2 FLAIR. Tiny well-defined areas of increased white matter (WM) signal (arrows). By our criteria, Less than 3 tiny (<2 mm) foci of increased WM signal were not deemed clinically important for follow-up in this study. They are presumed to represent small areas of gliosis but have undetermined etiology. They did not have the morphology or distribution to suggest demyelinating or neoplastic disease. They did not produce geometric distortion of brain morphology. b. Sagittal T1. Pineal cyst (arrows). c. Axial T1. Cavum septum pellucidum, a common normal variant. d. Sagittal T2 FLAIR. Small fluid signal focus in the pituitary between the anterior adenohypophysis and posterior neurohypophysis compatible with a normal variant pars intermedia cyst
Regarding findings deemed normal variants in this study (Class 1, Fig. 2), one or two <2 mm foci of increased signal in the subcortical white matter were not deemed clinically important for follow-up and were classified as normal variants (n = 5). They are presumed to represent small areas of gliosis but have undetermined etiology. These foci did not have the morphology or distribution to suggest demyelinating or neoplastic disease. They did not produce anatomic distortion of brain morphology. (Fig. 2a.). Other normal variants (Fig. 2b-d) encountered were a cavum septum pellucidum (1), pineal cyst (2), pars intermedia cyst (1), prominent cisterna magna (1), and slight mammillary body asymmetry without other abnormality (1).
Class 2 findings (possibly significant) were those findings that in and of themselves might not be clinically significant, but require interpretation within a clinical context and /or performance of follow-up clinical MRI to determine their significance. Class 2 findings among our study cohort included: multiple (>2 by study criteria) areas of white matter signal (more well-defined and pronounced than normal hypomyelination) were seen in 6 patients, typically in the subcortical and posterior periventricular regions (Fig. 3a). Callosal hypogenesis was seen in 2 cases, one with an associated lipoma (Fig. 3c,d). Two subjects had tonsillar ectopia >5 mm (Fig. 3b). One subject had a region of encephalomalacia adjacent to the right caudate head, likely the result of an old infarct (Fig. 3f). When sinus opacification was marked, or when there were air-fluid levels, clinical notification was performed (Fig. 3e). Mild mucosal thickening incompletely opacifying the paranasal sinuses (without air fluid level) (4 subjects) and mild symmetric prominence of normal lymph nodes in the upper neck (4 subjects) was identified, but not deemed clinically important. Based upon clinical experience, mild degrees of mucosal thickening and mild neck lymph node prominence are common in asymptomatic children in our region of the USA. All of the Class 2 brain findings were asymptomatic.
Examples of imaging findings deemed possibly significant (Class 2). a. Sagittal T2 FLAIR. Patchy confluent areas of abnormal T2 signal more well-defined and pronounced than normal hypomyelination (arrows), were seen in 6 subjects, of undetermined etiology. See Discussion for further details. b. Sagittal T1. Tonsillar ectopia (7 mm) with mild crowding of CSF spaces at the foramen magnum. c. Sagittal T1. Callosal hypogenesis (arrow). d. Sagittal T1. Callosal hypogenesis and associated callosal lipoma (arrow). e. Sagittal T2 FLAIR. Complete sphenoid sinus opacification (arrow). When sinus opacification was marked, or when there were air-fluid levels, clinical notification was performed. f. Axial T1. Area of localized encephalomalacia along the lateral margin of the right caudate head (arrow)
Class 3 findings (likely significant) occurred in two instances, a small fourth ventricular mass and a large sinonasal polyp with significant sinus opacification (Fig. 4). Neither of these findings caused significant anatomic distortion of supratentorial brain structures. Class 2 and 3 findings were slightly more prevalent in males than females, but did not reach statistical significance (7 (15.2 %) males, 7 (10.6 %) females; p = 0.56, Fisher’s exact).
Examples of imaging findings deemed likely significant (Class 3). a. Sagittal T1 (left), Sagittal T2 FLAIR (right). Well defined mass within the inferior fourth ventricle (arrows) possibly a small ependymoma or subependymoma. Currently undergoing clinical MRI follow-up. b. Sagittal T1 (left), Sagittal T2FLAIR (right). A large polyp was identified in the posterior nasal cavity (arrows) with associated complete opacification of the sphenoid, maxillary and ethmoid sinuses
All potentially clinically significant findings were communicated to the participant’s primary care provider (PCP) who discussed the findings and potential clinical significance with the participant/family. The site principal investigator followed up in writing with the participants/families and PCP to ensure the findings were communicated.
