Abstract
Carabid beetles, predatory insects, are abundant in forests and sensitive to environmental changes. The distribution patterns and diversity of carabid beetles in several natural forests were studied to provide a basis for evaluating the importance of a forest in the protection of carabid beetle diversity. Carabids were captured by pitfall traps during their seasonal activity from 2012 to 2013 in a poplar-birch forest, ash-walnut forest and broad-leaved Korean pine forest. A total of 5252 individuals, representing 21 species, were collected. Carabid abundance was highest in the broad-leaved Korean pine forest and lowest in the ash-walnut forest. Carabus billbergi Mannerheim and Pterostichus pertinax (Tschitscherine) were the dominant beetle species in each stand. Carabus canaliculatus Adams was dominant in the poplar-birch and ash-walnut forests, and Leistus niger Gebler was dominant in the ash-walnut forest. The carabids were affected differently by stand factors. C. billbergi and P. pertinax was positively correlated with mean DBH. C. canaliculatus and L. niger were not positively correlated with any stand factors. The broad-leaved Korean pine forest with greater age, large DBH and thick leaf litter fostered a high diversity of carabid species. The main yearly activity period for most carabids was during July. Different carabid species responded differently to seasonality, and the activity period of several species was relatively late (August) in the year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carabid beetles (Coleoptera: Carabidae) are a diverse, species-rich family of insects. Most species are predatory and are natural enemies of other forest insects. They provide effective population control of a number of pests (Thiele 1977; Magura et al. 2000; Liu et al. 2004; Zhang et al. 2012). They often travel on the ground, directly exposed to environmental factors, and their responses can indicate environmental changes. Carabid beetles have been widely studied and used as environmental indicators (Chen et al. 2009; Gao and Fu 2009; Eyre et al. 2016).
Ground vegetation is the main habitat of carabid beetles and their distribution is influenced by ground characteristics; species composition is significantly affected by soil moisture content, vegetation cover and biomass, litter thickness, and cover (Tyler 2008; Wang et al. 2009; He et al. 2011; Moraes et al. 2013; Yanahan and Taylor 2014; Kostova 2015; Spake et al. 2016). However, the responses of species in different habitats to environmental factors may differ (Yang et al. 2015; Hang et al. 2016). The composition and distribution of species are closely related to forest habitat, and their diversity is influenced by forest type, age, density of the canopy, and area of distribution (Yu et al. 2006; Jung et al. 2018; Homburg et al. 2019; Koivula et al. 2019; Okatsu and Tsutsumi 2019). The responses of different species to forest habitats vary and some species only inhabit specific types of forests (Qin et al. 2018). The abundance of ground vegetation affects species distribution but the results have varied in different studies (Jouveau et al. 2020). Carabid beetle diversity tends to be lower in heavily managed forests but increases as recovery time increases (Latty et al. 2006). Forest heterogeneity also contributes to increased species richness (Zou et al. 2014; Yang et al. 2017). Diversity is higher in native and more diverse forests than in monoculture plantations. Carabids are also more diverse in mixed conifer-hardwood forests than in pure coniferous forests (Fuller et al. 2008; Jung et al. 2014; Sun et al. 2018). Forest age also affects the distribution of carabid beetles by influencing climate and vegetation factors (Yu et al. 2002; Negro 2014; Johansson et al. 2017; Sun et al. 2018). However, this has been infrequently reported at the species level, and there are relatively few reports on the effects of forest age, stand types and structure on carabid distribution at the species level. A continuous collection of carabids beetles throughout their adult activity period from different stands will provide a more comprehensive scope of their distribution patterns in different stands. This study of the distribution patterns of carabid beetles in broad-leaved Korean pine mixed forests and other deciduous broad-leaved mixed forests at different stages of succession can provide a basis for estimating the response of carabids to forest succession. The major objectives of this study are: (1) to determine the response of carabids to changes in stand types and stand factors; and, (2) to determine the influence of time on carabids abundance.
