Abstract
We studied the impacts of liana cutting as a forest management tool on liana diversity (species richness, Shannon diversity index) and community structure (diameter distribution, basal area, species dominance) in the Asenanyo Forest Reserve, Ghana. Two types of silviculturally treated forests were studied: Logging treated (LT) and Tropical Shelterwood System (TSS) treated forests. An untreated primary forest was included as a control, resulting in three forest management systems. Lianas with diameter ≥2 cm were identified in ten 40 × 40 m2 plots within each management system. Liana cutting significantly reduced liana species richness, Shannon diversity index, and basal area in the LT forest after two decades. However, liana species richness and basal area were comparable in the TSS treated and untreated forests, indicating significant recovery in the former after over six decades. Sørensen similarity index of liana species composition between the untreated forest and each of the treated forests was moderate. Our findings suggest that liana cutting most likely influenced the dominance of some liana species. In view of the adverse impact of blanket liana cutting on liana diversity, selective liana cutting is recommended as a means of controlling liana numbers while maintaining liana diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical forests are important ecosystems as they support a significant proportion of global biodiversity (Douglas et al. 2005). They contain about two-thirds of known plant species and 90 % of known insect species (cf. Douglas et al. 2005). In view of their high biodiversity value and the severe anthropogenic threats they face, tropical forests are usually managed to conserve their biodiversity. Although there are several types of silvicultural methods, liana cutting remains one of the most preferred forms of silviculture used to manage tropical forests (Alvira et al. 2004; Addo-Fordjour et al. 2013).
Lianas have both positive and negative effects on tropical forest diversity and ecosystem functioning (Bongers et al. 2005; Addo-Fordjour et al. 2008; Schnitzer et al. 2011; Tang et al. 2012). For instance, lianas provide food resources for tree dispersers thereby helping to maintain diversity of trees and their dispersers (Bongers et al. 2005). On the other hand, by competing strongly with trees, lianas can impede tree growth and natural regeneration (Schnitzer et al. 2005; Schnitzer et al. 2011). Consequently, liana cutting is an important silvicultural treatment and forest management tool that can be used to maintain biodiversity in some tropical forests. Although liana cutting is the commonest silvicultural treatment for managing lianas in the tropics, uncontrolled burning of lianas has also been practiced in some forests (Parren 2003). The principal objective of liana cutting as a management tool is to reduce liana numbers and ultimately mitigate their impacts on trees (cf. Alvira et al. 2004). Even though much is known about liana cutting as a tool to reduce liana numbers (Gardette 1998; Foli and Pinard 2009; Addo-Fordjour et al. 2013) and enhance tree growth and reproduction (cf. Parren and Bongers, 2005), little is known about the impact of that activity on liana diversity. Only a few studies have assessed the impact of liana cutting on liana species richness (e.g. Gardette 1998; Gerwing and Vidal 2002; Campanello et al. 2012; Addo-Fordjour et al. 2014). The findings of the studies that assessed the impact of liana cutting on liana species richness have been mixed. Gerwing and Vidal (2002) reported that liana cutting was responsible for a considerable reduction in liana species richness 8 years after the activity. In contrast, Gardette (1998) reported that liana species richness in a Malayan Uniform System (MUS) treated forest that underwent complete liana cutting was comparable with that of an untreated forest 40 years after liana cutting. Campanello et al. (2012) reported that liana cutting had no impact on liana species richness in a subtropical forest 10 years after cutting. Addo-Fordjour et al. (2014) reported that liana cutting caused a significant reduction in liana species richness in a MUS treated forest (in relation to an untreated forest) 19 years after cutting. In a 42 year old MUS treated forest, liana species richness was restored to a level similar to that of an untreated forest (Addo-Fordjour et al. 2014). These studies suggest that the duration of liana cutting could be an important factor in recovery of liana species richness.
Most studies that assessed the impacts of liana cutting on liana community structure considered only liana abundance, neglecting equally important liana structural attributes such as stem basal area and species dominance. Some previous studies indicated that liana cutting resulted in a significant reduction in liana stem basal area after less than 10 years (Gerwing and Vidal 2002; Alvira et al. 2004), 10 years (Campanello et al. 2012), and 40 years (Foli and Pinard 2009). To date, there is no information about how liana cutting impacts liana species dominance in tropical forests. To gain better understanding of the impacts of liana cutting on liana assemblages, the role of liana cutting on liana species dominance must be studied, since species dominance provides a better estimate of plant species importance in a community than does abundance only.
