Abstract
Changbai Mountain, central in the distribution of Pinus koraiensis, supports a virgin Korean pine forest with vertical gradient distribution. Soil extracellular enzyme activity (EEA) and enzyme stoichiometry (ES) are reliable indicators of the energy and nutrients utilized by microbial communities and of soil nutrient changes. We measured four representative soil EEAs (sucrase, cellulase, urease, acid phosphatase) at two soil layers (A: 0–5 cm and B: 5–10 cm) beneath Korean pine forest at five elevations on Changbai Mountain during growing season. The vertical and seasonal variations of EEAs were analyzed by soil enzyme stoichiometry to quantify the role of soil microorganism in the nutrient cycling process. The activities of four soil extracellular enzymes and the ratios of enzyme activity to soil microbial biomass carbon (EA/ SMBC) did not vary with elevation. The first partition point of multiple regression trees was in September, and the second branch was split by elevation. Seasonal change had more influence on soil enzyme activity (A layer: 75.6%; B layer: 71.3%) than did change in elevation (A layer: 7.8%; B layer: 7.5%). Over one entire growing season, both vector length and vector angle were unchanged by elevation, but varied significantly by month. Among the soil physicochemical factors, available phosphorus and pH were the main factors affecting the four soil EEAs. The ratio of basal area of the coniferous tree to broad-leaved tree species (Scon/Sbr), soil microbial biomass carbon (MBC) and nitrogen (MBN) influenced the four soil EEAs. The results of vector analysis revealed that C and N sources were generally sufficient, but P was limiting (vector angle > 45°). The vector angle for September was significantly higher than for other months. This result verified that phosphorus was the limiting factor affecting soil microorganism function in nutrient metabolism and cycling. Soil enzyme stoichiometry proved to be an efficient index for quantifying soil microorganism-mediated nutrient cycling in the Korean pine ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In a forest ecosystem, microorganisms play important roles in soil C and N turnover by participating in the circulation of material and energy (Lucas et al. 2007; Boeddinghaus et al. 2015). Soil enzymes are important components of the forest ecosystem and important participants in the material cycle and energy flow of the forest ecosystem. The mineralization of organic matter by microorganisms is an important part of the biogeochemical cycle, and the degradation of complicated organic components is realized by secreting extracellular enzymes (Medeiros et al. 2018). Especially, the extracellular enzymes secreted by soil microorganisms are important decomposers of forest litter that is mainly composed of cellulose, lignin and chitin, and facilitate conversion of stable organic carbon to available organic carbon (Grandy et al. 2007; Veres et al. 2015). Four extracellular enzymes, cellulase (CL), sucrase (SC), urease (UE) and acid phosphatase (AP), are the most general indices for evaluating microorganisms related to the acquisition of C, N and P resources (Jian et al. 2016). Therefore, the activity of soil extracellular enzymes has important impacts on biochemical processes, which determine the rates of material circulation and directly affect the soil carbon cycle in forests. In this way, they are also important in global carbon balance and climate change.
Coniferous and broad-leaved mixed forests with Pinus koraiensis as the main constructive species are the most classic, diverse, and largest forest ecosystem among global temperate coniferous/broad-leaved mixed forests. They affect local air temperatures, precipitation, carbon dioxide, nitrogen deposition, and soil carbon storage. Meanwhile, they produce a redistribution effect on various climate factors, i.e., Pan Evaporation, the annual solar radiation amount (Wang et al. 2015). The ecological values of the Korean pine forest ecosystem highlight the importance of understanding soil enzyme characteristics, which represent a key link in the study of soil element circulation in the ecosystem. Spatial and seasonal variation are important factors driving differences in soil enzyme activity (Li et al. 2008; Uksa et al. 2015). However, there is little knowledge about the relative importance of time or space (Baldrian et al. 2013; Ali et al. 2015). Several studies have reported obvious seasonal variation of soil extracellular enzyme activity (Wang et al. 2013; Das and Mondal 2019), whereas other studies reported inconsistent results of soil extracellular enzyme activity at various elevations (Rastin et al. 1988; Pathan et al. 2017). Previous studies failed to compare the dynamic changes of enzyme activity from the perspectives of time and space, leaving a gap in our understanding of the enzymatic characteristics of the Korean pine forest.
