Abstract
Myrtle, Myrtus communis L. (Myrtaceae), an evergreen shrub also known as wild myrtle, has a history of use as a culinary and medicinal plant. To determine the diversity within the species, plant leaves of myrtle were collected in 12 natural habitats in Iran for investigation of chemical constituents in the essential oil. Extraction of the essential oils produced yields ranging from 0.7 to 1.5 mL per 100 g dry tissue. An analysis of the oils by GC and GC/MS revealed 40 compounds, constituting 90.1–99.9 % of the essential oils. Chemical constituents varied with the site of sample origin, although the principal essential oil components from all populations, were α-pinene (17.5–37.1 %), 1,8-cineole (9.9–29.8 %), linalool (7.0–23.1 %), and α-terpineol (5.3–8.3 %). Limonene (tr, 22.7 %) was a major constituent in three populations. Characterized chemotypes included Chemotype I: α-pinene/1,8-cineole/linalool, Chemotype II: α-pinene/linalool, Chemotype III: α-pinene/1,8-cineole, and Chemotype IV: α-pinene/1,8-cineole/limonene. The main source of variability in chemical composition and oil yield appeared to be differences in environmental conditions and chemotypes as plant populations collected from close geographical areas could be classified in a cluster.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myrtle (Myrtus communis L., Myrtaceae) is a wild evergreen shrub that grows primarily in Mediterranean climates. Although the plant is cultivated in Iran, the species, which is commonly known as “Mord or Mort,” grows wild throughout the Zagros Mountainous Range (Ghasemi Pirbalouti 2009). Due to the high essential oil content in leaves, flowers, and fruit, myrtle is an important medicinal and aromatic plant. Leaves and berries are sources of essential oil with medicinal properties, including activity as an antimicrobial (Atzei 2003; Zanetti et al. 2010; Mahboubi and Ghazian Bidgoli 2010; Djenane et al. 2011; Messaoud et al. 2012; Bajalan and Ghasemi Pirbalouti 2014; Ghasemi Pirbalouti et al. 2014), antioxidant and anti-mutagenic (Hayder et al. 2008; Messaoud et al. 2012), astringent, antiseptic, anti-hyperglycemic (Elfellah et al. 1984; Djenane et al. 2011; Messaoud et al. 2012), analgesic (Levesque and Lafont 2000), and anti-inflammatory (Rossi et al. 2009). The plant is also used as insecticide (Tavassoli et al. 2011; Motazedian et al. 2012), and nematicide (Barbosa et al. 2010; Oka et al. 2012). Leaf decoctions of myrtle are frequently employed in Iranian folk medicine as a treatment for skin and digestive disorders, as an astringent, as a bronchodilator, and as a hair conditioner (Zargari 1982–1992; Ghasemi Pirbalouti 2009).
The essential oil obtained from myrtle has been widely investigated and is known to vary in composition (Lawrence 1996), although 1,8-cineole is a major constituent (Bradesi et al. 1997). Depending on the amount of myrtenyl acetate, myrtle essential oils can be separated into two groups that can be further divided into two subgroups, depending on the relative ratios of α-pinene to myrtenyl acetate and α -pinene to 1,8-cineole (Flamini et al. 2004). A strong correlation exists between these groups and subgroups and the geographical origin of the plants (Bradesi et al. 1997).
Materials and methods
Plant material
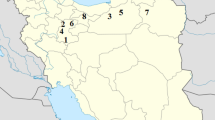
Wild populations of myrtle (Myrtus communis L.) were used in this study. Leaves (0.5 kg of terminal leafy twigs) were collected during the early flowering stage (10 June–5 July 2012) from myrtle plants growing in 12 localities in five provinces (Fars, Kohgelouyeh va Boyerahmad, Chaharmahal va Bakhtiari, Lorestan and Khuzestan) located in south and southwest Iran (Table 1; Fig. 1). The collection areas were geographically separate and included areas in which physical differences in plant characteristics were observed. Each collected sample was labeled and the location was recorded using a global positioning system (GPS, Vista Garmin) receiver. Plant identity was confirmed by H.A. Shirmardi, Ph.D., and voucher specimens (No. 231) were placed in the Herbarium of Islamic Azad University, Shahrekord Branch, Iran.