The aim of this study was to determine the impacts of liana cutting on liana diversity and community structure in silviculturally treated forests in the Asenanyo Forest Reserve, Ghana. The following research questions were addressed by the study: (1) what are the impacts of liana cutting on diversity of liana communities in treated forests? (2) what are the impacts of liana cutting on liana community structure in treated forests?
Materials and methods
Study area
Our study was conducted in the Asenanyo Forest Reserve, Ghana from August to December 2012. The Asenanyo Forest Reserve occurs in Nkwawie District (06°26′23″ N, 02°06′28″ W). It is comprised of lowland forests that are situated within the moist semi-deciduous forest zone in the country. There are old growth primary and secondary forests in the forest reserve. The secondary forest has the relics of both past and present human activities such as selective and illegal logging, and silvicultural treatments (logging and liana cutting). Celtis mildbraedii, Triplochiton scleroxylon, Albizia zygia and Cedrella odorata are the major tree species in the forest reserve. The forest reserve has daily temperatures ranging from 20 to 32.9 °C, and average annual rainfall of 1856 mm. The relative humidity for the forest reserve is about 91 %.
Site selection and sampling
Three different forest management regimes were selected for the current study, viz. Logging treated (LT) forest, Tropical Shelterwood System (TSS) treated forest, and an untreated primary forest. Logging in the LT forest during 1990–1992 was accompanied by extensive cutting of climbers (i.e. during-logging liana cutting). The TSS treated forest was silviculturally treated from 1949 to 1959 with the aim of improving tree regeneration. TSS involved a series of activities which lasted for a total of 10 years (Parren and de Graaf 1995; Sackey 2007). The activities included (a) climber cutting, (b) removal of lower storey non-valuable trees and larger crowned understorey trees in order to open the canopy, (c) cleaning over a number of years, (d) timber exploitation, and (e) finally climber cutting again (two stages of cutting). Thus, the TSS operations started and ended with climber cutting at two main stages, one before and two after harvesting of exploitable trees (Parren and de Graaf 1995). Consequently, the TSS treated forest experienced both pre-logging and post-logging climber cutting, and the LT forest underwent only one stage of climber cutting at the time of logging (hereafter, all are referred to as “liana cutting”). An untreated forest which was an old growth primary forest was added as another forest management system to serve as a control. The addition of the untreated forest made it possible to determine the extent of recovery of liana assemblages since the application of the silvicultural treatments in the treated forests. The LT forest covered a total area of about 16 ha in the forest reserve, and the TSS treated covered an area of 15 ha. The untreated forest was about 20 ha in area. The three forest management systems were separated from one another by a minimum distance of 4 km.
Each forest management system was represented by two independent replicate sampling sites of approximately equal size. Each site was separated by a distance ≥620 m from the other site within the same forest management system. Within a sampling site, five 40 × 40 m2 plots were randomly demarcated. Plots were separated by a minimum distance of 250 m. A total of 10 plots covering 1.6 ha were sampled in each forest management system. Within each sampling plot, lianas of diameter ≥2 cm that were hosted by trees (dbh ≥ 10 cm) were identified. Lianas were followed to the ground to determine whether they were attached to stems already enumerated. Only independent rooted lianas were considered as separate individuals. Plant identification was done with the assistance of plant taxonomists. Reference was also made to Floras (Arbonnier 2004; Hawthorne and Jongkind 2006). Nomenclature was in accordance with Jongkind (2005), and Hawthorne and Jongkind (2006). Voucher specimens were deposited at the herbarium of the Department of Theoretical and Applied Biology, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Data analyses
Differences in characteristics of biological assemblages in separate plots may cause sampling bias irrespective of whether the same sampling methods and efforts are used (Hortal et al. 2006). This type of bias can cause variations in species abundance within separate plots that can affect the number of species counted. For this reason, differences in species abundance of forest plots must be corrected using an appropriate statistical tool. Differences in liana species abundance among the forest management plots in the current study were corrected by conducting rarefaction analysis, which estimated species richness for a standardised number of individuals across all the forest plots. Rarefaction analysis was run using the software Estimate S (Colwell 2009). Although differences in species abundance can cause sampling bias, it can sometimes occur as a result of real and meaningful biological patterns in nature (Gotelli and Cowell 2001). When this happens, observed species richness values would be real and unbiased even when species abundance differences occur between separate plots. For this reason, we also recorded observed species richness for all sampling plots.