Materials and methods
Study area
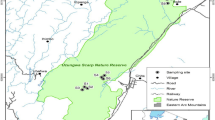
The study area was located in the Administration Bureau of the Jiaohe experimental area in the Changbai Mountains forest region, Jilin Province, northeast China (127º35′–127º51′ E, 43º51′–44º05′ N). Altitude ranged from 330 to 1176 m a.s.l. This region has a temperate continental monsoon mountain climate, and average annual temperature is 3.8 °C. Temperatures are highest in July (average 21.7 °C) and most precipitation occurs from June to August. More than 1000 plant species have been identified in this area. The representative vegetation types mostly include broad-leaved Korean pine mixed forests and broad-leaved mixed forests at different successional stages. The experimental area is used for research and teaching, and the forest is older and the area larger because of less logging and reduced human interference (Wang and Qin 2012). The main tree species include Pinus koraiensis Siebold et Zucc., Picea jezoensis var. komarovii Cheng et L.K.Fu, Abies holophylla Maxim., Abies nephrolepis (Trautv. ex Maxim.) Maxim., Juglans mandshurica Maxim., Phellodendron amurense Rupr., Fraxinus mandshurica Rupr., Tilia mandschurica Rupr. et Maxim., Tilia amurensis Pupr., Acer mono Maxim., Acer mandshuricum Maxim., Acer pseudo-sieboldianum (Pax.) Komarov, Betula platyphylla Suk., Betula costata Trautv., Populus davidiana Dode, Quercus mongolica Fisch.ex Turca., Ulmus davidiana Planch., and Maackia amurensis Rupr. et Maxim. Common shrubs include Lespedeza bicolor Turcz, Rhamnus davurica Pall., Syringa reticulate var. mandshurica (Maxim.) Hara), Lonicera ruprechtiana Regel., and Sorbaria kirilowii (Regel) Maxim.
Samples setting and specimen collection
Four representative natural stands were selected as study sites, with a size of 300 m × 400 m for each site (Table 1). Five 2 m × 2 m plots were arranged at the center point and in four vertices in the four stands. At the center of each plot and 1 m from the four sides, a pitfall trap was established. The pitfall trap spacing was 1 m and the size was three rows and three columns. A total of nine pitfall traps were set up in each plot and 45 in each stand. Plastic water cups were used as pitfall traps (depth 90 mm, top diameter 70 mm) to collect carabid specimens. A small hole in the top of each plastic trap prevented the loss of specimens due to rain. All traps were filled with slightly salty water, vinegar and alcohol.
Collection times and identification of specimens
The adult carabids were collected from middle May to late August in 2012 and 2013, and the collection interval covered the entire active period of all carabid beetles in this region. Carabids were retrieved from the trap cups seven times per season, late May (T1), middle June (T2), late June (T3), middle July (T4), late July (T5), middle August (T6), and late August (T7). All specimens collected were pinned and identified in the Insect Laboratory at the Forestry College of Beihua University.
Data analysis
Dominant species represented more than 10% of the total number of individuals collected. CCA (canonical correspondence analysis) and DCA (detrended correspondence analysis) were analyzed by Canoco 4.5 software (Biometris-Plant Research International, Wageningen, The Netherlands), and species and environmental data were transformed by square root. The collection times for each species were calculated quantitatively by ordinal date methods; the seven times were late May (T1) = 140, middle June (T2) = 150, late June (T3) = 170, middle July (T4) = 180, late July (T5) = 200, middle August (T6) = 210, late August (T7) = 230 (Hanks and Millar 2013; Liu et al. 2016). The number of species in different stands were statistically analyzed by one-way ANOVA.
Results
Species composition of carabid beetles
A total of 5252 carabid specimens representing 21 species were collected. Of these, 1130 were from the poplar-birch forest (PBF), 484 from the ash-walnut forest (AWF), 1802 from the broad-leaved Korean pine mixed forest 1 (BKP1), and 1836 from the broad-leaved Korean pine mixed forest 2 (BKP2). Carabus billbergi Mannerheim and Pterostichus pertinax (Tschitscherine) were the dominant species in each stand; Carabus canaliculatus Adams was dominant in PBF and AWF; Leistus niger Gebler was dominant in AWF (Table 2). Pterostichus (four species, 2996 individuals) and Carabus (four species, 1103 individuals) were dominant genera.