Environmental
The physical and chemical characteristics of the soil, including pH, electrical conductivity (EC), organic carbon (%OC), and texture at the sample collection sites, were measured along with climatic conditions as recorded at the nearest meteorology station (Table 1).
Essential oil extraction
For essential oil extraction, collected fresh myrtle leaves were dried for 1 week at room temperature (water content was approximately 75 % of plant fresh weight). The dried leaves were ground to a fine powder using a Moulinex food processor, and 100 g powdered leaf tissue was distillated with 1 L of water for 3 h using a Clevenger-type apparatus, following the method recommended in the European Pharmacopoeia (Council of Europe 1997). The essential oil yields were expressed in mL/100 g dry weight of plant material.
Oil constituency
GC and GC/MS analysis were used to determine the composition of the essential oils, using an Agilent Technologies 7890 gas chromatograph equipped with flame ionization detector (FID) and a HP-5MS 5 % capillary column (30 m × 0.25 mm, 0.25 µm film thicknesses). Oven temperature was programed 60 °C for an initial 4 min, and then raised 4 °C/min to 260 °C. Injector and detector temperatures were set at 290 and 300 °C, respectively. Helium was used as the carrier gas at a flow rate of 2 mL/min, and 0.1 µL samples were injected manually in the split mode. Peak areas were used for quantifying the constituent percentage of total oil.
Constituent identification was confirmed by coupling the gas chromatograph to an Agilent 5975 C (Agilent Technologies, Palo Alto, CA, USA) mass selective detector. Operating parameters for the EI-MS were: ionization voltage, 70 eV; ion source temperature, 200 °C. Retention indices were calculated for all components using a homologous series of n-alkanes (C5–C24) injected in conditions equal to the oil samples. Identification of oil components was accomplished based on comparison of retention times with those of authentic standards and by comparison of their mass spectral fragmentation patterns (WILLEY/ChemStation data system) (Adams 2007). The area percent was obtained electronically from the GC–FID response without the use of an internal standard or correction factors.
Statistical analysis
Data were statistically analyzed using a one-way ANOVA using the program SPSS (19.0). Means of the main constituents of the essential oils were compared and separated from each other using Duncan’s multiple range test at p ≤ 0.05 level. Analytical data for Hierarchical cluster analysis were treated by means of the SPSS statistical software.
Results
Essential oil yield
The essential oils extracted from the collected myrtle samples were a clear, light yellow to yellow color. Oil yields ranged from 0.7 to 1.5 mL/100 g of dry tissue and varied with the genotype (ecotype) and the environmental conditions at the collection site (Fig. 2). Oil content differed significantly (p ≤ 0.01) by geographic location. The 12 sampled populations ranked highest to lowest in terms of oil yield were: Dinarvand > Pelazh > Mongere > Chavoni > Seyedan > Dehdasth > Gachsaran = Simakan > Dezful > Cheshmeh–Ali > TangeHaft > Madan (Fig. 2).
Essential oil composition
The GC and GC-MS analysis identified 40 constituents in the essential oils (Table 2) that constituted the bulk of the oils, ranging from 90.1 to 99.9 % of the total oil. A total of 14 oil constituents were present at >1.0 % and 7 compounds were present at >5.0 % in oil from one or more locations. Some 26 oil constituents were at levels <1.0 %. A total of seven major oil constituents were detected: α -pinene (25.81 ± 7.64 %), 1,8-cineole (20.98 ± 9.59 %), linalool (23.38 ± 4.37 %), α-terpineol (6.99 ± 1.26 %), limonene (4.73 ± 8.52 %), linalyl acetate (5.82 ± 2.85), and geranyl acetate (2.08 ± 1.49). Of the top 12 oil constituents in the myrtle samples, 10 oil constituents varied by population source (Table 2).
Monoterpenes were the main constituents of the essential oil from the leaves of collected plants with 33 derivatives identified, representing 83.8–95.7 % of all oil, three sesquiterpenes accounted for 0.6–5.2 % of the oil. Essential oil composition varied by origin of the samples as evidenced by the highest and lowest concentrations of oxygenated monoterpenes being obtained from the Cheshmeh-Ali population (69.4 %), and the Dinarvand population (34.1 %), while the highest content of monoterpene hydrocarbons was obtained from the Dinarvand population (61.0 %) and the lowest content of monoterpene hydrocarbons (26.3 %) was in the Cheshmeh-Ali population (Table 2).