Discussion
Our study demonstrated that 14/112 or 12.5 % of neurologically normal children undergoing standardized high resolution brain imaging as part of a research protocol had unanticipated findings which were potentially clinically significant. A well-defined process of evaluation, notification, and follow-up was devised as part of the study procedure. This adds to the literature regarding unanticipated and potentially clinically significant findings in this vulnerable research population, and points out the necessity for following a robust assessment and notification protocol. We also provide templates for classification of imaging findings and details of a process for review and follow-up of research brain images.
Prior studies in children assessing unanticipated findings on research MR examinations are few but have shown a similar prevalence and distribution of potentially clinically important findings. Kim et al. (Kim et al. 2002), evaluated 225 pediatric MRI exams in neurologically normal children recruited for various fMRI studies over a 3-year period, with variable imaging techniques. They found a 12.4 % prevalence of normal variants and an 8 % prevalence of abnormalities requiring clinical referral with a similar distribution to our cohort (5 – significant sinus disease, 3 - WM abnormalities, 1 – tonsillar ectopia, 1 – possible mass). Kumra et al. (Kumra et al. 2006) found potentially clinically significant findings in 3/60 (5 %) children in a study evaluating both normal children and those with psychiatric conditions. In a study of normal African children, Potchen et al. (Potchen 2006) identified abnormal brain MRI findings in 16/96 children (17 %), the most common being WM signal abnormalities (7/96;7.3 %).Understanding the clinical significance of unanticipated brain imaging findings in neurologically and developmentally normal children can be challenging. Low cerebellar tonisllar position compatible with an imaging diagnosis of Chiari I malformation (Barkovich et al. 1998) was identified in 2/112 (1.8 %) of asymptomatic subjects in our study. Low cerebellar tonsil position (>5 mm) has been estimated to occur in up to one percent of the general population, (Aitken et al. 2009), similar to the prevalence in our normal population. In that study, only 63 % of children with tonsillar ectopia >/=5 mm had related clinical symptoms, most commonly headache. However, 21 % of asymptomatic children with tonsillar ectopia developed related symptoms on clinical follow-up (Aitken et al. 2009). We believe that follow-up is necessary to determine clinical significance of this abnormality in neurologically healthy children and our policy is to arrange for clinical follow-up with a PCP for low cerebellar tonsillar position >/=5 mm.
White matter signal abnormalities are often non-specific. Overall 11/112 (9.8 %) of children in our study cohort had WM signal foci detected (all on 3D T2 FLAIR sequences). These foci were poorly seen or not identified on the T1 sequences. Adjusted prevalence relating to those with the 3D T2 FLAIR sequence is 11/83 (13.2 %). Of these 11 patients, 6 were thought to be potentially clinically significant given extent and distribution, and the PCP and participant/family notification was made. Prior studies have documented white matter abnormalities in 1.3 –7.3 % of asymptomatic children (Kim et al. 2002; Potchen et al. 2013; Fisch et al. 2012). We postulate that the higher prevalence in our study is related to high resolution imaging using 3D T2 FLAIR sequences, and would anticipate that the detection of white matter abnormalities on research studies will continue to increase with continually improving imaging techniques and the application of clinical sequences in the research environment. Possible etiologies for these white matter abnormalities include gliosis from prior ischemia or inflammation, demyelination, or hypomyelination. While some of the signal abnormalities occupied the same distribution as normal hypomyelination in younger children, their well-defined nature, and markedly increased signal suggests a pathologic etiology. The ultimate etiology of white matter abnormalities in our normal population is unknown.
Paranasal and mastoid air cell opacification, as well as mild lymph node prominence in the upper neck, are common in asymptomatic children (Von Kalle et al. 2012). A total of 7 (5.4 %) of children in our cohort exhibited paranasal sinus opacification, and 3 were significant (one with a large associated antrochoanal polyp). Using clinical subjects (and excluding those with cystic fibrosis, where sinus opacification is universal), Von Kalle et al. (2012) found paranasal sinus, mastoid, or middle ear opacification in 61 % of clinically performed MRI exams. Major paranasal sinus or mastoid opacification was seen in 22 % of subjects. These findings were more common in younger patients with recent upper respiratory tract infections. Imaging findings did not correlate with reported ear, nose, and throat symptomatology, underscoring that sinusitis / mastoiditis is a clinical diagnosis and can only be suggested by imaging findings. Given the common finding of asymptomatic sinus and mastoid opacification in children, we only report cases in which there is marked opacification of a sinus, or if there is an air-fluid level. More minor degrees of sinus opacification are not reported.
