Abstract
The impact of oxygen impurity on phase relationships in the Zr-Mo-Fe system (Zr > 30 at.%) at 1000 °C was investigated by means of x-ray diffraction, scanning electron microscopy, and energy-dispersive spectroscopy analysis. An oxygen-stabilized ternary compound γ-Zr2(Mo,Fe), derived from Ti2Ni type Zr4Fe2O, with cell parameter of a = 1.2213 nm was observed to be present in the investigated Zr-Mo-Fe alloys. The phase relationships of the Zr-Mo-Fe (O) system at 1000 °C in the Zr-rich corner consist of 3 four-phase regions, i.e., [βZr + λ-Zr(Mo,Fe)2 + γ-Zr2(Mo,Fe) + ZrMo2], [βZr + λ-Zr(Mo,Fe)2 + γ-Zr2(Mo,Fe) + Liquid], and [λ-Zr(Mo,Fe)2 + γ-Zr2(Mo,Fe) + Liquid + ZrFe2]. The previously detected Zr9Mo4Fe compound was not observed in this work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Zirconium alloys are widely used as cladding and structural materials in nuclear reactors due to their low neutron absorption cross section, excellent corrosion and creep resistance in the harsh reactor environment.[1] In a loss-of-coolant accident (LOCA) scenario, the temperature of the reactor core rapidly increases, leading to the evaporation of coolant. During a LOCA transient, zirconium cladding tubes undergo a violent reaction with steam, resulting in the formation of a surface oxide layer of ZrO2, an oxygen-stabilized layer of α-Zr solution, and diffusion of oxygen into the underlying βZr substrate, if the core temperature is higher than that of the alpha-to-beta transformation.[2, 3] At 1000 °C, the oxygen content in the βZr can reach 0.7 wt.%.[2] The oxidation process can degrade the ductility of the cladding tube and increase its risk of failure.[4] In the development of a zirconium alloy, oxygen has always been used as a strengthening alloying element. The content of the diluted oxygen ranges from 0.06 to 0.16 wt.%, depending on the specific alloy series.[5] For example, the oxygen content in Zircaloy-2 and Zircaloy-4 ranges from 0.09 to 0.16 wt.%, while M5™ (a trademark of Framatome) and Zirlo™ (a trademark of Westinghouse) alloys have an oxygen content of 0.125 wt.%. E110 alloy has an oxygen content of 0.06 wt.%, and E635 alloy has an oxygen content of 0.08 wt.%, respectively.[5] The addition of oxygen as an alloying element is believed to fully dissolve in the Zr matrix and form a Zr(O) solution in the commercial zirconium alloys. There has been little research focusing on the phase relationships of zirconium alloys with varying levels of oxygen. It is interesting to investigate whether the precipitates in the cladding tubes change when the oxygen content increases during a LOCA transient. The present work will provide a new perspective on this issue.
The Zr-Mo-Fe system is an important subsystem in zirconium alloys. Previous studies showed that the addition of Mo improves the creep resistance of these alloys.[6,7,8] Zr-Excel alloys (Zr-3.5Sn-0.8Mo-0.8Nb-0.1Fe) have been found to exhibit excellent creep resistance.[9] Lee et al. conducted a comparative study of Zirlo™ alloys and Mo diluted Zirlo™ alloys.[10] Their findings showed that after recrystallization at 570, 600, and 650 °C for 8 h, the Zirlo™ alloy with Mo addition exhibited a higher corrosion rate in 360 °C water containing 0.01 mol/L LiOH under saturated pressure conditions at 18.8 MPa.[10] When the recrystallization time was extended to 32 h at 600 °C, the Mo-diluted Zirlo™ alloys showed corrosion resistance equivalent to that of the Zirlo™ alloys.[10] Recently, Niculina et al. observed that the 0.1% Mo addition in the E635 alloys (Zr-1.2Sn-1.0Nb-0.35Fe) greatly reduced its hydrogenation and propensity for nodular corrosion. The researchers found that Mo prefers to dissolve in Laves phase Zr(Nb,Fe)2 to form Zr(Nb,Fe,Mo)2 rather than forming cubic ZrMo2 compound.[11] Our previous research has shown that precipitate in the Zr-Excel alloy was ZrMo2.[12]
