Abstract
The aim of this work was to evaluate arbuscular mycorrhizal (AM) fungi as soil indicators and the mycorrhization of native and exotic tree species planted in the Acaraú basin, a transition area from the coast to the Brazilian semiarid region. Plots with 6-year-old trees of four native and three non-native species as well as one non-forested area were evaluated in terms of the diversity of AM fungi in the mycorrhizosphere and the root colonization by AM and ectomycorrhizal (EcM) fungi. Twenty-four AM fungi were identified; Claroideoglomus etunicatum, Glomus sinuosum, Paraglomus albidum, Acaulospora laevis, and Acaulospora brasiliensis were abundant in the forest soil. Diversity, dominance, evenness and richness indices of AM fungi were higher in plots with native trees. All root samples were colonized by AM fungi and only Anadenanthera colubrina, Acacia mangium, Casuarina equisetifolia and Eucalyptus urophylla formed associations with EcM fungi. Acaulospora morphotypes served as soil indicators for coverings with the native species Astronium fraxinifolium and Colubrina glandulosa. Exotic species may favor the proliferation of rarer AM fungi. These fungi–plant relationships may be important in the management of forest systems, and the evidence with mycorrhizal associations allows the inclusion of Brazilian species in tropical reforestation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tropical species are an important agribusiness in Brazil (Moreira et al. 2017) with large areas with Eucalyptus spp. (5.7 million ha), Pinus spp. (1.6 million ha) and other species (0.59 million ha) for the production of timber and cellulose (IBÁ 2017), in addition to vast natural forests which contain a great diversity of plants and tree species (Beech et al. 2017). Plantations are mainly concentrated in the Atlantic Forest and Cerrado biomes (IBÁ 2017). An intensification of forestry activities would be desirable in vulnerable semiarid areas which are found in nine Brazilian states and cover more than 10% of the country. While globally dry forests occupy the largest expanses of land (Bastin et al. 2017), they are very vulnerable to degradation. In the Brazilian semiarid region, there is anthropogenic pressure to convert forested areas into productive agro-systems (Silva et al. 2018). On coastal plateaus with sandy soils which are highly vulnerable to degradation (Mota and Valadares 2011), more conservative practices should be adopted in agricultural and afforestation systems. The planting of species such as the pink lapacho (Handroanthus impetiginosus (Mart. ex DC.) Mattos (Fonseca Filho et al. 2017), vilca (Anadenanthera colubrina (Vell.) Brenan (Monteiro et al. 2006) and other tree species (Silva et al. 2018) are recommended, given the specific objectives of the plantations (Chazdon et al. 2016).
At a regional level in semi-arid areas, there are some reforestation initiatives in Bahia (IBÁ 2017), although there is little information about forest management and the impact of these initiatives on soils. Forests affect soil properties (Aiala-Orozco et al. 2018), particularly biological components (Chandra et al. 2016) as edaphic organisms influence biogeochemical cycles of essential plant nutrients. To improve the understanding of the multi-functionality of agroforestry systems, symbioses with mycorrhizal fungi have been identified as vital (Smith and Read 2008), particularly on sites where crop productivity is limited by available phosphorus (Liu et al. 2018; Pedone-Bonfim et al. 2018).
Arbuscular mycorrhizal (AM) fungi are biotrophic symbionts associated with approximately 70% of vascular plants, while ectomycorrhizal (EcM) fungi form symbioses with forest species (Brundrett and Tedersoo 2018). Both AM and EcM types of symbiosis are associated with Eucalyptus spp. (Mello et al. 2006; Chen et al. 2007; Campos et al. 2011), Acacia spp. (Aggangan et al. 2010), Casuarina spp. (Diagne et al. 2013) and other genera and families of trees (Brundrett and Tedersoo 2018). AM symbioses are related the types of plant species in the Atlantic Forest (Zangaro et al. 2013) and the Caatinga vegetation types (Pagano et al. 2013; Souza and Freitas 2017; Pedone-Bonfim et al. 2018; Sousa et al. 2018). However, the diversity of fungi and symbioses of AM in plantations in the region, especially on arenosols or sandy-textured soils of the coastal tablelands is unknown.
The objective of this study was to evaluate the spore communities of AM fungi as indicators of forest soil fertility, and to determine the intensity of root colonization by AM and EcM fungi with native and exotic tree species planted in the Acaraú basin, a transition zone between the coast and semiarid region. We hypothesized that tree species naturally form symbiosis with AM fungal species that differ in sporulation, resulting in variations in the occurrence and frequency of propagules detected in the rhizosphere which allows for the detection of AM fungi indicative of different vegetative covers. However, some forest species also form EcM associations which may interfere with other plant-mycorrhizal interactions in tropical coastal forest ecosystems.