Changbai Mountain is well suited to study of climate change in the Northern Hemisphere (Jiang et al. 2014). Changbai Mountain National Nature Reserve has a complete vertical belt of mountain vegetation and is a typical representation of the northeast Asian forest ecosystem (Li et al. 2011). The Korean pine forest on Changbai Mountain is the most complete and representative vertically zoned forest, except for Xiao Xing′an Mountain, also in northeast China (Jiang et al. 2014; Lyu et al. 2017). Its Korean pine forest are mainly distributed at mid-high latitude elevations between 700 and 1300 m.
Because of the unique ecological value of the Korean pine forest ecosystem, we sampled plots at five elevations between 699 and 1177 m. We measured four representative EEAs associated with C, N, and P acquisition (cellulase, sucrase, urease and acid phosphatase) during one growing season. We analyzed the seasonal dynamic changes of soil EEAs over the elevation gradient to quantify the impacts of and season on four EEAs. We studied the relationships between soil EEAs and soil physicochemical properties or microbial biomass C and N pool sizes by use of multiple regression trees (MRT) and variation partition analysis (VPA). We used the soil enzyme stoichiometry of C, N, and P acquisition to analyze resource utilization by soil microorganisms and to evaluate the important role of soil microorganism in the process of element circulation.
Materials and methods
Study sites and sampling
Changbai Mountain National Nature Reserve is located in Jilin Province, China (41°41′49′′–42°25′18′′N, 127°42′55′′–128°16′48′′E). The site has a typical temperate continental monsoon climate, and soils are dark-brown. Annual precipitation is between 800 and 1800 mm. Annual relative humidity is 65–4%. Average annual temperature ranges from 4.9 to 7.3 °C (Zhao et al. 2004; Ping et al. 2017). In 2013, we selected five study sites in Korean pine forests at elevations of 699, 818, 937, 1044, and 1177 m on Changbai Mountain. Three parallel plots (20 × 20 m) separated by a gap of 20 m between each were sampled at each site. The vegetation survey of sample plots at five elevations is presented in Table 1.
We selected ten random observation quadrats (15 × 15 cm) for excavation of soil samples, which were mixed as a single sample for each plot. After removing the litter layer and soil animals on each quadrat, surface soils were sampled in May, July, August, and September in 2013 at depths of 0–5 (A) and 5–10 (B) cm by the natural soil profile sampling method. The soil samples were collected in aseptic bags and then stored with ice bags in an insulated cooler chest. Fresh soil samples, after removal of plant residues and roots, were divided into two subsamples in the laboratory: Some fresh soil was stored at 4 °C for later analysis of soil enzyme activities and microbial biomass, and the remainder was air-dried for determination of physicochemical properties and other soil enzyme activities.
Biomass investigation
In our three sample plots at each of three elevations, we identified to species and measured DBH (diameter at breast height) of living plants for which DBH was > 5 cm. We calculated Scon/Sbr for each sample plot (Table 1). The method used to calculate the basal area was as follows:
where S is the basal area, and DBH is DBH.
Soil physicochemical assays
Soil moisture was determined by use of the low temperature drying method (60 °C for 24 h). Soil pH was measured using a pH meter in soil slurry (soil: water = 1:2.5). Soil organic C content was analyzed with a carbon–nitrogen analyzer (Multi N/C 2 100 s). Soil available N content was measured by using the alkali N-proliferation method. Soil available P content was measured by the Olsen method (Lu 2000).