The Zr-Mo binary system and the Fe-Mo binary system have been extensively studied. The latest accepted Zr-Fe binary system is that reported by Stein et al.[13] The stable intermetallics in the Zr-Fe alloys include cubic ZrFe2, hexagonal ZrFe2, tetragonal Zr2Fe, and orthorhombic Zr3Fe. The previous known cubic Zr6Fe23 phase on the Fe-rich side has been shown to be stabilized by oxygen.[13] In the Zr-Mo system, there is only one intermediate phase, which is ZrMo2. This phase has C15 structure type and is formed by peritectic reaction: L + (Mo) → ZrMo2 at 1921 °C.[14] In the Fe-Mo system, there are four intermetallic phases, which are λ, R, μ, and σ.[15] The λ phase is stable below 927 °C with the constant composition of 33.3 at.% Mo. The R phase is stable from 1200 to 1488 °C with the maximum composition range from 33.9 to 38.5 at.% Mo. The μ phase is stable below 1370 °C in composition range from 39.0 to 44.0 at.% Mo. The σ phase is stable from 1235 to 1611 °C with the maximum composition range from 33.9 to 38.5 at.% Mo.[15]
Gruzdeva and Tregubov conducted a study on four partial isothermal sections in the Zr-rich corner of the Zr-Mo-Fe system at 1000, 900, 800, 700 °C, respectively.[16] No ternary compound was reported in this early work.[16] According to their results, bcc solid solution βZr is in equilibrium with ZrMo2 and liquid at 1000 °C. At 900 °C, the liquid disappears, and bcc βZr is in equilibrium with ZrMo2 and ZrFe2. At 800 °C, hcp αZr appears and equilibrates with βZr. βZr is still in equilibrium with ZrMo2 and ZrFe2 with its domain shrinking.[16] In 1973, Rogl et al. reported a ternary compound Zr9Mo4Fe.[17] This ternary compound has hexagonal Hf9Mo4B structure type with Hf and B positions occupied by Zr and Fe, respectively.[17] Another ternary phase λ-Zr(Mo,Fe)2with C14 hexagonal structure and Zr content at 33 at.% and Mo varying from 6 to 53 at.% was found in this system.[18] Zinkevich and Mattern conducted a thermodynamic assessment of this system based on limited experimental information.[18] In the process of preparing this manuscript, we found that Du et al. had reported an isothermal section of the Zr-Mo-Fe ternary system at 1000 °C. [19] They did not observe the ternary compound Zr9Mo4Fe, and did not report on the effect of impurity oxygen on the phase relationship in their work.[19]
The initial purpose of this work was to investigate the phase equilibria in the Zr-Mo-Fe system. The uptake of oxygen during the melting process results in a change of the phase relationship from the ternary Zr-Mo-Fe system to the quaternary Zr-Mo-Fe-O system. In light of the oxygen alloying effect and the rapid oxidation of zirconium-based cladding tubes during a transient LOCA scenario, it is important to develop an understanding of the phase relationship in Zr-Mo-Fe(O) ternary alloys.
2 Experimental Details
Nuclear grade sponge Zr (Zr > 99.8 wt.%, with main impurities of Fe 700 ppm, and O 700 ppm), pure Mo (99.95 wt.%), and Fe (99.95 wt.%) were used as starting materials. Ingots (4 g) were arc melted in an environment of pure argon (99.99 %). The alloy buttons were melted five times to guarantee homogeneity. After melting, the average weight gain rate of the alloy ingots is less than 0.5 wt.%. As-prepared buttons were sealed in vacuum quartz tubes with an inner diameter Ø10 mm and length 80 mm. The buttons were directly annealed at 1000 °C for 20 days, followed by a water quench. The quartz tubes were evacuated by using an Oil Sealed Rotary Vane Pump. The vacuum after evacuation was about 10 Pa. The quartz tubes were sealed by using oxy-acetylene flame, and preserved unbroken during the heat treatment. Thus, the influence of the retained oxygen in the sealed quartz tube on the amount of oxygen in the quenched alloy is negligible. The oxygen content for the selected alloys was measured by using inert gas fusion-infrared absorption method. The results show that the oxygen contents of three selected alloys are, 1200 ppm, 3400 ppm, and 4800 ppm, respectively. For the reader's convenience, a representation of the alloy compositions fabricated in this work was presented in Fig. 1. The designed alloy composition could be found in Table 1.