Material and methods
Plantation sites
This study was carried out in plantations on the coastal basin of Acaraú, a transition area from the coastal dune vegetation to the semi-arid region in Ceará, Brazil (3°6′638″S, 40°4′4.66″W). The climate is classified as Aw (tropical, with a dry winter) (Alvares et al. 2014), in which annual precipitation is almost 900 mm, with the most intense rainfall between February and June, and relative humidity 70%; evaporation can be as high as 1600 mm annually. The soil was classified as Dystrophic Sandy Gray Argisol and the upper horizons indicate a sandy texture with pH 5.5 and low levels of nutrients per kg (0.5 m molc K; 15.1 m molc Ca; 6.3 m molc Mg) (Weber et al. 2019) in addition to the low P content (9.12 mg kg−1). Sandy Argisols are common on the coastal tablelands of the northeast region (Bezerra et al. 2015), are vulnerable to degradation (Mota and Valadares 2011), but they can be used for agriculture with conservation practices, including reforestation.
In plantations on the Acaraú basin (3.6 ha), plots were selected and planted with 6-year-old trees of four native species: (1) Anadenanthera colubrina (Vellozo) Brenan (vilca), (2) Astronium fraxinifolium Schott. Ex Spreng. (kingwood), (3) Handroanthus impetiginosus (Mart. ex DC) Mattos (pink lapacho), (4) Colubrina glandulosa Perkins (sobrasil). An additional three plots were planted with exotic or introduced species: (5) Acacia mangium Willd., (6) Casuarina equisetifolia L., and (7) Eucalyptus urophylla S. T. Blake (clone GG 702). There was one non-forested plot. In the 270 m2 plots, there were 28–30 trees with a 3-m spacing between rows and 2 m between plants in the same row. Fertilization at the base of the seedlings was carried at the time of planting with 120 gof NPK (10:28:20) and 30 g per plant of fritted trace elements BR 12 (3.2% S, 1.8% B, 0.8% Cu, 2.0% Mn, 0.1% Mo, 9% Zn). At 6 months old, the seedlings were given an NPK supplement (50 g plant−1) to promote establishment. Herbaceous vegetation was left on the plots and in the rainy season, a grass cover from the genera Paspalum and Panicum (Poaceae) were present on the non-forested area. In areas protected by tree canopies, herbaceous species of to the genera Commelina (Commelinaceae), Momordica (Cucurbitaceae), Emilia (Asteraceae), among others, were common. This herbaceous stratum was similar to ones in areas of natural vegetation, and was left in place to mitigate the impact of rainfall maintain soil properties.
Soil and root samples
Field sampling took place in four periods, August and November 2016, February and May 2017. From each plot, four composite samples (one per period) were collected, each consisting of 12–15 samples taken from the soil up to 10 cm deep, keeping a minimum 1-m distance from the base of the trees. The samples were passed through a 2-mm sieve to remove roots, larger organic fragments, and mineral aggregates. The sieved samples were kept under refrigeration at 4 °C until analysis.
Fine roots were collected from the field from six to eight trees in each plot. For this collection, roots were followed from the base of the trunks to avoid collecting roots from invasive species. The roots were rinsed under tap water, separated, and placed in containers with a 60% commercial alcohol and 5% acetic acid solution to maintain root integrity.
Density of spores and identification of soil arbuscular mycorrhizal fungi
The spores of the mycorrhizal fungi were extracted from 50 g of soil using the wet-sieving and decanting technique, followed by centrifugation at 2000 RPM with spore suspension in 50% sucrose gradient (Brundrett et al. 1996). Spores were examined under a stereomicroscope (50× to 200×) and separated according to color, size, shape and the characteristics of their surface (Sieverding 1991). Morphological characteristics were available on the INVAM (International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi) located at https://invam.wvu.edu/ and descriptions of these species. Spores from various fungus morphotypes were placed on glass slides using PVLG (polyvinyl alcohol-lactic acid-glycerol) and PVLG + melzer reagent as fixatives in order to facilitate identification. This enabled calculation of the relative abundance (percentage of spores from fungal morphotypes in samples) as well as the relative frequency (percentage of samples from different covers in which fungal morphotypes were present).