Soil microbial biomass measurements
The soil microbial biomass C and N were measured by the chloroform-fumigation-extraction method (CFE) after Vance et al. (1987). We used these equations for calculating MBC and MBN:
where FC or FN is the difference of fumigated and unfumigated samples. KEC is the conversion coefficient of MBC, which was 0.45. KEN is the conversion coefficient of MBN, which was 0.54.
Enzyme activity assays
Sucrase and cellulase were assayed by using 3,5-dinitrosalicylic acid colorimetry. For surcease, enzyme activity was expressed as the quality of glucose in 1 g soil for 24 h. Cellulase was indicated by the quality of glucose in 10 g soil for 72 h. Urease was assayed by use of the colorimetric method of ammonium. Acid phosphatase was assayed by the 4-nitrophenyl phosphate disodium salt hexahydrate colorimetric method (Guan 1986).
Vector analysis of soil enzymes
Vector analysis quantifies the regulation capacity of soil microorganisms on C, N and P resources by using the untransformed proportion of enzyme activity related to C, N and P acquisition (e.g.[CL + SC]/[CL + SC + UE] or [CL + SC]/[CE + SU + AP]) (Moorhead et al. 2013). The vector lengths and angles of EEAs were calculated by using Microsoft Excel (Office for Windows 2013). The formulae for vector length and angel were:
where x is [CL + SC]/[CL + SC + AP], y is [CL + SC]/[CL + SC + UE] (Moorhead et al. 2016). Positive correlation between vector length and C-limitation yields a vector angle > 45°, indicating P-limitation. Conversely, a vector angle < 45° indicates N-limitation (Chen et al. 2018; Cui et al. 2018).
Statistical analyses
One-way ANOVA (SPSS 19.0, IBM Corp., Armonk, NY, USA) was used to assess differences in mean soil enzyme activity among samples from the five elevations and two soil layers. Least significant differences (LSD) were calculated to test equality of means of all the measured variables at an alpha level of 0.01. The relationship between soil environmental factors and soil enzyme activity was analyzed by VPA with the vegan package in R 3.4.3 (Vegan Community Ecology Package, https://CRAN.R-project.org/package=vegan) (Zhang et al. 2019). the mvpart package was used for MRT analysis in R (https://ftp.auckland.ac.nz/software/CRAN/src/contrib/Descriptions/mvpart.html) (Ge et al. 2008).
Results
Soil biotic and abiotic factors
All indicators of soil physicochemical properties differed significantly by elevation. Over the course of the growing season, all indicators increased with elevation from 699 to 937 m, where they peaked and then declined with further increase in elevation to 1044 and 1177 m, showing an inverted "V" shaped curve. Each index decreased with increasing soil depth, and all indicators differed by soil layer, except organic carbon at 1044 m and microbial biomass carbon at 699, 818, and 1177 m (Table 2).
Multivariate regression trees analysis
MRT analysis can visualize the relationships of four soil EEAs with environmental variables in a tree diagram with two or three branches based on months or elevations (Fig. 1a and b). For soil layer A, monthly and elevation explained 83.4% of the variance of the soil EEAs (Fig. 1a). The first branch of the MRT for soil EEAs was split by month. The soil EEAs for September were placed into a single group, and the soil EEAs for May, July and August were assigned to one group. This first split in this tree explained 63.3% of the variation in soil EEAs. The two groups were split further by sampling month and sampling elevation, which explained 12.3% and 7.8% of the variation, respectively. The tree of soil EEAs at soil layer B was also split by sampling month into two groups. Monthly dynamics and elevation explained 78.8% of the variance of the soil EEAs (Fig. 1b). The first partition form of soil layer B was the same as that of soil layer A, and the amount of explanation for the variance of the soil EEAs was 71.8%. Only in September, the second segmentation was carried out by 900 m as the node, and the amount of interpretation was 7.5%. Overall, seasonal dynamics was the main factor affecting soil EEAs.