Shannon diversity index was computed following Magurran (1988):
where pi is the proportion of the ith species, In pi is the natural log of pi.
Shannon diversity index for the forest management systems was estimated using PAST Programme (Version 2.15) (Hammer and Harper, Oslo, Norway). To test for significant differences in Shannon diversity index between the forest management systems, pairwise bootstrap tests were conducted using the PAST programme. Thus, we used liana Shannon diversity index and species richness (observed and rarefied species richness) as the measures of liana diversity.
Differences in liana species richness (observed and rarefied) and stem basal area between each treated forest and the control (untreated) forest were tested with t test. All the data fulfilled the normality and homogenous variance assumptions of t-test with the exception of basal area, which was log10 transformed. The t-test was conducted using the eleventh edition of GenStat Software (VSN International Ltd, Hemel Hempstead, UK) at a significant level of 5 %.
Liana community structure was represented by liana diameter distribution, basal area and species dominance. Liana species dominance was determined by computing the importance value index (IVI) of the liana species. The I VI of the species was calculated following Cottam and Curtis (1956):
where R D is relative density, R F is relative frequency, R BA is relative basal area.
Sørensen similarity index, S, was calculated and used to assess similarity of liana species composition between the untreated and treated forests. The following equation was used for the calculation of the index (Magurran 1988):
where C is number of species common to the two forests, a the number of species in forest A, and b is number of species in forest B.
Results
In total, we recorded 99 liana species, distributed in 51 genera and 24 families (Appendix S1 see Supplemental Data with online version of this article). There were 20, 18 and 10 liana species which exclusively occurred in the untreated, TSS treated and LT forests, respectively. Three liana families namely, Apocynaceae, Fabaceae and Celastraceae contributed the highest number of species in each of the forest management systems (LT forest: 10, 8 and 7 species, respectively; TSS treated forest: 10, 8 and 8 species, respectively; untreated forest: 11, 8 and 8 species, respectively). In addition, Combretaceae also contributed highly to liana species richness (10 species) in the untreated forest.
Liana species richness was significantly lower in the LT forest than in the untreated forest (Fig. 1a, p = 0.021 and 0.001 for observed and rarefied species richness, respectively). Similarly, liana Shannon diversity index was significantly lower in the LT forest than in the untreated forest (Fig. 1b, p = 0.005). Liana species richness in the TSS treated forest was similar to that in the untreated forest (Fig. 1c, p = 0.404 and 0.393 for observed and rarefied species richness, respectively). Nevertheless, Shannon diversity index of lianas was significantly higher in the untreated forest than in the TSS treated forest (Fig. 1d; p = 0.008). Similarity of liana species composition between the untreated and TSS treated forests was 0.62, and that between the LT and untreated forests was 0.50.