Diversity of arbuscular mycorrhizal fungi in soil and the mycorrhizal colonization of trees
Morphotypes and the relative abundance (Pi) of AM fungi enabled Shannon and Weiner’s indexes of diversity (H′), Simpson dominance (Si), evenness (J′) and richness (Ri) to be calculated, accordingto Mirzaei and Moradi (2017) and Magurran (2004). The formulae used were: H′ = − ∑(Pi ln Pi), Si = 1 − ∑(Pi2); J′ = H′lnS−1 and Ri = S(N−1/2), where N represents the number of spores and S the number of AM fungi per 50 g of soil.
Root colonization was evaluated after small roots had been placed in a 10% KOH solution (Phillips and Hayman 1970) and stained with blue aniline in lactoglycerol (875 mL of lactic acid, 63 mL of glycerin, 0.5 g of dye and 62 mL of distilled water). Segments of 1-mm thick stained roots were evaluated by the grid line intersect method according to Giovannetti and Mosse (1980) and Brundrett et al. (1996). Approximately 60 cm of fine roots were examined under a stereomicroscope at 200×; the presence of hyphae, vesicles and other internal root structures characteristic of AM fungi were confirmed under an optical microscope up to 400×. In other stained roots, morphological changes were noted by the presence of mantle and formation of root tips typical of ectomycorrhizae (Brundrett et al. 1996).
Analysis of mycorrhizal attributes
To obtain a better understanding of the mycorrhizal attributes under examination, an agglomerative hierarchical clustering analysis (AHC) was carried out according to the dissimilarities among the vegetation covers. The means of mycorrhizal attributes from the four samples were used to evaluate the vegetative cover. An abnormal distribution of the data (not shown) was observed, and therefore the Spearman dissimilarity matrix was used as an agglomeration technique of unweighted pair-groups (Unweighted Pair-Group Average, UPGMA), with centralized and reduced data. Principal component analysis (PCA) used was Spearman′s, enabling the identification of atypical attributes through average values from the soil analysis, and the separation of vegetation cover among the principal components. Calculations and graphics were generated by the XLSTAT © program version 2016.1 (Addinsoft Inc., Brooklyn, NY, USA).
An analysis of AM fungi species indicators (IndVal) of the soil was carried out (Dufrêne and Leandre 1997) to detect possible linkages between soil fungi and vegetation cover. IndVal values were measured, as was the significance (P) in the Monte Carlo test. Indival values ≥ 0.25 (Silva et al. 2017) and P ≤ 0.1 values were adopted for the indication of AM fungi in vegetation cover.
Results
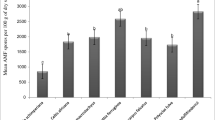
Twenty-four species of AM fungi distributed over seven families and 10 genera were identified (Table 1). The diversity was slightly higher in areas with native species: 17 morphotypes with A. fraxinifolium, 18 with A. colubrina and C. glandulosa, and 20 with H. impetiginosus, compared to areas with exotic species (15 morphotypes with C. equisetifolia and 16 with A. mangium and E. urophylla) or the unforested area (16 fungal morphotypes). These morphotypes were related to AM fungi and some new species could not be excluded. For some soil samples, it was difficult to separate the fungal spores, especially among the genera Acaulospora and Glomus based on their color, shape, size, surface and presence of self-sustaining hyphae. However, the description of fungal populations up to the level of genus may be sufficient for the rapid monitoring of soil AM fungi. In a complementary way, multiphasic analysis may be considered when specific fungus-plant relationships are assessed.
In this study, a variation in the relative abundance of AM fungi was evident throughout the different vegetation covers (Table 1), as well as the relative frequency of the morphotypes in the topsoil. In terms of frequency, the following were prominent: Claroideoglomus etunicatum > Aculospora laevis = Acaulospora brasiliensis > Paraglomus albidum > Acaulospora sp.3 > Glomus sinuosum > Scutellospora calospora. Rare fungi related to A. excavata were observed in the plot planted with E. urophylla, and Glomus aff. fasciculatum was detected in the soil under the C. glandulosa and A. colubrina canopies.