Multivariate regression tree of the soil enzyme activities with different sampling locations/elevations and sampling time. Note: The standardized enzyme activities were used to construct MRT. Bar plots show the multivariate means of enzyme activities at each branch, and the numbers of samples included in that splits are shown under bar plots. Jul, July; Aug, August; Sep, September. a the soil layer of 0−5 cm; b the soil layer of 5−10 cm
Factors affecting soil extracellular enzyme activity
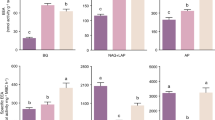
Soil microbial biomass C and available P explained the largest percentage of variation in sucrase activity, 18% and 18%, respectively. The effects of available N, water content, soil microbial biomass N and pH on sucrase were relatively homogeneous, with values of 15%, 15%, 13% and 12%, respectively (Fig. 2a). Scon/Sbr was the main factor affecting cellulase activity, and the interpretable variation was 66%. The interpretable variation in pH was 20% (Fig. 2b). The biotic factor was the main factor influencing the acid phosphatase activity. The effects values of Scon/Sbr, MBC and MBN on acid phosphatase were 19%, 16% and 15%, respectively (Fig. 2c). Among abiotic factors, pH explained most variation (21%), followed by available P, which at 17% (Fig. 2c). Available P explained the largest percentage of variation in urease activity (34%). Soil MBC explained 15% of the variation (Fig. 2d). There were some differences between the main factors influencing activities of the four enzymes and the explained degree of variation. However, MBC, MBN, Scon/Sbr, available phosphorus, and pH had significant effects on the four soil extracellular enzymes.
Ratios of extracellular enzyme activity to soil microbial biomass carbon
The soil microbial community and specific microbial metabolic processes are important in soil material cycling and organic C degradation. The four ratios (EA/SMBC) did not vary by elevation, indicating that elevation did not significantly affect the participation of microorganisms in soil carbon cycling (Table 3).
Vector analysis on soil extracellular enzyme activity
Over the entire growing season, the vector lengths were < 1 at all elevations, and the vector angles were > 45° (Fig. 3). This indicated that C and N resources were sufficient for soil microorganisms, but P was limited regardless of elevation. But month (a surrogate for season) did affect the utilization of nutrients for soil microorganisms. Among the sampling months, the vector length was significantly lower in September than in other months, and the vector angle was markedly higher in September than in other months. The results showed that the C source in September was relatively abundant at all elevations, and P was limited.
Vector lengths and angles in a whole growth season created from proportions of extracellular enzyme activities at different elevations. Note: Statistical characteristics of vector lengths and angles in sampling times created from proportions of extracellular enzyme activities at different elevations. a, c the soil layer of 0–5 cm; b, d: the soil layer of 5 –10 cm; all samples included values for cellulase (CL), sucrase (SC), urease (UE) and acid phosphatase (AP). All values are presented as the means ± standard errors (SE). Enzyme activities in mg kg−1 d−1, angles in degrees, lengths unitless. Calculations for vector length and angle described in text. Two-way ANOVA results are included in the figure to explore the effect of seasonal and elevations
Discussion
Effects of elevation on soil extracellular enzyme activity
Throughout the growing season, the effect of elevation on soil EEA were less than the effects of season. This corroborates the conclusions of Xun et al. (2015) and Peng and Wang (2016) who reported no differences in EEAs and EEAs/MBC by elevation. These results indicate that elevation change had no direct effect on soil EEAs or on the microbial ability to participate in soil nutrient cycling in ecosystems with the same dominant tree species. Others have reported that soil enzyme activity and the soil microbial biomass of forest ecosystems are determined by the dominant tree species (Saetre and Bååth 2000; Šnajdr et al. 2008; Saetre 2010). It has long been known that trees in late succession, especially dominant species, have a particularly homogenous impact on the soil environment.