Comparison of liana species richness (OSR observed species richness, RSR rarefied species richness) and Shannon diversity index in the LT and untreated forests (a and b, respectively), and TSS treated and untreated forests (c and d, respectively). Similar bars with different letters that fall under different forest management systems are significantly different at p < 0.05. Shannon diversity index does not have error bars because the values represent total Shannon diversity index (but not means) for each forest
Three liana species namely, Griffonia simplicifolia (IVI; LT forest = 31.32, TSS treated forest = 30.26 and untreated forest = 35.60), Millettia crhysophylla (IVI; LT forest = 30.07, TSS treated forest = 15.09 and untreated forest = 34.15) and Alafia barteri (IVI; LT forest = 45.10, TSS treated forest = 25.57 and untreated forest = 18.72) showed high IVI in all forest management systems (Appendix S1). The five most dominant liana species in the LT forest were A. barteri (45.10), G. simplicifolia (31.32), M. chrysophylla (30.07), Alafia whytei (23.83) and Calycobolus africanus (21.48). These liana species accounted for 50.6 % of the important value index in the LT forest. In the TSS treated forest, the five most dominant species were G. simplicifolia (30.26), A. barteri (25.57), M. chrysophylla (15.09), Motandra guineensis (14.67) and Landolphia dulcis (14.41), which together contributed 33.3 % of the important value index. In the case of the untreated forest, G. simplicifolia (35.60), M. chrysophylla (34.15), M. guineensis (30.01), A. barteri (18.72) and Combretum paradoxum (10.55) were the five most dominant species. These species contributed about 43.0 % of the IVI in the untreated forest. Liana species such as A. barteri, A. whytei, C. africanus, Salacia alata and Strophanthus sp. had higher IVI in the treated forests than in the untreated forest. On the other hand, liana species such as M. guineensis and M. chrysophylla recorded higher IVI in the untreated forest than in the treated forests. G. simplicifolia had high IVI in all three forest management regimes.
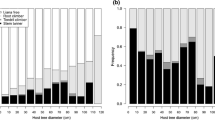
Liana diameter distribution followed an inverted J-curve for all three forest types (Fig. 2). For all diameter classes, there were more liana individuals in the untreated forest than in the LT forest (Fig. 2a). However, liana numbers at most of the diameter classes in the TSS treated forest were comparable with those in the untreated forest (Fig. 2b). Liana stem basal area was significantly lower in the LT forest than in the untreated forest (Fig. 3a, p = 0.005). Liana stem basal area in the TSS treated forest was comparable with that in the untreated forest (Fig. 3b, p = 0.816).
Discussion
Our results indicate that liana cutting impacted liana diversity (species richness and Shannon diversity index). The impact of the silvicultural activity was evident in the LT forest as its liana diversity was considerably lower than that in the untreated forest. However, lianas increased in species richness in the TSS treated forest to a level comparable with that in the untreated forest. A similar finding was also reported by Gardette (1998) in a lowland Malaysian forest, where liana species richness in a treated forest increased to a level comparable with that in an untreated forest after more than six decades. Our finding in the TSS treated forest and the report of Gardette (1998) suggest that following liana cutting, it can take a long time (>four decades) for pre-cutting liana diversity to restore. Thus, liana diversity in the LT forest might increase in future relative to that in the untreated forest. Since a pre-liana cutting inventory was not carried out for the untreated or treated forests prior to liana cutting, it is not possible to determine whether liana cutting eliminated sensitive liana species from the treated forests. However about 20 % of the species identified (20 species) in this study were exclusive to the untreated forest. It is possible that those species were initially present in the treated forest prior to liana cutting but were eliminated by cutting. Thus, about 20 % of the total liana species richness we recorded might have been adversely affected by liana cutting. Although the TSS treated forest recovered species richness, its liana Shannon diversity index still remained lower than that of the untreated forest as a result of lower species evenness. Analysis of liana species composition in this study indicated that similarity of liana species composition was moderate between the untreated forest and each treated forest. This finding indicates that although treated forests can recover species richness, their composition might differ from that of untreated forests due to the inability of some liana species to recover from cutting. This phenomenon was demonstrated by Parren and Bongers (2001) who reported that only 30 % of liana species survived after two years of liana cutting, and that the surviving species could explain a shift in species composition over time. Thus, the variations in liana species composition between the treated and the untreated forests in the current study suggest that liana species might have differed in their sensitivity to cutting, as suggested by Parren and Bongers (2001).