The annealed alloys were characterized by x-ray diffraction (XRD). The XRD was carried out on the XD–3 diffractometer or Bruker Advance-D8 with CuKα radiation and graphite monochromator. The indexing results were obtained by using Dicvol2006 software.[20,21,22] The metallographic specimens were prepared by mechanical grinding and polishing, but without etching. Microstructural observation and compositional analysis were carried out on a Scanning Electron Microscopy (SEM, EVO 18) coupled with an energy dispersive X–ray spectrometer (EDX, Bruker 5010). The compositions of the bulk alloys and the identified phases were obtained by area scan mode and dot scan mode, respectively, with ZAF corrections. Microstructure analyses of alloys 15-16 were performed by using electron probe microanalyzer (EPMA, JEOL JXA-8230). The acceleration volatage was 20 kV. Standards were used for the concentration calculation of the Zr, Mo, and Fe elements. For the element mapping an area of 200 × 200 μm was seleted. The composition point was designed as 500 × 500 by using one step per 0.4 μm. In the present work, element maps were presented along with the element intensity data-bar, which was generated by the system software based on the counts of characteristic x-ray lines. These values were used to specify the image color contour, which characterized the distribution of element concentration. For the metallographic quantitative analysis, software Fiji/Image J was utilized.[23]
3 Results and Discussions
Table 1 presents the results of alloys investigated using XRD and SEM/EDS. The composition of alloys 15-16 was measured using EPMA. A new oxygen-stabilized ternary compound, denoted as γ-Zr2(Mo,Fe), was observed in present work.
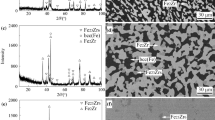
Large amount of phase γ-Zr2(Mo,Fe) and minor fraction of λ-Zr(Mo,Fe)2 were observed in alloy 2. This alloy was annealed at 900 °C for 10 days. This temperature was chosen to ensure the synthesis of solid γ-Zr2(Mo,Fe) as liquid would appear at 1000 °C. Figure 2(a) is the back-scattered electron (BSE) image of the microstructure of this alloy, in which two phases are clearly identified. The white-gray phase with a composition of Zr66.5Mo4.5Fe29.0 repesents ternary γ-Zr2(Mo,Fe) compound, while the black-gray phase with a composition of Zr36.5Mo10.8Fe52.7 is identified as λ-Zr(Mo,Fe)2 with C14-type hexagonal structure. Figure 2(b) is the x-ray diffraction pattern of this alloy. By subtracting the peaks of λ-Zr(Mo,Fe)2 the remaining diffraction peaks were found to match well with those of the Ti2Ni type compound Zr4Fe2O.[24] Considering the oxygen contamination present in this work, which will be discussed in detail later in the text, we suggest that the γ-Zr2(Mo,Fe) compound is derived from Zr4Fe2O with Fe partially replaced by Mo. Dicvol[20,21,22] software was used to index the observed 14 peaks for this γ phase. The results showed that γ phase has a fcc structure with a cell parameter of a = 1.2213 nm, as shown in Table 2.
This Ti2Ni type compound was first observed in the as-cast alloy 1 (Zr62Mo13Fe25), as shown in Fig. 3(a). The composition of the dendrite γ phase in this alloy is Zr57.5Mo16.7Fe25.8. No the ternary phase with such composition was reported in the Zr-Mo-Fe system. Thus, this phase was initially thought to be a new ternary compound. Apart from γ phase, λ-Zr(Mo,Fe)2 with the composition of Zr36.1Mo37.4Fe26.5, βZr with the composition of Zr90.4Mo4.7Fe4.9, and Zr2Fe with the composition of Zr72.7Mo0.8Fe26.5 were observed in the as-cast alloy. After being subjected to heat treatment at 1000 °C for 20 days, a microstructure with coarse and well-rounded grains was obtained for alloy 1, as shown in Fig. 3(b). This microstructure indicates the thermodynamic equilibrium has been reached for this alloy, with λ-Zr(Mo,Fe)2 and γ observed in equilibrium with βZr. The compositions of the equilibrated phases are provided in Table 1.