The influence of stand factors on carabid species
Canopy density, litter thickness, herb coverage, trees/ha, shrub cover, and average DBH in each stand were recorded (Table 3). The number of individuals and the mean values of the six environmental factors were transformed by square root. Canonical correspondence analysis (CCA) was carried out with the number of individuals, four stand types and six factors in the different forests (Fig. 1). The contribution rate of the first axis was 73.8%, the contribution rate of the second was 15.6%, and cumulative contribution rate was 89.4% and the Monte Carlo Permutation Test P = 0.05, PBF was positively correlated with the first axis and negatively with the fourth axis in the fourth quadrant. AWF was in the first quadrant and positively correlated with both the first and second axes. In the second quadrant, BKP1 was negatively correlated with the first axis and positively correlated with the second axis. BKP2 was in the third quadrant and was negatively correlated with the first and second axes.
Canonical correspondence analysis between carabid species, four stands, and six stand factors. 1: Calosoma cyanescens Motschulsky, 2: Carabus billbergi Mannerheim, 3: Carabus granulatus Linnaeus, 4: Carabus seishinensis Lapouge, 5: Carabus constricticollus Kraatz, 6: Chlaenius sericimicans Chaudoir, 7: Cychrus morawitzi Géhin, 8:Cymindis sp., 9: Carabus vietinghoffi Adams, 10: Harpalus simplicidens Schauberger, 11: Harpalus ussuriensis Chaudor, 12: Leistus niger Gebler, 13: Carabus canaliculatus Adams, 14: Pristosia vigil Tschistcherine, 15: Pterostichus interruptus (Dejean), 16: Pterostichus adstrictus Eschscholtz, 17: Pterostichus pertinax (Tschitscherine), 18: Pterostichus sp., 19: Poecilus reflexicollis Gebler, 20: Synuchus sp., 21: Trichotichnus coruscus (Tschitscherine), same to below
Among the six environmental factors, herb coverage (X3), trees per hectare (X4) and shrub coverage (X5) were positively correlated with the first axis; the correlation coefficients were 0.9766, 0.9958, and 0.9316. Canopy density (X1), litter thickness (X2), average DBH (X6) were negatively correlated with the first axis, and the correlation coefficients were − 0.9836, − 0.9056, and − 0.997. BKP1 and BKP2 were strongly correlated with canopy density (X1), litter thickness (X2) and average DBH (X6). There was a strong correlation between PBF, AWF and herb cover (X3), trees per hectare (X4) and shrub cover (X5).
C. cyanescens (1) was strongly correlated with average DBH (X6), canopy density (X1), and litter thickness (X2). P. adstrictus (16), C. billbergi (2), Cymindis sp. (8), P. pertinax (17), P. vigil (14) showed a strong correlation with average DBH (X6). C. granulatus (3), C. morawitzi (7), P. interruptus (15) were correlated with number of trees per hectare (X4) and shrub coverage (X5). C. vietinghoffi (9) and Pterostichus sp. (18) were strongly correlated with herb cover (X3). Other species did not show obvious correlations with environmental factors.
Influence of stand type on the dominant species
In four stands, the number of P. pertinax was the highest of all species, reaching 51.0% of the total, and was significantly higher in BKP1 and BKP2 than in PBF and AWF (F = 73.86; df = 3, 16; P < 0.001) (Fig. 2a). C. billbergi number reached 17.3% of the total, and was also significantly higher in BKP1 and BKP2 than in PBF and AWF (F = 53.60; df = 3, 16; P < 0.001) (Fig. 2b). C. canaliculatus was highest in AWF (F = 1.427; df = 3, 16; P > 0.05) (Fig. 2c), and L. niger was most common in BKP1 (F = 1.525; df = 3, 16; P > 0.05) (Fig. 2d).