The results of the present study suggest that liana community structure was also influenced by liana cutting. The diameter distribution pattern recorded in the TSS treated forest showed that this forest type largely recovered its large diameter lianas (diameter ≥ 5 cm) when compared with the untreated forest. However, the number of very large lianas (diameter > 11 cm) in the TSS treated forest was about half that in the untreated forest, indicating that the TSS treated forest probably needed more time to recruit more lianas into the very large diameter class. The response of liana stem basal area to the silvicultural treatments was similar to that exhibited by liana species richness described above. The LT forest did not fully recover its liana basal area, which was significantly lower than that in the untreated forest. Thus, the impact of liana cutting on liana basal area was still evident in the LT forest two decades after cutting. Nonetheless, the TSS treated forest recovered its liana basal area to a level comparable with that in the untreated forest. The recovery trend observed in the TSS treated forest differs from the result of a similar study conducted in Ghana in which treated forests did not recover their liana basal area over the long term (Foli and Pinard 2009). The silvicultural activities in the treated forests most probably influenced liana species dominance in the current study. The dominance of some liana species was lower in the treated forests than in the untreated forest, while the dominance of other species was higher in the treated forests than in the untreated forest. The variation in liana species dominance between the untreated and treated forest management regimes might be partly due to differences in the ability of various liana species to tolerate cutting (Parren and Bongers 2001). The patterns of liana species dominance in the treated and untreated forests might reflect differences and similarities in liana species composition, distribution, abundance, and basal area among the forests.
Implications for conservation
Although liana cutting is generally used as a form of silvicultural treatment to control liana numbers and enhance tree diversity, it might adversely affect liana diversity. This study demonstrated that liana cutting significantly reduced liana diversity in the LT forest. Since lianas contribute greatly to plant diversity in tropical forests (Schnitzer and Bongers 2002; Addo-Fordjour et al. 2008), liana cutting can reduce the overall plant diversity of treated forests in the tropics. Judging from the liana diversity recovery patterns in the LT and TSS treated forests, we conclude that treated forests take many years to recover their pre-cutting liana diversity. Thus, indiscriminate liana cutting can affect forest biodiversity in totality, since apart from adding to forest diversity themselves, lianas generally play an integral role in maintaining diversity in tropical forest ecosystems (Schnitzer and Bongers 2002; Tang et al. 2012). For this reason, wholesale liana cutting should be discouraged and selective liana cutting should be encouraged in tropical forest ecosystems. Selective liana cutting can reduce liana numbers while maintaining liana diversity in treated forests. Selective liana cutting can be applied to the most abundant liana species in a particular forest so as to reduce their numbers (Addo-Fordjour et al. 2014). Furthermore, liana cutting could be limited to liana species that cause most damage to their host trees. In this case, mechanical property tests should be carried out on liana species to determine which species inflict most damage to trees.
Conclusion
Liana cutting contributed to significant reductions in liana diversity and basal area in the LT forest after two decades. However, the TSS treated forest recovered its liana species richness and basal area to levels similar to those in the untreated forest after more than six decades of liana cutting. Liana cutting probably influenced liana species dominance in the treated forests. In view of the important ecological roles of lianas in tropical forests, it is recommended that selective liana cutting be used to control lianas because this can reduce the effects of liana cutting on liana diversity.
References
Addo-Fordjour P, Anning AK, Atakora EA, Agyei PS (2008) Diversity and distribution of climbing plants in a semi-deciduous rain forest, KNUST Botanic Garden. Ghana Int J Bot 4(2):186–195
Addo-Fordjour P, Rahmad ZB, Asyraf AMS (2013) Impacts of forest management on liana abundance and liana-tree relationships in a tropical forest, Malaysia: implications for forest conservation. Int J Biodivers Sci Ecosyst Serv Manag 9(1):13–20
Addo-Fordjour P, Rahmad ZB, Shahrul AMS (2014) Impacts of forest management on community assemblage and carbon stock of lianas in a tropical lowland forest. Malaysia. Trop Conserv Sci 7(2):244–259