Effects of seasonal variability on soil enzyme activity
Seasonal dynamics of soil enzyme activity in forests are fundamental to the evaluation of soil quality and material cycles in a regional ecosystems (Medeiros et al. 2018). We detected inconsistency in the seasonal dynamics of soil enzymes at different elevations (Fig. S1). Therefore, we adopted an advanced statistical analysis (multivariate regression trees, MRT) to directly examine the relative importance of elevational and seasonal variation on soil enzyme activity. In our study, MRT was divided for the first time according to a time dimension (month), resulting in significantly different vector lengths and vector angles by month at different elevations. This indicated that seasonal dynamics was an important factor affecting enzyme activity. This is consistent with the result of many studies that the change of soil extracellular enzyme activity in the forest ecosystem is closely related to seasonality ( Rastin et al. 1988; Henry et al. 2012 Siles et al. 2017;). The vector analysis simultaneously showed that the soil microorganisms had relatively stable utilization efficiency of C and sufficient C sources throughout the entire growing season. In contrast to the adequate concentrations of C, soil microorganisms are mainly limited by P resources on Changbai Mountain. This corroborates the findings of Vitousek et al. (2010) who used vector analysis to conclude that soil microorganisms were less limited by C than P resources in tropical rainforest. In fact, it is generally considered that P is the limiting factor of soil microorganism in forest ecosystems (Waring et al. 2014; Chen et al. 2018).
Soil EEA and the vector angle in September were significantly greater than in May, July and August, while the vector length in September was significantly shorter than in other months. Because the litter content increases greatly in autumn, and the "detonator effect" of litter leads to an increase of soil microbial activity and metabolism, soil microbial communities are metabolically active to degrade stabilizing organic C (e.g., cellulose, lignin, and chitin) into active organic C in September. In particular, the significant increases of acid phosphatase and urease activity have been shown to promote P and N cycling, while the organic products could also be used as C sources that enhance microbial decomposition capacity (Šnajdr et al. 2008; Stone et al. 2014; Fan et al. 2014). This result also proved that the dynamic changes of acid phosphatase and urease were closely related to soil C metabolism, which is basically consistent with the continuous decay process of litter.
The role of enzyme stoichiometry in soil environmental assessment
Forest soil enzymes, as products of soil microbial activities, are influenced by ecological factors, such as the physicochemical properties of forest soil, substrate quality, vegetation type, and biodiversity (Salazar et al. 2011; Ge et al. 2012). All of soil extracellular enzymes participate in the process of C metabolism in forest ecosystems and can serve as indicators to reveal the status of C pools and soil microbial communities (Moorhead et al. 2016; Peng and Wang 2016). Most researchers used statistical methods such as canonical correlation analysis (CCA), redundant analysis (RDA), and principal component analysis (PCA) to study relations between soil extracellular enzymes and soil biotic and abiotic factors for exploring the response of soil extracellular enzymes to ecosystem change (Ginrich 1967; Evans et al. 2006; Pietikäinen et al. 2007; Stone et al. 2014; Hendriksena et al. 2016). However, these methods only indicated the correlation and relative influence of various environmental factors on soil extracellular enzymes. The status of the ecosystem was not evaluated and given feedback by the metabolic capacity of the soil extracellular enzymes, and the assessment of a single enzyme activity level has failed to explain the patterns of material circulation in forest ecosystems (Salazar et al. 2011; Chen et al. 2017). Therefore, since the 1990s, Sinsabaugh et al. have tried to use enzyme stoichiometry to assess the regulation of soil microbial metabolism on nutrient cycling and energy flows (Sinsabaugh et al. 2009; Sinsabaugh and Shah 2012; Waring et al. 2014). With the optimization of the enzyme stoichiometry analysis method, the complementary role of enzyme stoichiometry and conventional analytical methods in ecological research have become more apparent.