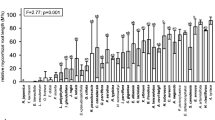
Different spore populations and ecological indices of AM fungi were detected among the different vegetation covers (Table 2), as well as variations in the intensity of root colonization by AM and EcM fungi. Higher values of H′ (1.94) and Si (0.81) occurred in plots with A. fraxinifolium and C. Glandulosa (H′ = 1.92; Si = 0.83), while these indices were particularly low in the area planted with C. equisetifolia (H′ = 1.41; Si = 0.65), an exotic species that substantially reduced AM fungi sporulation in the root zone. Root colonization by AM fungi was evident in all species (Table 3), in particular by the presence of fungal mycelium as well as other fungal structures (vesicles and arbuscules) found in the fine roots. Howewer, the intensity of colonization was higher in the roots of native species, reaching 57% in H. impetiginosus. The AM symbiosis may help to explain the adaptation and performance of native species to edaphic and climatic conditions in the study area. In turn, the colonization of roots by EcM fungi was greater in exotic species, up to 29.9% in E. urophylla. Additionally, reproductive structures typical of EcM fungi or fruiting bodies were also noted under canopies of A. colubrina and exotic species.
The ecological indices of AM fungi and the levels of AM and EcM colonization (Table 2) made it possible to carry out hierarchical cluster analysis (HCA) (Fig. 1) and principal component analysis (PCA) (Fig. 2). Three groups were formed, species in cluster 2 were the most similar (node level 0.268), A. colubrina (native) and A. mangium, both belonging to the Fabaceae (Mimosoideae) family, and able to establish AM and EcM symbioses in addition to nitrogen-fixing bacteria in root nodules (not measured). Cluster 1 group ( node level 0.357) included E. urophylla (Myrtaceae) and C. equisetifolia (Casuarinaceae) (Fig. 1), which showed high levels of root colonization by EcM soil fungi. Cluster 3 species (node level 0.415) included A. fraxinifolium and H. impetiginosus and C. glandulosa, which were similar to the non-forested area.
Dendrogram constructed by UPGMA using mean values of arbuscular mycorrhizal and ectomycorrhizal attributes assessed in forested and non-forested areas; plantations separated by the cluster C1 [Eu (Eucalyptus urophylla), and Ce (Casuarina equisetifolia)]; cluster C2 [Ac (Anadenanthera colubrina), and Am (Acacia mangium)]; and cluster C3 [Hi (Handroanthus impetiginosus), Af (Astronium fraxinifolium), Cg (Colubrina glandulosa), and a non-forested (NF) area]
Principal component analysis using the mean values of arbuscular mycorrhizal and ectomycorrhizal attributes in forested and non-forested areas. Plantations with native species: Ac (Anadenanthera colubrina), Af (Astronium fraxinifolium), Hi (Handroanthus impetiginosus), Cg (Colubrina glandulosa); and exotic species: Am (Acacia mangium), Ce (Casuarina equisetifolia) and Eu (Eucalyptus urophylla); N (fungal spores 50−1 g soil), Ri (Richness), J′ (Evenness), Si (Simpson index), and H′ (Shannon–Wiener index) for AM fungi; root colonization by AM and ectomycorrhizal (EcM) fungi
In Fig. 2, the first two PCA components represent 80.8% of the information and can explain most of the total variance, showing a distinction between plantations, forming groups like the clusters obtained in the HCA. The F1 component separated the native species except for A. colubrina that was colonized by EcM fungi, from the exotic species, placing them on the right side of the graph. There was no clear difference between the non-forested site and plots with C. glandulosa, H. impetiginosus and A. fraxinifolium (cluster 3), particularly in relation to the population of fungal spores (N) and the H′ and Si indices. The F2 component separated the plantations regarding J′ versus Ri with species from cluster 2 (A. colubrina and Acacia mangium), showing higher values for J′ and lower values for Ri than species from cluster 1 (E. urophylla and C. equisetifolia). Root colonization by AM fungi did not contribute significantly to differentiate the plots.
There was a positive correlation between AM spore populations and the value Si, the index of which was also correlated with H′ (Table 3). In turn, richness of AM fungi was inversely correlated with the evenness of the soil fungi. A significant inverse correlation also ocurred between the indices H′ and Si in AM fungi and radicular colonization by EcM soil fungi. This last plant–mycorrhizal ratio was undetected in three of four forest species; some nutritional competition in symbioses with trees should not be disregarded.
The relationship between soil AM fungi and vegetation cover was confirmed (Table 4). Values of IndVal (> 0.4) with high probability (P < 0.05) were found for fungi related to Acaulospora sp1 and Acaulospora sp2 on sites planted with C. glandulosa and A. fraxinifolium. In the cover with native trees, other morphotypes of the genera Glomus, Claroideoglomus and Ambispora frequently occurred, although they did not reach the desired level of significance (P < 0.1). A relationship between E. urophylla and G. margarita was also identified (Table 4), a combination which should be considered in future studies. In addition to the afforestation areas, spores related to S. calospora and R. intraradices were representative for the control area covered with Paspalum and Panicum.