Cluster analysis
Correlation and cluster analyses of compounds were computed to determine the relationship between chemical components in myrtle essential oil and the environmental conditions of sampled habitats. Compounds, such as α -pinene, 1,8-cineole, linalool, α-terpineol, limonene, linalyl acetate, linalool oxide, and geranyl acetate were present in oils of myrtle. The highest negative correlation (p ≤ 0.05) was between relative humidity and linalyl acetate (−0.683), followed by precipitation and geranyl acetate (−0.658) (Table 3).
Hierarchical cluster analysis of all identified components grouped essential oil of the 12 populations into four distinct clusters (Fig. 3). The first cluster formed by oils of five populations (Gachsaran, Dehdasht, Cheshmeh-Ali, Madan and Seyedan) and contained α -pinene (17.5–25.7 %), 1,8-cineole (19.9–26.9 %), linalool (12.1–23.1 %), and limonene (0–0.6 %). This cluster had a higher linalool concentration as compared with other clusters (Chemotype I: α-pinene/1,8-cineole/linalool). The plant populations in the first cluster were collected from nearby geographical areas. For intra-population tests, genetic distances were estimated as described in the second cluster formed by oil of one population (Seyedan), which contained α -pinene (24.6 %), linalool (15.3 %), 1,8-cineole (10.59 %), and limonene (1.8 %) (Chemotype II: α-pinene/linalool). Isolation of this population was probably due to the low elevation in which the plant grows in comparison with the other geographic regions.
The third cluster formed by oils of three populations (Dezful, Mongere, and Chavoni) contained the highest α -pinene (29.7–37.1 %) and 1,8-cineole (21.9–29.8 %) concentrations in comparison with the other clusters (Chemotype III: α-pinene/1,8-cineole). The populations of these plants were collected from nearby geographical areas and classified in a cluster. The fourth cluster was formed by essential oils of three populations (Dinarvand, Tangehaft, and Pelazh) and contained α -pinene (26.4–28.6 %), and 1,8-cineole (9.9–23.0 %) and limonene (12.9–22.7 %). This cluster had higher limonene in comparison with the other clusters (Chemotype IV: α-pinene/1,8-cineole/limonene).
Pearson’s correlation indicated a negative correlation (p ≤ 0.05) between annual precipitation and oxygenated monoterpenes (−0.596) (Table 4). To examine the relationships between the different populations of myrtle, PCA was applied to the volatile oil constituent data, and the first four eigenvalues corresponded to 33, 29, 15, and 9 % of the total variance (Table 5). The first principal component (PC1) explained 33 % of the variation and had an eigenvalue of 3.96, which consisted of α -pinene and 1,8-cineole as major constituents (Table 5). PC2 accounted for 29 % of the variation, with an eigenvalue of 3.54. PC2 was bipolar with linalool, linalool oxide, linalyl acetate, and geranyl acetate. PC3 accounted for 15 % of the variation, and had an eigenvalue of 1.78. Limonene, a major volatile oil constituent, was responsible for this bipolar vector. PC4 explained 9 % of variation, mainly due to limonene, terpinolene, linalool, geranyl acetate.
Discussion
The chemical differentiation observed in the myrtle samples sourced in this study is in agreement with the interpopulation chemical polymorphism observed by other researchers (Boelens and Jimenez 1991; Chalchat et al. 1998; Jerkovic et al. 2002; Messaoud et al. 2005; Gardeli et al. 2008; Mulas and Melis 2011). The plant populations having significantly higher percentages of the major components in the essential oil obtained from different populations of myrtle contained monoterpenes, oxygenated monoterpenes, hydrocarbons, phenylpropanoids, and sesquiterpenes. The essential oil of the Pelazh population had the highest concentration of phenylpropanoids [methyl eugenol (2.1 %) and methyl chaviol (2.1 %)]. In contrast, samples from Fars province, including the Dehdasth (5.2 %) and Simakan (5.1 %) populations, had the highest concentrations of sesquiterpenes.