With improving brain imaging technology, there will likely be an increasing rate of unexpected but potentially clinically significant findings in research studies of otherwise healthy children. As recommended by the 2005 NIH workshop (Illes et al. 2006) and corroborated by our study, this mandates establishing policies for timely review and disclosure of these findings. IRB informed consent processes should be designed to explain the likelihood of unanticipated findings, as the frequency may meet or exceed 12 % in healthy populations. Despite this finding rate, subjects must be informed in the consent process that most unanticipated findings are asymptomatic and will require minimal follow-up.
Disclosure of unanticipated findings can be a cause of undue psychological stresses (Schmidt et al. 2013). The results of this study in adults indicate that up to 20 % of individuals undergoing research MRI imaging experienced significant psychosocial distress upon learning the results of unanticipated findings, regardless of severity. Additionally, 25 % of participants did not fully understand the medical terminology and severity of results upon disclosure of unanticipated findings by investigators (Schmidt et al. 2013). These numbers might be expected to be larger in a pediatric imaging study where results about un-emancipated minors are disclosed to parents. It is our hope that with proper consenting processes that explain the likelihood of unanticipated findings and define a clear follow-up process, some of the stress concerning unanticipated findings can be avoided.
In addition to being timely, follow-up for unanticipated findings must also be clinically correlated, as our study participants were asymptomatic at image acquisition, per study protocol. Our follow-up scheme as outlined in the methods section addresses the follow-up process and defines roles for each member of the study team. The primary responsibility falls on the principal investigator (as study initiator and liaison to the IRB that approved the study) to assure that families are notified of unanticipated findings and the need for clinical correlation through appropriate channels of communication. The PI and/or delegates are responsible for informing both the family of the study participant and the participant’s PCP. Ultimately, it is the responsibility of the PI as study originator to close communication loops and ensure that the follow-up process is complete. Steps to ensure completeness of communication include follow-up notices to families using phone or email contacts. Written documentation of the follow up process is also necessary.
The responsibility of the study participant’s PCP is to correlate unanticipated neuroimaging findings with the clinical examination and symptoms, and discuss the findings and potential clinical significance with the participant and family. The PCP has a greater knowledge of the participant’s clinical history, a personal relationship with the family, and can schedule a dedicated appointment with the participant to follow up on the findings associated with the study. As primary identifier of the abnormality, the study radiologist (or other qualified clinical imaging interpreter) should be available and willing to discuss interpretation of unanticipated findings with the PCP to help place findings in clinical context, as well as provide guidance in recommending follow-up clinical studies. Unfortunately, many unanticipated findings will have no clear clinical correlation, which could lead to undue stress on the part of the research subject and the subject’s family. Typically, the study radiologist and/or neurologist will confer with the PCP to make a referral for any follow-up diagnostic testing that might be appropriate given the incidental findings. This process should be handled by the clinical professionals involved in the study.
Several ethical questions arise concerning the follow-up process for unanticipated findings encountered on research MRI studies. First, what about families who have no access to primary care, and/or access to imaging facilities should the need arise for a follow-up scan? This issue may arise frequently, considering that one of the main reasons for enrolling in imaging studies is monetary compensation (Kirschen et al. 2006). With high incidental finding rates, investigators should anticipate unexpected findings and have a defined action plan for designating primary care providers and imaging follow-up for families with financial need. Investigators might consider dedicating funds to follow-up of imaging findings. As an example, many major children’s hospitals operate a Primary Pediatric Care clinic that can serve as a PCP-replacement for study participants with no other access to healthcare. In addition, larger pediatric institutions have capabilities to provide follow-up imaging to families with demonstrable financial need.
Second, what if families/participants have no desire to know the results of the study? Questions of disclosure have been addressed in previous ethical studies, where 5 % of research participants did not want researchers to communicate study findings with them (Illes et al. 2004a, b). There are well-described ethical arguments in the genetics and bioethics literature supporting a subject’s right not to know (Wilson 2005). The desire to know or not know the results of a study is the parent/guardian and/or participant's choice, best understood under the ethical principle of autonomy. This principle extends to our study, where parents of un-emancipated minors have the right to non-disclosure of results of MR imaging of their children. To our knowledge, no parent indicated that they preferred non-disclosure of results in the informed consent process.