Figure 4(a) shows a backscattered electron (BSE) image of alloy 3, where the co-existence of γ-Zr2(Mo,Fe), λ-Zr(Mo,Fe)2, and solidified liquid can be observed. The domain where the white-gray phase mixes with the black-gray phase was the liquid phase. The white-gray phase has a βZr structure with a composition of Zr92.1Mo4.1Fe3.8, while the black-gray phase has a Zr2Fe structure with a composition of Zr67.5Mo0.3Fe32.2. These phases are the solidification products of the liquid phase after quenching. Figure 4(b) shows the x-ray diffraction (XRD) pattern of this alloy, which is in agreement with the observations made using SEM/EDS. After being annealed at 1000 °C for 20 days, alloy 4 exhibited an equilibrium between γ-Zr2(Mo,Fe), β-Zr, and λ-Zr(Mo,Fe)2, as can be seen in Fig. 5. The compositions of γ-Zr2(Mo,Fe), λ-Zr(Mo,Fe)2 and βZr in this alloy are Zr63.2Mo12.0Fe24.8, Zr38.0Mo39.0Fe23.0 and Zr89.5Mo7.7Fe2.8, respectively. Figure 5(b) shows the XRD pattern of this alloy, which is consistent with the observation made using SEM/EDS.
Alloy 6 exhibits an equilibrium between γ-Zr2(Mo,Fe), ZrMo2, and βZr, as shown in Fig. 6. The microstructures and x-ray diffraction patterns of this alloy confirm that γ-Zr2(Mo,Fe) coexisted with ZrMo2 and βZr. The XRD analysis verifies the existence of γ-Zr2(Mo,Fe), ZrMo2, and βZr in this alloy. In alloy 9, an equilibrium among γ, λ, and ZrFe2 phases was observed, as shown in Fig. 7. X-ray diffraction peaks of ZrFe2 overlap with those of Zr(Mo,Fe)2. Identification of ZrFe2 is mainly based on the composition analysis. It is worth noting that the presence of un-melted Mo was observed in alloy 5 due to the high melting point of molybdenum and the short melting time or insufficient melting current used. Along with Mo, γ-Zr2(Mo,Fe), βZr, and ZrMo2 phases were detected in this alloy, as shown in Fig. 8. With the exception of alloy 5, none of the fabricated alloys showed the presence of un-melted Mo.
Figure 9 displays the tie-triangles determined for alloys 4 and 6, where the tie-triangle of [γ + βZr + λ] partially overlaps with that of [γ + βZr + ZrMo2]. Likewise, the tie-triangles of [γ + λ + ZrFe2] and [γ + λ + liquid] determined for alloys 3 and 9 are presented in Fig. 9 and overlap with each other as well. In the ternary Zr-Mo-Fe system, if equilibrium is attained after the heat treatment, a tie-triangle of either [γ + βZr + λ] or [γ + βZr + ZrMo2] is anticipated for alloys 4 and 6. The same holds true for alloys 3 and 9. After being annealed for an additional 10 days, x-ray diffraction patterns of alloys 4 and 6 were found to remain unchanged. Well-defined phase boundaries in alloys 4 and 6 indicate that both alloys have attained equilibrium. The oxygen contents in alloys 2, 5 and 6 are 1200 ppm, 3400 ppm and 4800 ppm, respectively. Thus, oxygen is thought to be the reason of overlap of the tie-triangles.