Alvira D, Putz FE, Fredericksen TS (2004) Liana loads and post-logging liana densities after liana cutting in a lowland forest in Bolivia. For Ecol Manag 190(1):73–86
Arbonnier M (2004) Trees, shrubs and lianas of West African dry zones. CIRAD, MARGRAF Publishers, Montpellier, pp 166–515
Bongers F, Parren MPE, Swaine MD, Traoré D (2005) Forest climbing plants of West Africa: introduction. In: Bongers F, Parren MPE, Traoré D (eds) Forest climbing plants of West Africa: diversity, ecology and management. CAB International, Wallingford, pp 5–18
Campanello PI, Villagra M, Garibaldi JF, Ritter LJ, Araujo JJ, Goldstein G (2012) Liana abundance, tree crown infestation, and tree regeneration 10 years after liana cutting in a subtropical forest. For Ecol Manag 284:213–221
Colwell RK (2009) EstimateS: statistical estimation of species richness and shared species from samples. Version 8.2. User’s guide and application. http://viceroy.eeb.uconn.edu/estimates
Cottam G, Curtis JT (1956) The use of distance measurement in phytosociological sampling. Ecology 37(3):451–460
Douglas EM, Sebastian K, Vörösmarty CJ, Wood S, Chomitz KM (2005) The role of tropical forests in supporting biodiversity and hydrological integrity. World Bank Policy Research Paper 3635. Science Publishers, Enfield, pp 1–12
Foli EG, Pinard MA (2009) Liana distribution and abundance in moist tropical forest in Ghana 40 years following silvicultural interventions. Ghana J For 25:1–12
Gardette E (1998) The effect of selective timber logging on the diversity of woody climbers at Pasoh. In: Lee SS, Dan YM, Gauld ID, Bishop J (eds) Conservation, management and development of forest resources. Forest Research Institute, Kepong, pp 115–125
Gerwing JJ, Vidal E (2002) Changes in liana abundance and species diversity 8 years after liana cutting and logging in an eastern Amazonian forest. Conserv Biol 16(2):544–548
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4(4):379–391
Hawthorne WD, Jongkind C (2006) Woody plants of western African forests: a guide to the forest trees, shrubs and lianes from Senegal to Ghana. Royal Botanic Gardens, Kew, pp 14–1022
Hortal J, Borges PAV, Gaspar C (2006) Evaluating the performance of species richness estimators: sensitivity to sample grain size. J Anim Ecol 75(1):274–287
Jongkind CC (2005) Checklist of climber species in Upper Guinea. In: Bongers F, Parren MPE, Traoré D (eds) Forest climbing plants of West Africa: diversity, ecology and management. CAB International, Wallingford, pp 231–264
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, New Jersey, pp 34–95
Parren MPE (2003) Lianas and logging in West Africa., Tropenbos-Cameroon Series 6Wageningen, Tropenbos International, p 116
Parren MPE, Bongers F (2001) Does climber cutting reduce felling damage in southern Cameroon? For Ecol Manag 141(3):175–188
Parren MPE, Bongers F (2005) Management of climbers in the forest of West Africa. In: Bongers F, Parren MPE, Traoré D (eds) Forest climbing plants of West Africa: diversity, ecology and management. CAB International, Wallingford, pp 217–229
Parren MPE, de Graaf NR (1995) The quest for natural forest management in Ghana, Côte d’Ivoire and Liberia, vol 13., Tropenbos SeriesWageningen, Tropenbos Foundation, pp 106–107
Sackey ANA (2007) Assessment of forest management practices in Ghana—a case study of some forest districts in Ghana. Int Manag Resour Environ J 1(2)
Schnitzer SA, Bongers F (2002) The ecology of lianas and their role in forests. Trends Ecol Evol 17(5):223–230
Schnitzer SA, Kuzee M, Bongers F (2005) Disentangling above- and below-ground competition between lianas and trees in a tropical forest. J Ecol 93(6):1115–1125
Schnitzer SA, Bongers F, Wright SJ (2011) Community and ecosystem ramifications of increasing lianas in neotropical forests. Plant Signal Behav 6(4):598–600
Tang Y, Kitching RL, Cao M (2012) Lianas as structural parasites: a re-evaluation. Chin Sci Bull 57(4):307–312
Acknowledgments
This study was supported by TWAS-USM Postgraduate Fellowship and Research University Grant (RU) (1001/PBIOLOGI/815086). We are grateful to Mr. Ntim Gyakari of the Forestry Commission of Ghana for plant species identification.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project Funding: This study was supported by TWAS-USM Postgraduate Fellowship and Research University Grant (RU) (1001/PBIOLOGI/815086).
The online version is available at http://www.springerlink.com
Corresponding editor: Zhu Hong
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Addo-Fordjour, P., Rahmad, Z.B., S Shahrul, A.M. et al. Impacts of forest management on liana diversity and community structure in a tropical forest in Ghana: implications for conservation. J. For. Res. 27, 147–153 (2016). https://doi.org/10.1007/s11676-015-0163-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-015-0163-4