Distribution of ordinal date of species
There were significant differences in the adult activity period of carabid beetles. The main month of activity of adults was July (Table 4). C. billbergi, C. canaliculatus, C. vietinghoffi, P. interruptus, P. pertinax, Pterostichus sp., and P. vigil were found in all seven collection times; and their activity periods were relatively long. Nine species were active in early spring from mid-May to the end of May. C. constricticollus, L. niger, T. coruscus were active from early June to late August. H. ussuriensis, Synuchus sp., H. simplicidens, C. seishinensis had small populations and a later active period mainly in August. The number of C. billbergi, P. pertinax, P. vigil, P. adstrictus, T. coruscus varied greatly in each sample period as indicated by the SD values (Table 4).
Detrended correspondence analysis between species and time
The individual numbers of 21 carabid species were square root transformed. Detrended correspondence analysis was established between the 21 species collected over the seven dates (Fig. 3). The contribution rate of the first axis was 63.1%, the second 10.9%, and the cumulative contribution rate was 74.0%. Early May, early and late June were in the second quadrant, negatively correlated with the first axis and positively correlated with the second. Early July and late July were in the fourth quadrant and positively correlated with the first axis and negatively correlated with the second. Early August and late August were in the first quadrant and positively correlated with both the first and second axes.
The relationship between late May (T1) and each carabid beetle were relatively weak. There was a strong correlation between August (T6 and T7) and H. simplicidens (10), C. seishinensis (4), H. ussuriensis (11), and Synuchus sp. (20). Compared with other species, the relationship between June (T2 and T3) and P. adstrictus (16), C. billbergi (2), P. reflexicollis (19), and C. cyanescens (1) were stronger. The relationship was stronger between July (T4 and T5) and Cymindis sp. (8), C. morawitzi (7), C. constricticollus (5), C. sericimicans (6), L. niger (12), and C. granulatus (3). C. canaliculatus (13), C. vietinghoffi (9), P. interruptus (15), P. pertinax (17), T. coruscus (21), Pristosia vigil (14), and Pterostichus sp. (18) did not correspond with any collection time.
Dominant species temporal dynamics
There were large numbers of (a) P. pertinax, (b) C. billbergi, (c) C. canaliculatus, (d) L. niger collected at every sample time (Fig. 4). P. pertinax was the largest number, accounting for more than 50% of the total, and the number was unimodal. Its active peak period was in early July, followed by late July (Fig. 4a). The adult activity period of C. billbergi was mainly before early July; the peak period was late June (Fig. 4b). C. canaliculatus active period was from early July to late August, with a peak was in early July (Fig. 4c). L. niger active peak period was from late July to late August, with a peak was in late July (Fig. 4d).
Discussion
Forest ecosystems are an important habitat of carabid beetles. Forest type, tree species composition and structure affect their distribution by influencing the ground environment (Li et al. 2017; Vician et al. 2018). This study identified substantial differences in the composition of the carabid communities in broad-leaved Korean pine mixed forest, poplar-birch forest and ash-walnut forest in the Changbai Mountains. Carabid abundance was higher in the mature broad-leaved Korean pine mixed forest than in the poplar-birch and ash-walnut forests in earlier successional stages. This is consistent with the high abundance of beetles in mature forests (Zou et al. 2015; Liu et al. 2018b). The vegetation composition of the mature forest is complex which can increase the diversity of carabid beetle species, and the mature forest appears to play an important role in their protection (Fuller et al. 2008; Jung et al. 2014; Zou et al. 2019). We found that there were unique characteristics in the carabid composition in each stand. The number of P. pertinax was the largest, and C. billbergi, C. canaliculatus, and L. niger were also numerous. At the genus level, Carabus and Pterostichus were the main assemblages in the forests, which is consistent with other studies in which these genera were dominant (Gnetti et al. 2015; Sun et al. 2018).