Discussion
This study showed a high diversity of AM fungi in the topsoil under a plantation can be associated with root diversity of the mycotrophic plants and improvement in the physical and chemical properties of the soil. Soil attributes, diversity of roots and environmental factors affect the proliferation of fungal spores and subsequent diversity of AM fungi (Mirzaei and Moradi 2017; Brundrett and Tedersoo 2018).
Some of the spore morphotypes detected could not be related to the fungal species described to this point. They were related to the genera Glomus and Acaulospora. It should be noted that in tropical forest systems new species of AM fungi can occur. Thus, the identification of the fungal spores up to the level of the genus can be considered, especially for monitoring forest systems, similarly to that carried out in natural environments of the semiarid region (Pagano et al. 2013; Zangaro et al. 2013; Retama-Ortiz et al. 2017; Souza and Freitas 2017; Sousa et al. 2018). However, to characterize fungus-plant relationships in a better way, molecular analyses of the symbionts can be used (Dawkins and Esiobu; 2017; Sheldrake et al. 2017; Toju and Sato 2018) as they are complementary to the morphological descriptors used in the monitoring of AM fungal communities.
Fungal spores such as Claroideoglomus, Acaulospora, Paraglomus, Glomus and Scutellospora were frequent within forest covers. The species C. etunicatum is common in semiarid regions and usually included in artificial inoculation tests, as positive responses of these plants to symbiosis have been observed (Pedone-Bonfim et al. 2018). Other fungal species with high frequencies in the soil under forest cover (Table 1) have already been detected in the Caatinga environments, apart from Paraglomus (= Glomus) albidum (Sousa et al. 2018) and Acaulospora (= Ambispora) brasiliensis. The occurrence of A. brasiliensis has been reported in agroforestry systems of the Atlantic Forest and Cerrado biomes, as well as P. albidum in forest succession ecosystems in the Amazon region (Winagraski et al. 2019).
Equally important were the rare fungi associated with A. excavata and G. margarita, detected in areas planted with A. mangium and E. urophylla, respectively, in addition to G. fasciculatum detected within two forest covers (C. glandulosa and A. colubrina). This may be related to litter type and the consequent increase of soil carbon as detected in a forest fragment of Bahia State by Pereira et al. (2018). Microenvironmental factors also affect the proliferation of spores in the rhizosphere. It is recognized that AM fungi are not specific plant hosts but are responsive to the environmental conditions where the host grows (Winagraski et al. 2019), so different tree species could benefit from native mycorrhizal associations. Camara et al. (2017) observed the beneficial response of C. glandulosa seedlings to inoculation with mixtures of Rhizoglomus clarum, G. margarita and Dentiscutata heterogama in an organo-mineral substrate. Pedone-Bonfim et al. (2013) also found that seedlings of A. colubrina responded positively to inoculation with a mixture of Gigaspora albida and A. longula in soil with low availabilty of P (up to 15 mg dm−3 soil).
The AM fungal community status as observed through the ecological indeces (Table 2) could explain the adaptability of the Brazilian species to the soil conditions of this study. Souza and Freitas (2017) also observed higher indices of diversity and greater richness of AM fungal species in the root zones of native Mimosa tenuiflora than in the rhizosperic soil of exotic species such as Prosopis juliflora. However, the exotic species also showed root colonization by EcM fungi in addition to native trees of A. colubrina. EcM symbiosis is common in Casuarina (Diagne et al. 2013), Eucalyptus (Campos et al. 2011), Acacia (Aggangan et al. 2010), and other families and genera of tree species (Brundrett and Tedersoo 2018), suggesting the possibility of the management of mycorrhizal associations in plantations in the region.
The ecological indices of soil AM fungi and the intensities of root colonization by AM and EcM fungi allowed the formation of three clusters: A. colubrina and A. mangium; E. urophylla and C. equisetifolia; and other vegetation covers including the non-forested area. Distinctions between tree covers could also be made by PCA analysis (Fig. 2). Trees of H. impetiginosus showed AM fungal richness and high intensity of root colonization by AM fungi, grouped with C. glandulosa and A. fraxinifolium, presenting high populations of fungal spores and high values of Simpson and Shannon–Wiener indices. Data from the clusters may be suitable for management of symbioses in any new reforestation program in the region.