In our study, the results of MRT and vector analysis were identical. Seasonal dynamics had significant impacts on soil EEAs, but elevation had no impact on soil EEAs. Furthermore, we found that soil available P may be a key factor affecting enzyme activity. Because the results of VPA showed that the effect of available P on soil EEAs was relatively higher than for other physicochemical factors. This was verified by the vector analysis that P source was the limiting factor affecting the soil microbial metabolism level. Therefore, the complementation of soil EEA analysis and ES indicates the P-limitation in the Korean pine forest ecosystem, which effectively proved that soil microorganisms play a significant role in regulating the material cycle of the soil ecosystem by secreting extracellular enzymes (Vitousek et al. 2010; Salazar et al. 2011; Waring et al. 2014; Chen et al. 2018). Dong et al. (2019) found that the content of soil P was significantly and positively correlated with soil EEA, a result inferred by vector analysis and leading to the supposition that increasing P-limitation may be the principal reason for grassland degradation. We conclude that the application of enzyme stoichiometry helps explain the nutrient limitation and energy flows of forest ecosystems.
Conclusion
In the Korean pine forest ecosystem on Changbai Mountain, change in elevation had no direct impact on soil EEA and microbial participation ability in soil nutrient cycling. This verified the conclusion that a constructive species has a profound and consistent impact on the soil environment. Seasonal change also had significant impacts on soil EEA. Over the entire growing season, the C and N resources of Korean pine forest were sufficient, but P was the limiting factor for soil microorganism participating in the material metabolism cycle. Enzyme stoichiometry proved to be an important method to study the regulation of soil microorganisms on nutrient metabolism and cycles.
References
Ali RS, Ingwcersen J, Demyan MS, Funkuin YN, Wizemann HD, Kandeler E, Poll C (2015) Modelling in situ activities of enzymes as a tool to explain seasonal variation of soil respiration from agro-ecosystems. Soil Biol Biochem 81:291–303
Baldrian P, Šnajdr J, Merhautová V, Dobiášová P, Cajthaml T, Valášková V (2013) Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol Biochem 56:60–68
Boeddinghaus RS, Nunan N, Berner D, Marhan S, Kandeler E (2015) Do general spatial relationships for microbial biomass and soil enzyme activities exist in temperate grassland soils? Soil Biol Biochem 88:430–440
Chen H, Luo P, Wen L, Yang LQ, Wang KL, Li DJ (2017) Determinants of soil extracellular enzyme activity in a karst region, southwest China. Eur J Soil Biol 80:69–76
Chen H, Li DJ, Xiao KC, Wang KL (2018) Soil microbial processes and resource limitation in karst and non-karst forests. Funct Ecol 32:1400–1409
Cui YX, Fang LC, Guo XB, Wang X, Zhang YJ, Li PF, Zhang XC (2018) Ecoenzymatic stoichiometry and microbial nutrient limitation in rhizosphere soil in the arid area of the northern loess plateau, China. Soil Biol Biochem 116:11–21
Das C, Mondal NK (2019) Temporal and vertical variation of selected extracellular enzyme activities on tree litter degradation of a subtropical forest. Agric Res 8:84–91
Dong CC, Wang W, Liu HY, Xu XT, Zeng H (2019) Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition: evidence from soil extracellular enzyme stoichiometry. Ecolo Indic 101:453–464
Evans LS, Harnett J, Kahn-Jetter Z (2006) Procedures to determine the amount of plant cover/basal area in field plots. Environ Exp Bot 58(1):180–187
Fan YC, Wang PC, Xiao WF, Zeng LX, Huang ZL, Lei JP (2014) Seasonal dynamics of soil enzymes and its relationship with nutrients for two forests in Three Gorges Reservoir Area. J Huazhong Agri Univ-China 33(4):39–44
Ge Y, He JZ, Zhu YG, Zhang JB, Xu ZH, Zhang LM, Zheng YM (2008) Differences in soil bacterial diversity: driven by contemporary disturbances or historical contingencies? ISME J 2(3):254–264
Ge XG, Xiao WF, Zeng LX, Huang ZL, Huang LL, Tan BW (2012) Relationships between soil nutrient contents and soil enzyme activities in Pinus massoniana stands with different ages in Three Gorges Reservoir Area. Chin J Appl Ecol 23(2):445–451
Ginrich SF (1967) Measuring and evaluating stocking and stand density in upland hardwood forests in the central states. For Sci 13(1):38–53
Grandy AS, Neff JC, Weintraub MN (2007) Carbon structure and enzyme activities in alpine and forest ecosystems. Soil Biol Biochem 39(11):2701–2711
Guan SY (1986) Soil enzyme and its research method. Beijing Agriculture Press, Beijin, China, pp 260–339 (in Chinese).