Figure 10 shows the BSE image, x-ray diffraction, and element maps of alloy 15. This alloy (Zr63Mo13Fe24) has a composition slightly different to that of alloy 1 (Zr63Mo12Fe25) and was produced in a different melting batch. The aim was to investigate if it was possible to obtain βZr and λ-Zr(Mo,Fe)2 without the presence of γ-Zr2(Mo,Fe) by carefully controlling the melting process to eliminate oxygen contamination. Unexpectedly, a four-phase equilibrium of liquid, βZr, γ-Zr2(Mo,Fe), and λ-Zr(Mo,Fe)2 was observed in this alloy, as shown in Fig. 10(a). Upon quenching, the liquid solidified into a mixture of Zr2Fe, βZr, and γ-Zr2(Mo,Fe). The black area in the liquid domain represents Zr2Fe. The compositions of the precipitates can be found in Table 1. Figure 10(c), (d), (e), (f) and (g) display the element maps of O, Zr, Fe, and Mo for this alloy. As shown in Fig. 10(c), oxygen preferred to segregate in βZr, regardless of whether it is an equilibrium phase or a solidification product. The equilibrium phases ranked by oxygen concentration from highest to lowest are βZr, liquid, γ-Zr2(Mo,Fe), and λ. It is worth noting that a certain amount of oxygen dissolves in the γ phase, and oxygen in λ is negligible.
Figure 11 dipicts the BSE image, x-ray diffraction, and element maps of alloy 16, in which equilibrium of γ-Zr2(Mo, Fe), liquid, and λ-Zr(Mo,Fe)2 was observed. The element maps showed that oxygen preferred to segregate in liquid. Minor oxygen was detected in γ-Zr2(Mo, Fe) and λ-Zr(Mo,Fe)2. The oxygen content in γ-Zr2(Mo, Fe) is slightly higher than that in λ-Zr(Mo,Fe)2.
The oxygen content in alloy 2 was determined to be 1200 ppm. Area fractions of γ-Zr2(Mo, Fe) and λ-Zr(Mo, Fe)2 in this alloy were measured to be 88 % and 12 %, respectively, and were directly treated as the volume fraction. The cell parameter of λ-Zr(Mo, Fe)2 is a = 0.507 nm, c = 0.833 nm. The relative molecular weights and the densities of γ-Zr2(Mo, Fe) and λ-Zr(Mo, Fe)2 are 243 g/mol and 219 g/mol, and 7.12 g·cm−3 and 7.84 g·cm−3, respectively. The volume fractions for both phases were converted into mass fractions, which were 87% for γ-Zr2(Mo, Fe), and 13% for λ-Zr(Mo, Fe)2, respectively. The element maps of alloy 15 showed that oxygen in λ-Zr(Mo,Fe)2 is nearly undetected, so it will not introduce significant error by assuming that all oxygen dissolves in γ-Zr2(Mo, Fe) for alloy 2. Thus, the oxygen concentration in the γ-Zr2(Mo, Fe) compound is calculated to be approximately 2.2 at.%.
Alloy 15 presents a microstructure of four-phase equilibrium of [βZr, liquid, λ-Zr(Mo, Fe)2, γ-Zr2(Mo, Fe)]. βZr, liquid, λ-Zr(Mo, Fe)2 are stable equilibrium phases in the Zr-Mo-Fe alloy.[18,19] Thus, it is reasonable to conclude that γ-Zr2(Mo, Fe) is formed by the introduction of oxygen. This conclusion is also supported by the results of above element maps and the oxygen content calculation for γ-Zr2(Mo, Fe). The designed composition of alloys 1 and 15 is nearly identical and very close to the measured composition of γ phase. Apart from phases γ, βZr, and λ-Zr(Mo,Fe)2, which were found in alloy 1, additional liquid appeared in alloy 15. This difference might be due to the varying oxygen content between alloys 1 and 15, as the oxygen uptake varied from alloy to alloy during the melting procedure.
The γ phase is an oxygen-stabilized compound in the Zr-Mo-Fe (O) system. Thus, results obtained from alloys 4 and 6 defined a four-phase equilibrium of [βZr + λ-Zr(Mo,Fe)2 + γ + ZrMo2] in this quarternary system. The results obtained from alloys 3 and 9 specified another four-phase region [λ-Zr(Mo,Fe)2 + γ + Liquid + ZrFe2].