In forest stands with reduced human interference, factors such as canopy density, litter thickness, herb cover, and number of trees per unit area, shrub cover and average DBH are closely related to stand composition and age. Differences in stand composition and structure leads to differences in stand factors. However, the influence of these on carabid species was also variable. Abundances of P. pertinax, C. billbergi and P. vigil were closely related to the average DBH of the stand. This increase in average stand diameter helped increase carabid species abundance, which is consistent with findings that large trees in a forest help to protect the diversity of carabid beetles and increase species abundance (Jouveau et al. 2020). Herb coverage affected the distribution of carabid communities, and the species and numbers of individual carabid species were greater in habitats with high herb cover (Eyre et al. 2004). Some species prefer to move into areas with high herb density (Brose 2003; Eyre et al. 2004; Tyler 2008). In this study, there was a strong correlation between herb cover and C. granulatus, C. morawitzi, C. vietinghoffi, and Pterostichus sp. This may be because these species prefer to feed and move in herbaceous areas.
Forest litter is the main factor that affects the distribution of carabid beetles. Litter helps to prevent the ground temperature from losing warmth and provides a habitat for beetles to hunt and hide (Luff et al. 1989; Irmler 2000). Litter thickness has a strong positive effect on carabid abundance (Sroka and Finch 2006), and mature stands with large diameter trees tend to accumulate a thick litter layer and have higher species abundance (Liu et al. 2018a). However, at the species level, the results of this study show that only some carabid species abundance were correlated with litter thickness. Of the four dominant species, only P. pertinax and C. billbergi were positively correlated while C. canaliculatus and L. niger were not correlated with environmental factors. This may be because carabid species exhibit diverse habitat preferences and adaptations at the species level.
Seasonality affects forest temperatures, humidities and ground vegetation, and thus influences the distribution patterns of carabid beetles. Only seven species were collected in all seven time periods from late May to late August, while the activity periods of the other species differed. Most studies have shown a significant positive correlation between carabid abundance and temperatures in the forest, with peak numbers occurring in July (Liu et al. 2018a; Sun et al. 2018). However, the four dominant species in this study responded differently to the season, which may reflect differentiated adaptability to seasonality at the species level (Yu et al. 2002). The activity peak of C. billbergi was relatively early (late June), consistent with its time distribution in the Changbai Mountain Reserve (Wang et al. 2014). This may be related to the biological characteristics of overwintering in adults (Jennings et al. 1986). When temperatures rise in the forest, the adults become active. The peak activity of P. pertinax and C. canaliculatus were in early July and related to higher forest temperatures and the abundance of edible animals and plants (Yu et al. 2002). Some studies have also shown that the higher ground temperatures at this time of year is an important reason for increased carabid abundance (Magura et al. 2004). The activity peak of L. niger was relatively late (late July) and was associated with a late emergence period. The temporal dynamics of the four dominant species were all unimodal, consistent with the finding that the activity period of most carabid species is unimodal in the forests of northern China (Wang et al. 2014).
Conclusion
As one of the representative vegetation types in this region, the broad-leaved Korean pine mixed forest is older, has less human disturbance, a larger diameter class and more litter accumulated, all of which have a positive effect on the abundance and richness of carabid beetles populations. Thus, the broad-leaved Korean pine mixed forest can play an important role in protecting the diversity of carabids. Carabids are more active in July, with different species sensitive to time changes. The results also suggest that the abundance and diversity of carabid beetles were affected by seasonal changes.