The 2005 NIH consensus panel, while recommending that someone (be it a neurologist, radiologist, or researcher) with imaging expertise review scans to make sure findings requiring urgent follow-up were not missed (Illes et al. 2006), was inconclusive on recommending a specific professional. Commentary included that review of all research imaging by a neuroradiologist may not be necessary, as the primary goal of research imaging is not to identify existing pathology (Illes et al. 2006). The radiologist, as a physician with expertise in interpretation of diagnositic images, may be the ideal professional to evaluate imaging, identify abnormalities, produce reports, and direct appropriate follow-up and clinical referral, as these duties are a core part of their everyday professional activities. A radiologist can become a key liaison with the PCP in order to provide explanation of the clinical findings and help direct follow-up, particularly in studies where the principal investigator is not a medically-trained clinical professional. Identifying significant structural abnormalities that might impact group analyses or complicate or influence performance of BOLD, DTI, or perfusion data is another role in which the radiologist offers a unique contribution with scientific impact on the intergrity of the study. How to compensate this professional for their time and expertise is a difficult issue, and will vary based upon the sponsoring institution, research protocol, and radiologist availability. In our institution, research image review by radiologists is supported during the pilot phase of an imaging study by the clinical department as a part of its overall institutional support of imaging research. As pilot studies develop into NIH-funded projects, radiologists are included in the research budget along with other study personnel. If the radiologist’s evaluation is of the data collected and analyzed as part of a funded research project, then strong consideration should be given to budgeting this individual as part of the grant submission. At our institution, scanner costs for research done on the clinical MRI scanners has a built-in professional fee in addition to the technical fee, which covers imaging evaluation.
We agree with the NIH consensus opinion that research MRI examinations are designed to answer specific research questions and should in no way be considered clinical scans. An abbreviated number of sequences, many of which may have no defined clinical utility, are typically performed. This should be clearly reviewed with the subject and family as part of the informed consent process. Research image review is meant to provide an assessment for any revealed abnormalities that might be clinically important, not a comprehensive clinical evaluation. Questions regarding medico-legal liability if there is a missed finding, or whether a doctor-patient relationship is established if a radiologist reviews imaging, become clearer when the research scans are specifically documented as for research use only.
An important practical question related to the clinical care of subjects with unanticipated findings is in what manner the research images should be made available for potential clinical use. The research-obtained images, while potentially having clinically important imaging findings, are not clinical images. Research sequences often are only limited, or use sequences that are investigational in nature, not suitable for clinical decision-making. It is our strong feeling that any imaging finding identified as potentially clinically significant on research-obtained imaging, and thought to be important after being placed in clinical context by the PCP, be corroborated on clinically-obtained imaging prior to final clinical decision-making. In our institution, for studies requiring radiologic follow-up, subsequently obtained clinical imaging is typically compared with the research imaging stored on a separate research-only PACS system. The research-obtained imaging can also be placed in clinical PACS under the patient's clinical medical record number for easier comparison, but only if clinical imaging is also obtained. It must be emphasized that research-obtained images should not be used in isolation for clinical care. The research imaging report with key images (Fig. 1) is made available for review by the subject’s primary care physician, and if requested, the entire anatomic data set, on CD with a viewer, is made available with the clear disclaimer that these are research images and are not suitable in isolation for clinical decision-making.
Pediatric imaging research studies includes a vulnerable population as defined by FDA regulations. Children may be subject to undue coercion or influence by parents or other adults to participate in an imaging study. Vulnerability can also be considered as a limitation to autonomy. Pediatric imaging studies should limit risk to a population that cannot reasonably provide full informed consent under law and should also address the issue of “assent” in a pediatric population in a thoughtful manner. Assent is the agreement of a child to participate in research. Assent does not include failure to object to a study, nor is it applicable to all children, as certain ages and developmental stages may not be able to provide assent. However, assent remains an important concept in pediatric ethical discussion and must be obtained in all study participants where feasible. Despite assent to participate, it may not be reasonable to expect a child to understand the implications of unanticipated findings during a brain imaging research study. Appropriately trained study coordinators should take the time to communicate clearly with the participant, detailing what the process will be for disclosing any abnormal brain imaging findings to parents and physicians, should they occur.