It is well known that impurities such as O and N can stabilize intermetallics Zr2M (M = Fe, Co, Ni, etc.) and result in the formation of partially filled cubic Ti2Ni structure-type ternary compounds, such as Zr4Fe2O,[24] Zr4Co2O,[25] Zr4Ni2O[26,27] and Zr4Pt2O.[28] Two ternary compounds with similar composition and with identical Ti2Ni structure, τ1 (Zr65Mo18−xNi16.5+x) and ω (approximately Zr66Mo12Co22), have been reported in the Zr-Mo-Ni and Zr-Mo-Co ternary systems, respectively.[29,30] These compounds were treated as stable phase in these ternary systems. Given the similarity of the composition between γ-Zr2(Mo,Fe), τ1 (Zr65Mo18−xNi16.5+x) and ω (approximately Zr66Mo12Co22),[29,30] and the similarity of Fe, Co and Ni, further work needs to be done to clarify whether τ1 (Zr65Mo18−xNi16.5+x) and ω (approximately Zr66Mo12Co22) are stabilized by oxygen.
To verify the existence of the ternary compound Zr9Mo4Fe, alloy 6 was designed to be located in the region where Zr9Mo4Fe equilibrates with λ-Zr(Mo,Fe)2, based on the thermodynamical assessment of the Zr-Mo-Fe system.[17] However, Zr9Mo4Fe was not observed in this alloy. To further verify the existence of Zr9Mo4Fe, additional alloy 8 with a nominal composition of Zr65.2Mo28.0Fe6.7, nearly identical to the composition of Zr9Mo4Fe, was arc melted and subjected to heat treatment. The phase ZrMo2 grains, the deep-gray phase γ-Zr2(Mo,Fe), and the gray-matrix phase βZr were observed in this alloy, as shown in Fig. 12. It is clear that Zr9Mo4Fe was not observed in this study. These results are consistent with recently reported isothermal section of the Zr-Mo-Fe ternary system at 1000 °C by Du et al.[19] They also did not observe the Zr9Mo4Fe compound in their study.[19]
The present results show that there are 3 four-phase regions in the Zr-Mo-Fe (O) system in Zr-rich portion at 1000 °C, which are [βZr + λ-Zr(Mo,Fe)2 + γ + ZrMo2], [βZr + λ-Zr(Mo,Fe)2 + γ + Liquid], and [λ-Zr(Mo,Fe)2 + γ + Liquid + ZrFe2], repsectively. Phase relationships in the Zr-Mo-Fe (O) system in the Zr-rich portion at 1000 °C were illustrated and projected along the oxygen composition direction, as presented in Fig. 13. The circles in Fig. 13 represent the compositions of identified phases in the investigated alloys. When referring to Fig. 13, it should be noted that the projected phase regions overlap each other. For instance, the four-phase region [βZr + λ-Zr(Mo,Fe)2 + γ + ZrMo2], bounded by lines ab → bc → cd → da, overlaps with the projection of the three-phase region [βZr + λ-Zr(Mo,Fe)2 + γ], which is bounded by lines be → ef → fh → hd → db. Similarly, the projections of the three-phase region [βZr + λ-Zr(Mo,Fe)2 + γ] and four-phase region [βZr + λ-Zr(Mo,Fe)2 + γ + Liquid] overlap each other, too. It is important to note that the end-points of the tie-tetrahedron in Fig. 13 were arbitrarily determined. Excluding the oxygen-stabilized γ phase, tie-triangles in the oxygen-free Zr-Mo-Fe system in Zr-rich portion at 1000 °C are [βZr + λ-Zr(Mo,Fe)2 + ZrMo2], [βZr + λ-Zr(Mo,Fe)2 + Liquid], and [λ-Zr(Mo,Fe)2 + Liquid + ZrFe2].