Earlier reports (see Table 6) on the chemical constituents in various ecotypes, cultivars, and varieties from Iran, plus other countries were comparable with the chemical compounds in myrtle oil obtained from our populations. The recurring polymorphism observed within the genus produces a large number of subspecies, varieties, and plant forms in varying degrees of abundance that produce essential oils with different chemical composition. In most cases hydrocarbons monoterpenes (α-pinene) and oxygenated monoterpenes (linalool, 1,8-cineole, limonene, myrtenyl acetate, and linalyl acetate) and phenylpropanoid (methyl chavicol, methyl cinnamate, eugenol, and methyl eugenol) are the major components of the oils.
The secondary metabolite production is believed to be stimulated by stressful environmental conditions such as water deficiency (Sangwan et al. 2001). The strongest positive correlation (p ≤ 0.01) was between the relative linalool (C10H18O) and linalool oxide (C10H18O2) levels, compounds that have similar structure with only a difference is in the position of the oxygen group (O). In addition, the strongest positive correlations were between the relative linalool, linalool oxide, and linalyl acetate (C12H20O2). Linalyl acetate is the acetate ester of linalool, and the two often occur in conjunction (Ghasemi Pirbalouti 2010). The highest negative correlations were between α -pinene and linalool, α-pinene and linalool oxide and α -pinene and linalyl acetate. Linalool can be synthesized by a-pinene through kinetic peculiarities of catalytic steps (Semikolenov et al. 2001). We found negative correlation between 1,8-cineol and limonene. 1,8-cineole and limonene are both correlated strongly with various formylated phloroglucinol compounds in eucalyptus (Moore et al. 2004).
The clusters of plants with similar essential oil profiles may be due to enzymatic reactions induced by shortage of water or other stress that leads to the conversion of oxygenated monoterpenes to monoterpene hydrocarbons. While little experimental data is available to support this notion, Hendawy and Khalid (2005) have reported variations in essential oil yield and composition due to environmental effects on enzyme activity and metabolic pathways. The highest negative correlation was between oxygenated monoterpenes and monoterpenes hydrocarbons, a relationship that could be explained by a change in the biosynthetic pathway for monoterpene hydrocarbons and oxygenated monoterpenes (Selmar and Kleinwächter 2013).
Conclusion
Essential oil components of wild populations of myrtle varied by chemotype, environmental conditions, and geographic origin. Essential oils of myrtle leaves were characterized by high levels of oxygenated monoterpenes, hydrocarbon monoterpenes, phenylpropanoids, and sesquiterpenes. Monoterpenes were the main constituents of the essential oil of the leaves of the collected plants. The level of oxygenated monoterpenes, including 1,8-cineole, linalool, and α-terpineol, were higher than the monoterpene hydrocarbons, such as α-pinene and limonene. These monoterpenes are widespread components of the essential oils and used as fragrances and flavors in the cosmetic, perfume, drug and food industries.
Our results suggest that samples of myrtle used in this study can be assigned to chemotypes, α -pinene/linalool α-pinene/1,8-cineole/linalool, α -pinene/1,8-cineole, and α-pinene/1,8-cineole/limonene due to the relatively high grouping of these chemical constituents in the essential oil. The variation in oil composition and oil yield of myrtle can result from genetic diversity, differences in environmental conditions, and diversity/environment interactions. The main source of variability in chemical composition and oil yield of the studied populations seemed to be due to differences in environmental conditions and chemotypes. Plant populations collected from close geographical areas could be classified in a cluster.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured Publishing Corporation, Carol Stream