In summary, previous ethics discussions at the NIH (Illes et al. 2006) recommended that neuroimaging researchers should: anticipate unanticipated findings; explain the likelihood of incidental findings as well as associated follow-up procedures during the informed consent process; arrange for qualified, timely review of images; communicate findings to participants in a timely way; and support this process by ensuring that IRB protocols and consents lay out a plan for handling unanticipated findings transparently. Through this study, we have accomplished two main goals: First, confirming the abnormal finding rate of previous studies, indicating that researchers should anticipate findings at a rate of 8–12 % in future MRI research studies involving brain imaging in asymptomatic children. Second, defining a reporting process which meets the goals of previous NIH consensus recommendations. By defining roles for the PI, radiologist, PCP, and patient’s family, future follow-up of unanticipated findings can be streamlined and effectively handled. Brain imaging research in healthy children is necessary to improve our understanding of child development and developmental diseseases, but researchers have a duty to safeguard the rights of pediatric subjects as a vulnerable population, and to communicate unanticipated findings in a timely and effective manner to maximize the beneficence toward subjects.
References
Aitken, L. A., Lindan, C. E., Sidney, S., Gupta, N., Barkovich, A. J., Sorel, M., & Wu, Y. W. (2009). Chiari type I malformation in a pediatric population. Pediatric Neurology, 40(6), 449–54.
Barkovich, A. J., Wippold, F. J., Sherman, J. L., & Citrin, C. M. (1998). Significance of cerebellar tonsillar position on MR. AJNR. American Journal of Neuroradiology, 7(5), 795–9.
Brown, D. A., & Hasso, A. N. (2008). Toward a uniform policy for handling incidental findings in neuroimaging research. AJNR. American Journal of Neuroradiology, 29(8), 1425–7.
Fisch, N., Konen, O., Halevy, A., Cohen, R., & Shuper, A. (2012). Incidental multifocal white matter lesions in pediatric magnetic resonance imaging. Pediatric Neurology, 47(1), 7–12.
Illes, J., Kirschen, M. P., Edwards, E., Stanford, L. R., Bandettini, P., Cho, M. K., Ford, P. J., Glover, G. H., Kulynych, J., Macklin, R., Michael, D. B., & Wolf, S. M. (2006). Working Group on Incidental Findings in Brain Imaging Research.Ethics. Incidental findings in brain imaging research. Science, 311(5762), 783–784.
Illes, J., Kirschen, M. P., Karetsky, K., Kelly, M., Saha, A., Desmond, J. E., Raffin, T. A., Glover, G. H., & Atlas, S. W. J. (2004a). Discovery and disclosure of incidental findings in neuroimaging research. Magnetic Resonance Imaging, 20(5), 743–747.
Illes, J., Rosen, A. C., Huang, L., Goldstein, R. A., Raffin, T. A., Swan, G., & Atlas, S. W. (2004b). Ethical consideration of Incidental findings on adult brain MRI in research. Neurology, 62(6), 888–890.
Katzman, G. L., Dagher, A. P., & Patronas, N. J. (1999). Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA, 282, 36–9.
Kim, B. S., Illes, J., Kaplan, R. T., Reiss, A., & Atlas, S. W. (2002). Incidental findings on pediatric MR images of the brain. AJNR. American Journal of Neuroradiology, 23(10), 1674–7.
Kirschen, M. P., Jaworska, A., & Illes, J. (2006). Subjects’ expectations in neuroimaging research. Journal of Magnetic Resonance Imaging, 23(2), 205–9.
Kumra, S., Ashtari, M., Anderson, B., Cervellione, K. L., & Kan, L. (2006). Ethical and practical considerations in the management of incidental findings in pediatric MRI studies. Journal of the American Academy of Child and Adolescent Psychiatry, 45(8), 1000–6.
Potchen, M. J., Kampondeni, S. D., Mallewa, M., Taylor, T. E., & Birbeck, G. L. (2013). Brain imaging in normal kids: a community-based MRI study in Malawian children. Tropical Medicine and International Health, 18(4), 398–402.
Schmidt, C. O., Hegenscheid, K., Erdmann, P., Kohlmann, T., Langanke, M., Völzke, H., Puls, R., Assel, H., Biffar, R., & Grabe, H. J. (2013). Psychosocial consequences and severity of disclosed incidental findings from whole-body MRI in a general population study. European Radiology, 23(5), 1343–51.