Some of the mycorrhizal variants have positive correlations (Simpson dominance and Shannon and Weiner’s diversity or population of soil AM fungal spores), and others negative correlations (colonization by EcM fungi and Simpson or Shannon and Weiner’s indices). There is possibly a nutritional competition in the mycorrhization of tree species. According to Liu et al. (2018), plants colonized by AM and EcM fungi share sources of P in the soil (dissolved phosphate, simple phosphate monoesters, and inositol phosphate) and both tree symbioses favor the coexistence of plant species.
The relation between two native species (C. glandulosa and A. fraxinifolium), and Acaulospora could be, along with Glomus, environmental indicators in coastal ecosystem (restinga) vegetation in northeast Brazil (Silva et al. 2017). Otherwise, G. margarita, which showed a low frequency of spores, has been reported in forest fragments cultivated with the introduced E. urophylla in Bahia State (Santos et al. 2013), and in Eucalyptus plantations in southern Brazil (Mello et al. 2006). In addition to the three AM morphotypes, indicative of forest covers, we detected relationships between S. calospora and R. intraradices in the non-forested area covered in the wet season with Paspalum and Panicum. The two fungal species were equally common in the sandy, unfertile soils in the coastal ecosystems in Cuba (González et al. 2016), and they could be considered for the management of pastureland in the region.
To guarantee high diversity of AM fungi in the topsoil under a plantation, mycotrophic native species such as A. colubrina, H. impetiginosus and A. fraxinifolium can be introduced. The first also associates with EcM and potencially with rhizobia, which can result in a more resilient plant cover.
Conclusions
Plantations of native Brazilian species favor the maintenance of the diversity of AM fungi in sandy Argisol of tropical coastal regions. Moreover, in these ecosystems, exotic species of A. mangium, E. urophylla and C. equisetifolia form symbiotic relationships with AM and EcM fungi. The most frequent AM fungi in plantations of A. colubrina, A. fraxinifolium, H. impetiginosus, C. glandulosa, A. mangium, C. equisetifolia, E. urophylla are Acaulospora, Claroideoglomus, Paraglomus, and Scutellospora. On one hand, morphotypes of fungi related to Acaulospora served as indicators of the covers with native A. fraxinifolium and C. glandulosa. On the other hand, Gigaspora was an indicator of tree covers with Eucalyptus, and Rhizoglomus and Scutellospora were indicators of the non-forested area.
The fungus-plant relationships may be considered in the management of forest systems, and the evidence from mycorrhizal associations reinforce the inclusion of the Brazilian species in reforestation programs for tropical regions. In addition, the selection will depend on the desired economic and environmental benefits. Evidence of mycorrhizal associations may lead to improvements in plant growth as well as benefits for nutrient cycling.
References
Aggangan NS, Moon HK, Han SH (2010) Growth response of Acacia mangium Willd. seedlings to arbuscular mycorrhizal fungi and four isolates of the ectomycorrhizal fungus Pisolithus tinctorius (Pers.) Coker and Couch. New For 39(2):215–230
Aiala-Orozco B, Gavito ME, Mora F, Siddique I, Balvanera P, Jaramillo VJ, Cotler H, Romero-Duque LP, Martínez-Meyer E (2018) Resilience of soil properties to land-use change in a tropical dry forest ecosystem. Land Degrad Dev 29(2):315–325
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2014) Köppen’s climate classification map for Brazil. Meteorol Z 22(6):711–728
Bastin JF, Berrahmouni N, Grainger A, Maniatis D, Mollicone D, Moore R, Patriarca C, Picard N, Sparrow B, Abraham EM, Aloui K, Atesoglu A, Attore F, Bassüllü C, Bay A, Garzuglia M, García-Montero LG, Groot N, Guerin G, Lastadius L, Lowe AJ, Mamane B, Marchi G, Patterson P, Rezende M, Ricci S, Salcedo I, Diaz ASP, Stolle F, Surappaeva V, Castro R (2017) The extent of forest in dryland biomes. Science 356(6338):635–638