References
Brose U (2003) Bottom–up control of carabid beetle communities in early successional wetlands: mediated by vegetation structure or plant diversity? Oecologia 135:687–703
Chen YQ, Li Q, Wang SM (2009) Diversity of ground–dwelling beetles in lac–plantation–farmland ecosystem: a case study in Luchun, Yunnan, South–western China. Acta Entomol Sin 52(12):1319–1327 (in Chinese)
Eyre MD, Rushton SP, Luff ML, Telfer MG (2004) Predicting the distribution of ground beetle species (Coleoptera, Carabidae) in Britain using land cover variables. J Environ Manage 72(3):163–174
Eyre MD, McMillan SD, Critchley CNR (2016) Ground beetles (Coleoptera, Carabidae) as indicators of change and pattern in the agroecosystem: Longer surveys improve understanding. Ecol Ind 68:82–87
Fuller RJ, Oliver TH, Leather SR (2008) Forest management effects on carabid beetle communities in coniferous and broadleaved forests: implications for conservation. Insect Conservat Divers 1(4):242–252
Gao GC, Fu BQ (2009) Advances of research on carabid beetles as bioindicators. Chin Bull Entomol 46(2):216–220 ((in Chinese))
Gnetti V, Bombi P, Taglianti AV, Bologna MA, D’Andrea E, Cammarano M, Bascietto M, Cinti BD, Matteucci G (2015) Temporal dynamic of a ground beetle community of eastern Alps (Coleoptera, Carabidae). Bull Insectol 68(2):299–309
Hang J, Shi Y, An JJ, He DH (2016) Selection of microhabitat of carabid beetles (Coleoptera: Carabidae) in different ecological restored habitats in the Hilly and Gully Area of Loess Plateau, Ningxia. China Sci Silvae Sini 52(1):71–79 (in Chinese)
Hanks LM, Millar JG (2013) Field bioassays of cerambycid pheromones reveal widespread parsimony of pheromone structures, enhancement by host plant volatiles, and antagonism by components from heterospecifics. Chemoecology 23(1):21–44
He Q, Wang XP, Yang GJ (2011) Species diversity of carabid beetles in desert–steppe in Yanchi of Ningxia. China Acta Ecol Sini 31(4):923–932 ((in Chinese))
Homburg K, Drees C, Boutaud E, Nolte D, Schuett W, Zumstein P, Ruschkowski E, Assmann T (2019) Where have all the beetles gone? Long–term study reveals carabid species decline in a nature reserve in Northern Germany. Insect Conservat Divers 12(4):268–277
Irmler U (2000) Environmental characteristics of ground beetle assemblages in northern German forests as basis for an expert system. Zeitschrift Fuer Oekologie Naturschutz 8:227–237
Jennings DT, Houseweart MW, Dunn GA (1986) Carabid beetles (Coleoptera: Carabidae) associated with strip clearcut and dense spruce–fir forests of maine. Coleopt Bull 40(3):251–263
Johansson T, Gibb H, Hjältén J, Dynesius M (2017) Soil humidity, potential solar radiation and altitude affect boreal beetle assemblages in dead wood. Biol Cons 209:107–118
Jouveau S, Toïgo M, Giffard B, Castagneyrol B, Halder IV, Vétillard F, Jactel H (2020) Carabid activity–density increases with forest vegetation diversity at different spatial scales. Insect Conservat Divers 13:36–46
Jung JK, Kim ST, Lee SY, Park CK, Park JK, Lee JH (2014) A comparison of diversity and species composition of ground beetles (Coleoptera: Carabidae) between conifer plantations and regenerating forests in Korea. Ecol Res 29:877–887
Jung JK, Lee SK, Lee S, Lee JH (2018) Trait–specific response of ground beetles (Coleoptera: Carabidae) to forest fragmentation in the temperate region in Korea. Biodivers Conserv 27:53–68
Koivula MJ, Venn S, Hakola P, Niemelä J (2019) Responses of boreal ground beetles (Coleoptera, Carabidae) to different logging regimes ten years post- harvest. For Ecol Manage 436:27–38
Kostova R (2015) Ground beetles (Coleoptera Carabidae) diversity patterns in forest habitats of high conservation value, Southern Bulgaria. Biodivers J 6:341–352