Von Kalle, T., Fabig-Moritz, C., Heumann, H., & Winkler, P. (2012). Incidental findings in paranasal sinuses and mastoid cells: a cross-sectional magnetic resonance imaging (MRI) study in a pediatric radiology department. Röfo, 184(7), 629–34.
Weber, F., & Knopf, H. J. (2006). Incidental findings in magnetic resonance imaging of the brains of healthy young men. Neurological Sciences, 240, 81–4.
Wilson, J. (2005). To know or not to know? Genetic ignorance, autonomy, and paternalism. Bioethics, 19, 492–504.
Acknowledgments
Acknowledgement: This works was sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Contract title: The Pediatric Functional Neuroimaging Research Network, # HHSN275200900018C.
Disclosures
Drew Kaiser, James Leach, Jennifer Vanest, Mark Schapiro, and Scott Holland declare that they have no conflicts of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
*The CMIND (Cincinnati MR Imaging of NeuroDevelopment) Authorship Consortium
Scott K. Holland, Ph.D.1,6,9,10
Jennifer Vannest, Ph.D.1,5
Vincent J. Schmithorst, Ph.D.1,2
Mekibib Altaye, Ph.D.1,7
Gregory Lee, Ph.D.1,6
Luis Hernandez-Garcia, Ph.D.3
Michael Wagner, Ph.D.1,8
Arthur Toga, Ph.D.12,13
Jennifer Levitt, MD14
Anna W. Byars, Ph.D1,5
Andrew Dimitrijevic, Ph.D.9,10
Nicolas Felicelli8
Darren Kadis, Ph.D.1,5
James Leach, MD1,6
Katrina Peariso, MD, Ph.D.5
Elena Plante, Ph.D.4
Akila Rajagopal, M.S.1
Andrew Rupert, M.S.8
Mark Schapiro, MD1,5
Ronald Ly14
Petros Petrosyan12
JJ Wang, Ph.D.11
Lisa Freund, Ph.D.15
1Pediatric Neuroimaging Research Consortium
Cincinnati Children’s Hospital Medical Center
University of Cincinnati, 3333 Burnet Ave.
Cincinnati, OH 45229
2Pediatric Imaging Research Center, Dept. of Radiology
Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA
3Functional MRI Laboratory, Dept. of Biomedical Engineering
University of Michigan, Ann Arbor, MI
4Dept. of Speech, Language, and Hearing Sciences
University of Arizona, Tucson, AZ
5Div. of Neurology, Dept. of Pediatrics
Cincinnati Children’s Hospital Medical Center
University of Cincinnati, 3333 Burnet Ave.
Cincinnati, OH 45229
6Dept. of Radiology
Cincinnati Children’s Hospital Medical Center
University of Cincinnati, 3333 Burnet Ave.
Cincinnati, OH 45229
7Div. of Biostatistics and Epidemiology, Dept. of Pediatrics
Cincinnati Children’s Hospital Medical Center
University of Cincinnati, 3333 Burnet Ave.
Cincinnati, OH 45229
8Div. of Biomedical Informatics, Dept. of Pediatrics
Cincinnati Children’s Hospital Medical Center
University of Cincinnati, 3333 Burnet Ave.
Cincinnati, OH 45229
9Dept. of Otolaryngology
Cincinnati Children’s Hospital Medical Center
University of Cincinnati, 3333 Burnet Ave.
Cincinnati, OH 45229
10Communication Sciences Research Center
Cincinnati Children’s Hospital Medical Center
University of Cincinnati, 3333 Burnet Ave.
Cincinnati, OH 45229
11Dept. of Neurology, UCLA, Los Angeles, CA
12Laboratory of Neuroimaging, Keck School of Medicine of USC
Los Angeles, CA
13Departments of Ophthalmology, Neurology, Psychiatry, and the
Behavioral Sciences, Radiology and Engineering
Keck School of Medicine of USC, Los Angeles, CA
14Psychiatry and Biobehavioral Sciences, UCLA, Los Angeles, CA
15Eunice Kennedy Shriver National Institute of Child Health and
Human Development, Bethesda, MD
Rights and permissions
About this article
Cite this article
Kaiser, D., Leach, J., Vannest, J. et al. Unanticipated findings in pediatric neuroimaging research: Prevalence of abnormalities and process for reporting and clinical follow-up. Brain Imaging and Behavior 9, 32–42 (2015). https://doi.org/10.1007/s11682-014-9327-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-014-9327-7