Beech E, Rivers M, Oldfield S, Smith PP (2017) Global tree search: the first complete global database of tree species and country distributions. J Sustain For 36(5):454–489
Bezerra CEE, Ferreira TO, Romero RE, Mota JCA, Vieira JM, Duarte LRS, Cooper M (2015) Genesis of cohesive soil horizons from northeast Brazil: role of argilluviation and sorting of sand. Soil Res 53(1):43–55
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbiosis and global host plant diversity. New Phytol 220(4):1108–1115
Brundrett MC, Bougher N, Dell B, Grove T, Malajcczuk N (1996) Working with mycorrhizas in forestry and agriculture. AClAR, Canberra. Monograph 32
Camara R, Fonseca Junior AM, Sousa ACO, Pereira MG, Oliveira Júnior JQ (2017) Influência do substrato e inoculação micorrízica na produção de mudas de Colubrina glandulosa Perkins. Rev Floresta 47(4):449–458
Campos DTS, Silva MCS, Luz JMR, Telesfora RJ, Kasuya MCM (2011) Colonização micorrízica em plantações de Eucalipto. Rev Árvore 35(5):965–974
Chandra LR, Gupta S, Pande V, Singh N (2016) Impact of forest vegetation on soil characteristics: a correlation between soil biological and physico-chemical properties. 3 Biotech 6:188. https://doi.org/10.1007/s13205-016-0510-y
Chazdon RL, Brancalion PHS, Laestadius L, Bennett-Curry A, Buckingham K, Kumar C, Moll-Rocek J, Vieira ICG, Wilson SJ (2016) When is a forest a forest? Forest concepts and definitions in the era of forest and landscape restoration. Ambio 45(5):538–550
Chen YL, Liu S, Dell B (2007) Mycorrhizal status of Eucalyptus plantations in south China and implications for management. Mycorrhiza 17(6):527–535
Dawkins K, Esiobu N (2017) Arbuscular and ectomycorrhizal fungi associated with the invasive Brazilian pepper tree (Schinus terebintholius) and two native plants in South Florida. Front Microbiol 8:665
Diagne N, Diouf D, Svistoonoff S, Kane A, Noba K, Franche C, Bogusz D, Duponnois R (2013) Casuarina in Africa: distribution, role and importance of arbuscular mycorrhizal fungi and Frankia on development. J Environ Manag 128:204–209
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for flexible asymmetrical approach. Ecol Monogr 67(3):345–366
Fonseca Filho IC, Bomfim BLS, Farias JC, Vieira FJ, Barros RFM (2017) Pau-d’arco-roxo (Handroanthus impetiginosus (Mart. Ex DC.) Mattos: conhecimento e uso madeireiro em comunidades rurais do Nordeste do Brasil. Gaia Sci 11(2):57–70
Giovannetti M, Mosse EB (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84(3):489–500
Gonzalez SG, Torres-Arias Y, Ortega-Fors R, Gómes EF (2016) Hongos micorrizógenos arbusculares (Glomeromycota) de laplaya Santa Maríadel Mar, Cuba. Rev Jardin Botánico Nacional 37:81–84
IBÁ (Indústria Brasileira de Arvores) (2017) Brazilian tree industry—Report 2017. https://iba.org/images/shared/Biblioteca/IBA_RelatorioAnual2017.pdf. Accessed 10 Apr 2018
Liu X, Burslem DFRP, Taylor JD, Taylor AFS, Khoo E, Majalap-Lee N, Helgarson T, Johnson D (2018) Partitioning of soil phosphorus among arbuscular and ectomycorrhizal trees in tropical and subtropical forests. Ecol Lett 21(5):713–723
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Malden and Oxford
Mello AH, Antoniolli ZI, Kaminski J, Souza EL, Oliveira VL (2006) Fungos arbusculares e ectomicorrízicos em áreas de eucalipto e de campo nativa em solo arenoso. Ciência Florestal 16(3):293–301
Mirzaei J, Moradi M (2017) Relationships between flora biodiversity, soil physiochemical properties, and arbuscular mycorrhizal fungi (AMF) diversity in a semi-arid forest. Plant Ecol Evol 150(2):151–259
Monteiro JM, Almeida CFCB, Albuquerque UP, Lucena RFP, Florentino ATN, Oliveira RIC (2006) Use and traditional management of Anadenanthera colubrina (Vell.) Brenan in semi-arid region of northeastern Brazil. J Ethnobiol Ethnomed 2(6):1–7
Moreira JMMA, Simion FJ, Oliveira EB (2017) Importância e desempenho das florestas plantadas no contexto do agronegócio brasileiro. Floresta 47(1):85–94