Latty EF, Werner SM, Mladenoff DJ, Raffa KF, Sickley TA (2006) Response of ground beetle (Carabidae) assemblages to logging history in northern hardwood–hemlock forests. For Ecol Manage 222:335–347
Li WB, Liu NY, Wu YH, Zhang YC, Xu Q, Chu J, Wang SY, Fang J (2017) Community composition and diversity of ground beetles (Coleoptera: Carabidae) in Yaoluoping national nature reserve. J Insect Sci 17(6):1–8
Liu YH, Yu ZR, Liu Y (2004) Temporal and spatial structure of carabid community in agricultural landscape of Dongbeiwang, Beijing. Chin J Appl Ecol 15(1):85–90 (in Chinese)
Liu SD, Meng X, Meng QF, Li Y (2016) Cerambycidae species diversity and temporal dynamics of adult emergence in broad–Leaved Korean pine forest of southern Zhangguangcai Mountains. Sci Silvae Sini 52(2):74–81 (in Chinese)
Liu SD, Meng QF, Gao WT, Li Y, Zhao HR, Li TQ (2018a) An analysis of temporal dynamics of litter–layer beetles in broad–leaved Korean pine forest in Jiaohe. Jilin Prov Acta Ecol Sini 38(7):2462–2470 (in chinese)
Liu SD, Meng X, Meng QF, Li Y, Zhao HR, Gao WT (2018b) Influence of different stands on ground–dwelling beetle community in broad–leaved Korean pine forests. Sci Silvae Sini 54(2):110–118 (in Chinese)
Luff ML, Eyre MD, Rushton SP (1989) Classification and ordination of habitats of ground beetles (Coleoptera, Carabidae) in north–east England. J Biogeogr 16(2):121–130
Magura T, Tóthmérész B, Bordán Z (2000) Effects of nature management practice on carabid assemblages (Coleoptera: Carabidae) in a non–native plantation. Biol Cons 93(1):95–102
Magura T, Tóthmérész B, Molnár T (2004) Changes in carabid beetle assemblages along an urbanisation gradient in the city of Debreeen, Hungary. Landsc Ecol 19:747–759
Moraes RM, Mendonça JMS, Ott R (2013) Carabid beetle assemblages in three environments in the Araucaria humid forest of southern Brazil. Revista Brasilra De Entomol 57:67–74
Negro M, Vacchiano G, Berretti R, Chamberlain DE, Palestrini C, Motta R, Rolando A (2014) Effects of forest management on ground beetle diversity in alpine beech (Fagus sylvatica L.) stands. For Ecol Manag 328:300–309
Okatsu Y, Tsutsumi T (2019) Carabid beetle (Coleoptera: Carabidae) assemblages in the intermediate successional stage after Mt. Bandai eruption of, 1888: effects of environmental variables on carabid beetles in the Urabandai area. Entomol Sci 22(2):182–193
Qin WC, Tian HG, Wang XP, Yang GJ (2018) Community diversity of carabid beetles in the Luo mountain national nature reserve in Ningxia. J Northwest A F Univ (Nat Sci Ed) 46(5):109–117
Spake R, Barsoum N, Adrian C, Newton AC, Doncaster CP (2016) Drivers of the composition and diversity of carabid functional traits in UK coniferous plantations. For Ecol Manage 359:300–308
Sroka K, Finch OD (2006) Ground beetle diversity in ancient woodland remnants in north–western Germany (Coleoptera: Carabidae). J Insect Conserv 10:335–350
Sun XJ, Yang X, Sang WG (2018) Effects of temporal and spatial variation on the diversity of carabid assemblages in different forest types of Zhangguangcai mountain. Ecol Environ Sci 27(12):2185–2192 (in Chinese)
Thiele HU (1977) Carabid Beetles in their environments–a study on habitat selection by adaptations in physiology and behavior. Springer-Verlag, New York, pp 1–2
Tyler G (2008) The ground beetle fauna (Coleoptera: Carabidae) of abandoned fields, as related to plant cover, previous management and succession stage. Biodivers Conserv 17:155–172
Vician V, Svitok M, Michalková E, Lukáčik I, Stašiov S (2018) Influence of tree species and soil properties on ground beetle (Coleoptera: Carabidae) communities. Acta Oecol 91:120–126