Aidi Wannes WA, Mhamdi B, Sriti J, Marzouk B (2010) Changes in essential oil composition of Tunisian Myrtus communis var. italica L. during its vegetative cycle. J Essent Oil Res 22:13–18
Asllani U (2000) Chemical composition of albanian myrtle oil (Myrtus communis L.). J Essent Oil Res 12:140–142
Atzei AD (2003) Le piante nella tradizione popolare della Sardegna. Carlo Delfino Editore, Sassari, pp 319–323
Bajalan I, Ghasemi Pirbalouti A (2014) Variation in antibacterial activity and chemical compositions of essential oil from different populations of myrtle. Ind Crop Prod 61:303–307
Barbosa P, Lima AS, Vieria P, Dias LS, Tinoco MT, Barroso JG, Pedro LG, Figueiredo AC, Mota M (2010) Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursaphelenchus xylophilus. J Nematol 42:8–16
Boelens MH, Jimenez R (1991) The chemical composition of Spanish myrtle leaf oils. Part I. J Essent Oil Res 3:173–177
Bradesi TP, Casanova J, Costa J, Bernardini AF (1997) Chemical composition of myrtle leaf oil from Corsica (France). J Essent Oil Res 9:283–288
Chalchat JC, Garry RP, Michet A (1998) Essential oil of Myrtle (Myrtus communis L.) of the Mediterranean Littoral. J Essent Oil Res 10:613–617
Council of Europe (1997) European Pharmacopoeia, 3rd edn. Council of Europe, Strasbourg, pp 121–122
Djenane D, Yangüela J, Amrouche T, Boubrit S, Boussad N, Roncalés P (2011) Chemical composition and antimicrobial effects of essential oils of Eucalyptus globulus, Myrtus communis and Satureja hortensis against Escherichia coli O157:H7 and Staphylococcus aureus in minced beef. Food Sci Technol Int 17:505–515
Elfellah MS, Akhter MH, Khan MT (1984) Anti-hyperglycaemic effect of an extract of Myrtus communis in streptozotocin induced diabetes in mice. J Ethnopharmacol 11:275–281
Farah A, Afifi A, Fechtal M, Chhen A, Satrani B, Talbi M, Chaouch A (2006) Fractional distillation effect on the chemical composition of Moroccan myrtle (Myrtus communis L.) essential oils. Flavour Frag J 21:351–354
Flamini G, Cioni PL, Morelli I, Maccioni S, Baldini R (2004) Phytochemical typologies in some populations of Myrtus communis L. on Caprione Promontory (East Liguria, Italy). Food Chem 85:599–604
Gardeli C, Vassiliki P, Athanasios M, Kibouris T, Komaitis M (2008) Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: evaluation of antioxidant capacity of methanolic extracts. Food Chem 107:1120–1130
Ghasemi Pirbalouti A (2009) Medicinal plants used in Chaharmahal and Bakhtyari districts, Iran. Herba Pol 55:69–75
Ghasemi Pirbalouti A, Mirbagheri H, Hamedi B, Rahimi E (2014) Antibacterial activity of the essential oils of myrtle leaves against Erysipelothrix rhusiopathiae. Asian Pac J Trop Biomed 4:505–509
Hayder N, Skandrani I, Kilani S, Bouhlel I, Abdelwahed A, Ben Ammar R, Mahmoud A, Ghedira K, Chekir-Ghedira L (2008) Antimutagenic activity of Myrtus communis L. using the Salmonella microsome assay. S Afr J Bot 74:121–125
Hendawy SF, Khalid KA (2005) Response of sage (Salvia officinalis L.) plants to zinc application under different salinity levels. J Appl Sci Res 1:147–155
Jerkovic I, Radonic A, Borcic I (2002) Comparative study of leaf, fruit and flower essential oils from Croatian Myrtus communis L. during a 1-year vegetative cycle. J Essent Oil Res 14:266–270
Lawrence BM (1996) Progress in essential oils. Myrtle oil Perfum Flavor 21:57–58
Levesque H, Lafont O (2000) Aspirin throughout the ages: a historical review. La Revue de Medecine Interne 21:8–17
Mahboubi M, Ghazian Bidgoli F (2010) In vitro synergistic efficacy of combination of amphotericin B with Myrtus communis essential oil against clinical isolates of Candida albicans. Phytomedicine 17:771–774
Messaoud C (2001) Polymorphisme isoenzymatique et variabilité de quelques constituants chimiques de l’huile essentielle chez le Myrte commun (Myrtus communis L.) en Tunisie. Mémoire de DEA, Facultédes Sciences Bizerte Tunisia, pp 80