Mota LHSO, Valladares CS (2011) Vulnerabilidade à degradação dos solos da Bacia do Acaraú, Ceará. Rev Ciência Agron 42(1):39–50
Pagano MC, Zandavalli RB, Araújo FS (2013) Biodiversity of arbuscular mycorrhizas in three vegetational types from the semi-arid of Ceará state, Brazil. Apl Soil Ecol 67:37–46
Pedone-Bonfim MVL, Lins MA, Coelho IR, Santana AS, Silva FSB, Maia LC (2013) Mycorrhizal technology and phosphorus in the production of primary and secondary metabolites in cebil (Anadenanthera colubrina (Vell.) Brenan) seedlings. J Sci Food Agric 93:1479–1484
Pedone-Bonfim MVL, Silva DKA, Maia LC, Yano-Melo AM (2018) Mycorrhizal benefits on native plants of the Caatinga, a Brazilian dry tropical forest. Symbiosis 74(2):79–88
Pereira JES, Barreto-Garcia PAB, Scoriza RN, Saggin Júnior OJ, Gomes VS (2018) Arbuscular mycorrhizal fungi in soils of arboreal Caatinga submitted to forest management. Rev Bras Ci Agrar 13(1):e5497
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55(1):158–161
Retama-Ortiz Y, Ávila-Bello CH, Alarcón A, Ferrera-Cerrato R (2017) Effectiveness of native arbuscular mycorrhiza on the growth of four tree forest species from Santa Marta Mountain, Veracruz (Mexico). For Syst 26(1):1–9
Santos RS, Scoriza RN, Ferreira JS (2013) Fungos micorrízicos arbusculares em diferentes coberturas florestais de Vitória da Conquista Bahia. Floresta Ambiente 20(3):344–350
Sheldrake M, Rosenstock NP, Revillini D, Olsson PA, Mangan S, Sayer EJ, Wallander H, Turner BL, Tanner EV (2017) Arbuscular mycorrhizal fungal community composition is altered by long-term litter removal but not litter addition in a lowland tropical forest. New Phytol 214(1):455–467
Sieverding E (1991) Vesicular–arbuscular mycorrhiza management in tropical agrosystems. GTZ, Eschborn
Silva IR, Silva DKA, Souza FA, Oehl F, Maia LC (2017) Changes in arbuscular mycorrhizal fungal communities along a river delta island in northeastern Brazil. Acta Oecol 79:8–17
Silva UBT, Dalgado-Jaramillo M, Aguiar LMS, Bernard E (2018) Species richness, geographic distribution, pressures, and threats to bats in the Caatinga drylands of Brazil. Biol Conserv 221:312–322
Smith SR, Read D (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, New York. p, p 787
Sousa NMF, Veresoglou SD, Oehl F, Rillig MC, Maia LC (2018) Predictors of arbuscular mycorrhizal fungal communities in the Brazilian tropical dry forest. Microb Ecol 75(2):447–458
Souza TAF, Freitas H (2017) Arbuscular mycorrhizal fungal community assembly in the Brazilian tropical dry forest. Ecol Process 6(3):2–10
Toju H, Sato H (2018) Root-associated fungi shared between arbuscular mycorrhizal and ectomycorrhizal conifers in a temperate forest. Front Microbiol 9(433):1–11
Weber OB, Silva MCB, Silva CF, Sousa JA, Taniguch CAK, Garruti DS, Romero RE (2019) Biological and chemical attributes of soils under forest species in Northeast Brazil. J For Res. https://doi.org/10.1007/s11676-019-00982-1
Winagraski E, Kaschuk G, Monteiro PHR, Auer CG, Higa AR (2019) Diversity of arbuscular mycorrhizal fungi in forest ecosystems of brazil: a review. CERNE 25(1):25–35
Zangaro W, Rostirola LV, de Souza PB, de Almeida AR, Lescano LE, Rondina AB, Nogueira MA, Carrenho R (2013) Root colonization and spore abundance of arbuscular mycorrhizal fungi in successional stages from Atlantic rainforest biome in southern Brazil. Mycorrhiza 23(3):221–233
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This work was financially supported by SINDIMÓVEIS (Sindicato das Indústrias de Móveis do Ceará), ADECE (Agência de Desenvolvimento do Estado do Ceará), and Embrapa (code 12.13.07.006.0002).
The online version is available at http://www.springerlink.com.
Corresponding editor: Yanbo Hu.
Rights and permissions
About this article
Cite this article
Weber, O.B., da Silva, M.C.B., da Silva, C.F. et al. Diversity of mycorrhizal fungi and soil indicative species in coastal plantations of northeast Brazil. J. For. Res. 32, 1203–1211 (2021). https://doi.org/10.1007/s11676-020-01190-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-020-01190-y