Wang H, Qin SL (2012) Forest resource status and sustainable management countermeasures of Jiaohe forestry experimental region Administration of Jilin Province. For Investig Des 162(2):2–5 (in Chinese)
Wang Y, Gao GC, Fu BQ, Wu Z (2009) Composition and spatial distribution pattern of ground–dwelling beetle communities in Yeyahu Wetland. Beijing Biodivers Sci 17(1):30–42 (in Chinese)
Wang XW, Müller J, An L, Ji LZ, Liu Y, Wang XG, Hao ZQ (2014) Intra–annual variations in abundance and species composition of carabid beetles in a temperate forest in Northeast China. J Insect Conserv 18:85–98
Yanahan AD, Taylor SJ (2014) Vegetative communities as indicators of ground beetle (Coleoptera, Carabidae) diversity. Biodivers Conserv 23:1591–1609
Yang GJ, Jia YX, Wang XP, Zhang DZ (2015) Diversity of carabid beetles community across alfalfa–desert grassland ecotone in Yanchi of Ningxia. China J Environ Entomol 37(3):483–491 (in Chinese)
Yang YC, Yang GJ, Wang J (2017) Effects of topographic factors on the distribution pattern of carabid species diversity in the Helan Mountains, northwestern China. Acta Entomol Sin 60(9):1060–1073 (in Chinese)
Yu XD, Zhou HZ, Luo TH (2002) Distribution patterns and their seasonal changes of carabus beetles in Dongling Mountain region near Beijing. Acta Ecol Sin 22(10):1724–1733 (in Chinese)
Yu XD, Luo TH, Zhou HZ (2006) Distribution of carabid beetles among regenerating and natural forest types in Southwestern China. For Ecol Manage 231:169–177
Zhang XZ, Chang H, Zhang X, Duan MC, Li X, Yu ZR, Liu YH (2012) Temporal patterns of carabid beetle diversity in agro–landscape in relation to landscape structure. Chin J Ecol 31(12):3127–3132 (in Chinese)
Zou Y, Sang WG, Zhou HC, Huang LY, Axmacher JC (2014) Altitudinal diversity patterns of ground beetles (Coleoptera: Carabidae) in the forests of Changbai Mountain. Northeast China. Insect Conservat Divers 7(2):161–171
Zou Y, Sang WG, Wang SZ, Warren-Thomas E, Liu YH, Yu ZR, Wang CL, Axmacher CJ (2015) Diversity patterns of ground beetles and understory vegetation in mature, secondary, and plantation forest regions of temperate northern China. Ecol Evol 5(3):531–542
Zou Y, Sang W, Bai F, Brennan E, Diekman M, Liu YH, Li LT, Marples A, Shi HL, Sui ZZ, Sun XJ, Wang CL, Wang X, Warren–thomas E, Yang X, Yu ZR, Axmacher JC (2019) Large–cale α–diversity patterns in plants and ground beetles (Coleoptera: Carabidae) indicate a high biodiversity conservation value of China’s restored temperate forest landscapes. Divers Distrib 25:1613–1624
Acknowledgements
We would like to thank Prof. Hongbin Liang (Institute of Zoology, Chinese Academy of Sciences) and Dr. Hongliang Shi (Beijing Forestry University) for helping to identify carabid specimens.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This research was supported by grants from the National Natural Science Foundation of China (31600517), the Science and Technology Development Project of Jilin Province (20180201059NY), Science and Technology Research Project of Jilin Provincial Education Department (JJKH20190651KJ), Open Project of Key Laboratory of Geographical Processes and Ecological Security of Changbai Mountains, Ministry of Education (GPES202003), and National College Students' Innovation and Entrepreneurship Training Program (202110201030).
The online version is available at http://www.springerlink.com
Corresponding editor: Yanbo Hu.
Rights and permissions
About this article
Cite this article
Liu, S., Dong, S., Liu, R. et al. The distribution patterns and temporal dynamics of carabid beetles (Coleoptera: Carabidae) in the forests of Jiaohe, Jilin Province, China. J. For. Res. 33, 333–342 (2022). https://doi.org/10.1007/s11676-021-01367-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-021-01367-z