Hendriksena NB, Creamerb RE, Stone D, Winding A (2016) Soil exo-enzyme activities across Europe-the influence of climate, land-use and soil properties. Appl Soil Ecol 97:44–48
Henry HAL (2012) Soil extracellular enzyme dynamics in a changing climate. Soil Biol Biochem 47:53–59
Husson F, Pagès J, Lê S (2014) Exploratory Multivariate Analysis by Example Using R. CRC Press, Boca Raton, USA
Jian S, Li J, Ji C, Wang G, Mayes MA, Dzantor KE, Hui D, Luo YJ (2016) Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: a meta-analysis. Soil Biol Biochem 101: 32–43.
Jiang YF, Yin XQ, Wang FB (2014) Impact of soil mesofauna on the decomposition of two main species litters in a Pinus koraiensis mixed broad-leaved forest of the Changbai Mountains. Acta Ecol Sinica 34(2):110–115
Li GQ, Bau F, Sang WG (2011) Different responses of radial growth to climate warming in Pinus koraiensis and Picea jezoensis var. komarovii at their upper elevational limits in Changbai Mountain. China Chin J Plant Ecol 35:500–511
Li W, Yu LJ, Li T, Xiang J, Wu G (2008) Seasonal and spatial dynamics of soil enzyme activities and its relationship to soil fertility in Karst ecosystem−a case study of Guilin Yaji Karst Experimental Site. J Agro-Environ Sci 27(1):260–0266
Loeppmann S, Semenov M, Blagodatskaya E, Kuzyakov Y (2016) Substrate quality affects microbial-and enzyme activities in rooted soil. J Plant Nutr Soil Sc 179(1):39–47
Lu RK (2000) Analytical methods for soil and agro-chemistry. China Agricultural Science and Technology Press, Beijing, China, pp 12–111
Lucas RW, Casper BB, Jackson JK, Balser TC (2007) Soil microbial communities and extracellular enzyme activity in the New Jersey Pinelands. Soil Biol Biochem 39(10):2508–2519
Lyu S, Wang X, Zhang Y, li Z, (2017) Different responses of Korean pine (Pinus koraiensis) and Mongolia oak (Quercus mongolica) growth to recent climate warming in northeast China. Dendrochronologia 45:113–122
Medeiros EVD, Duda GP, Junior MAL, Hammecker C (2018) Seasonal effect of land use type on soil absolute and specific enzyme activities in a Brazilian semi-arid region. CATENA 172(1):397–407
Moorhead DL, Rinkes ZL, Sinsabaugh RL, Weintraub MN (2013) Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: informing enzyme-based decomposition models. Fron Microbiol 4(226):1–12
Moorhead DL, Sinsabaugh RL, Brain HH, Weintraub MN (2016) Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol Biochem 93:1–7
Pathan SI, Žifčáková L, Ceccherini MT, Pantani OL, Větrovský T, Baldrian P (2017) Seasonal variation and distribution of total and active microbial community of β-glucosidase encoding genes in coniferous forest soil. Soil Biol Biochem 105:71–80
Peng XQ, Wang W (2016) Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol Biochem 98:74–84
Pietikäinen J, Tikka PJ, Valkonen S, Isomäki A, Fritze H (2007) Is the soil microbial community related to the basal area of trees in a Scots pine stand? Soil Biol Biochem 39:1832–1834
Ping Y, Han DX, Wang N, Hu YB, Mu LQ, Feng FJ (2017) Vertical zonation of soil fungal community structure in a korean pine forest on Changbai Mountain. China World J Microb Bio 33(1):12
Rastin N, Rosenplänter K, Hüttermann A (1988) Seasonal variation of enzyme activity and their dependence on certain soil factors in a beech forest soil. Soil Biol Biochem 20:637–642
Saetre P (2010) Spatial patterns of ground vegetation, soil microbial biomass and activity in a mixed spruce-birch stand. Ecography 22(2):183–192