Messaoud C, Zaouali Y, Salah AB, Khoudja ML, Boussaid M (2005) Myrtus communis in Tunisia: variability of the essential oil composition in natural populations. Flavour Fragr J 20:577–582
Messaoud C, Laabidi A, Boussaid M (2012) Myrtus communis L. infusions: the effect of infusion time on phytochemical composition, antioxidant, and antimicrobial activities. J Food Sci 77:941–947
Moore BD, Foley WJ, Wallis IR, Cowling A, Handasyde KA (2005) Eucalyptus foliar chemistry explains selective feeding by koalas. Biol Lett 1:64–67
Motazedian N, Ravan S, Bandani AR (2012) Toxicity and repellency effects of three essential oils against Tetranychus urticae Koch (Acari: Tetranychidae). J Agr Sci Tech 14:275–284
Mulas M, Melis RAM (2011) Essential oil composition of myrtle (Myrtus communis) leaves. J Herbs Spices Med Plants 17:21–34
Oka Y, Ben-Daniel B, Cohen Y (2012) Nematicidal activity of the leaf powder and extracts of Myrtus communis against the root-knot nematode Meloidogyne javanica. Plant Pathol 61:1012–1020
Ozek T, Demirci B, Baser KHC (2000) Chemical composition of Turkish myrtle oil. J Essent Oil Res 12:541–544
Pereira PC, Cebola MJ, Bernardo-Gil MG (2009) Evolution of the yields and composition of essential oil from Portuguese Myrtle (Myrtus comunis L.) through the vegetative cycle. Molecules 14:3094–3105
Pirbalouti AG (2010) Iranian Medicinal Plants (Introduction and Application), 2nd edn. I.A.U. Publisher, Shahrekord
Rasooli I, Moosavi ML, Rezaee MB, Jaimand K (2002) Susceptibility of microorganisms to Myrtus communis L. essential oil and its chemical composition. J Agric Sci Technol 4:127–133
Rossi A, Paola RD, Mazzon E, Genovese T, Caminiti R, Bramanti P, Pergola C, Koeberle A, Werz O, Sautebin L, Cuzzocrea S (2009) Myrtucommulone from Myrtus communis exhibits potent anti-inflammatory effectiveness in vivo. J Pharmacol Exp Ther 329:76–86
Sangwan NS, Farooqi AHA, Shabih F, Sangwan RS (2001) Regulation of essential oil production in plants. Plant Growth Regul 34:3–21
Selmar D, Kleinwächter M (2013) Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crop Prods 42:558–566
Semikolenov VA, Ilyna II, Simakova IL (2001) Linalool synthesis from α-pinene: kinetic peculiarities of catalytic steps. Appl Catal A 211:91–107
Tavassoli M, Shayeghi M, Abai MR, Vatandoost HV, Khoobdel M, Salari M, Ghaderi A, Rafi F (2011) Repellency effects of essential oils of myrtle (Myrtus communis), marigold (Calendula officinalis) compared with DEET against Anopheles stephensi on human volunteers. Iran J Arthropod-Borne Dis 5:10–22
Tuberoso CIG, Barra A, Angioni A, Sarritzu E, Pirisi FM (2006) Chemical composition of volatiles in Sardinian myrtle (Myrtus communis L.) alcoholic extracts and essential oils. J Agric Food Chem 54:1420–1426
Yadegarinia D, Gachkar L, Rezaei MB, Taghizadeh M, Alipoor Astaneh S, Rasooli I (2006) Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry 67:1249–1255
Zanetti S, Cannas S, Molicotti P, Bua A, Cubeddu M, Porcedda S, Marongiu B, Sechi LA (2010) Evaluation of the antimicrobial properties of the essential oil of Myrtus communis L. against clinical strains of Mycobacterium spp. Interdiscip Perspect Infect Dis 2010:1–3
Zargari A (1982–1992) Medicinal Plants, vol 1–6. University Publication, Tehran
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding: This work was supported by Deputy of Researches and Technology, Islamic Azad University of Shahrekord Branch, Iran (No Grant., IAUSHK-6121).
The online version is available at http://www.springerlink.com
Corresponding editor: Hu Yanbo
Rights and permissions
About this article
Cite this article
Ghasemi Pirbalouti, A., Craker, L.E. Diversity in chemical compositions of essential oil of myrtle leaves from various natural habitats in south and southwest Iran. J. For. Res. 26, 971–981 (2015). https://doi.org/10.1007/s11676-015-0094-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-015-0094-0