Saetre P, Bååth E (2000) Spatial variation and patterns of soil microbial community structure in a mixed spruce–birch stand. Soil Biol Biochem 32:909–917
Salazar S, Sánchez LE, Alvarez J, Valverde A, Galindo P, Igual JM, Peix A, Santa-Regina I (2011) Correlation among soil enzyme activities under different forest system management practices. Ecol Eng 37(8):1123–1131
Siles JA, Cajthaml T, Filipová A, Minerbi S, Margesin R (2017) Altitudinal, seasonal and interannual shifts in microbial communities and chemical composition of soil organic matter in alpine forest soils. Soil Biol Biochem 112:1–13
Sinsabaugh RL, Hill BH, Shah JJF (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798
Sinsabaugh RL, Shah JJF (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–342
Šnajdr J, Valášková V, Merhautová VR, Herinková J, Cajthaml T, Baldrian P (2008) Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol Biochem 40:2068–2075
Stone MM, DeForest JL, Plante AF (2014) Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol Biochem 75:237–247
Tabatabai MA, Bremner JM (1972) Assay of urease activity in soils. Soil Biol Biochem 4:479–487
Uksa M, Schloter M, Kautz T, Athmann M, Köpke U, Fischer D (2015) Spatial variability of hydrolytic and oxidative potential enzyme activities in different subsoil compartments. Biol Fert Soils 51(4):517–521
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Veres Z, Kotroczó Z, Fekete I, Tóth JA, Lajtha K, Townsend K, Tóthmérész B (2015) Soil extracellular enzyme activities are sensitive indicators of detrital inputs and carbon availability. Appl Soil Ecol 92:18–23
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15
Wang CY, Lü YN, Lei W, Liu XY, Tian XJ (2013) Insights into seasonal variation of litter decomposition and related soil degradative enzyme activities in subtropical forest in China. J Forestry Res 24(4):683–689
Wang N, Wang MJ, Li SL, Wang NN, Feng FJ, Han SJ (2015) Effects of precipitation variation on growing seasonal dynamics of soil microbial biomass in broadleaved Korean pine mixed forest. Chin J Appl Ecol 26(5):1297–1305
Waring BG, Weintraub SR, Sinsabaugh RL (2014) Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 117:101–113
Xun W, Huang T, Zhao J, Ran W, Wang BR, Shen QR, Zhang RF (2015) Environmental conditions rather than microbial inoculum composition determine the bacterial composition, microbial biomass and enzymatic activity of reconstructed soil microbial communities. Soil Biol Biochem 90:10–18
Zhang MM, Wang N, Zhang JY, Hu YB, Cai DJ, Guo JH, Wu D, Sun GY (2019) Soil physicochemical properties and the rhizosphere soil fungal community in a mulberry (Morus alba L.)/Alfalfa (Medicago sativa L.) intercropping system. Forests 10(2):167
Zhao SQ, Fang JY, Zong ZJ, Zhu B, Shen HH (2004) Composition, structure and species diversity of plant communities along an altitudinal gradient on the northern slope of Mt. Changbai. Northeast China Biodivers Sci 12:164–173
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding editor: Yanbo Hu.
The online version is available at http://www.springerlink.com.
Project funding: This work was supported by the National Natural Science Foundation of China (No. 31670496).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, N., Zhang, M., Zhao, N. et al. Season-dependence of soil extracellular enzyme activities in a Pinus koraiensis forest on Changbai Mountain. J. For. Res. 32, 1713–1722 (2021). https://doi.org/10.1007/s11676-020-01213-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-020-01213-8