Phase relationships of the Zr-Mo-Fe (O) system in the Zr-rich corner at 1000 °C—Projection along the oxygen composition direction. Along the projection direction, the solid lines represent the visible edges of equilibrium tetrahedron, and the dashed lines represent the hidden edges of equilibrium tetrahedron
One of the interesting findings of this study is the presence of the oxygen-stabilized compound γ-Zr2(Mo,Fe) in all investigated alloys, regardless of the phase region they are located in. Research on the oxidation of the Zr-base alloys mainly focused on the oxidation of Zr-matrix and its precipitates.[31] However, there has been limited research on how the nature of the precipitates changes during the oxidation process. This discovery serves as a reminder that γ-Zr2(Mo,Fe) may form in Zr-Mo-Fe based alloys as the ingress of oxygen increases during service. The current element mapping has shown that oxygen tends to segregate in the βZr phase, while minor or very limited oxygen was observed in the intermetallics. This result is consistent with the oxidation process of zirconium-based alloys, in which zirconium prefers to oxidize and the intermetallics exhibit delayed oxidation.[32]
Based on the compositions listed in Table 1, the maximum solubilities of Mo in the ZrFe2 and Fe in ZrMo2, are 5 at.% and 12 at.%, respectively. The solubility of Mo in ZrFe2 in this study is quite consistent with that (4.9 at.%) reported in Ref 19. The composition range of λ-Zr(Mo,Fe)2 varies from 7 to 39 at.% Mo with Zr at approximately constant 36 at.%. Current composition range of λ-Zr(Mo,Fe)2 is consistent with that reported by Du et al., which is 8-40.7 at.% Mo.[19]
4 Conclusions
-
(1)
When the amount of oxygen increases in the Zr-Mo-Fe alloys, a oxygen-stabilized compound was observed to be present in this system. This compound was identified as γ-Zr2(Mo,Fe) with cell parameter of a = 1.2213 nm, with an approximate composition of Zr63Mo12Fe25.
-
(2)
The previously reported ternary compound Zr9Mo4Fe was not observed in the current work.
-
(3)
For the Zr-Mo-Fe (O) system at 1000 °C, three four-phase regions, which are [βZr + λ-Zr(Mo,Fe)2 + γ + ZrMo2], [βZr + λ-Zr(Mo,Fe)2 + γ + Liquid], and [λ-Zr(Mo,Fe)2 + γ + Liquid + ZrFe2], are identified in the Zr-rich corner.
References
A.T. Motta, Waterside Corrosion in Zirconium Alloys, JOM, 2011, 63(8), p 63–67.
J.P. Abriata, J. Garcés, and R. Versaci, The O-Zr (Oxygen-Zirconium) system, Bull. Alloy Phase Diagr., 1986, 1986(7), p 116–124.
M. Billone, Y. Yan, T. Burtseva, R. Daum, Cladding Embrittlement during Postulated Loss-of-Coolant Accidents, NUREG/CR-6967, July 31, 2008; United States. (https://digital.library.unt.edu/ark:/67531/metadc901576/)
G. Hache and H.M. Chung, The History of LOCA Embrittlement Criteria, NUREG/CP-0172, pp. 205-237 (2001)
M. Slobodyan, Arc Welding of Zirconium and Its Alloys: A Review, Prog. Nucl. Energy, 2021, 133, p 103630.
M. Pahutova, K. Kucharova, and J. Cadek, Creep and Creep Fracture in Zr-Sn-Mo and Zr-Sn-Mo-Nb Alloys at Temperatures of 623–823 K—II, Creep Fract. Kovove Mater., 1976, 14(6), p 702–716.
M. Pahutová, J. Čadek, and V. Černý, Creep and Creep Rupture of a Zr-6%Sn-1%Mo Alloy, J. Nucl. Mater., 1977, 68(1), p 111–121.
M. Pahutová, and J. Čadek, Effect of Molybdenum on Some Basic Creep Characteristics of Alpha Zirconium in a Temperature Interval of 350 to 600 °C, Mater. Sci. Eng. C, 1975, 20, p 277–285.
B. Cheadle, R. Holt, V. Fidleris, A. Causey, V. Urbanic, High-strength, creep-resistant excel pressure tubes, zirconium in the nuclear industry. in 4–7 Aug. 1980 (Boston), ASTM Committee B-10 on Reactive and Refractory Metals and Alloys (Sponsor), ed. by D. Franklin, West Conshohocken, PA, ASTM International, pp. 193–207 (1982).
J.H. Lee, and S.K. Hwang, Effect of Mo Addition on the Corrosion Resistance of Zr-Based Alloy in Water Containing LiOH, J. Nucl. Mater., 2003, 321(2), p 238–248.
A.V. Nikulina, V.F. Konkov, M.M. Peregud, and E.E. Vorobev, Effect of Molybdenum on Properties of Zirconium Components of Nuclear Reactor Core, Nucl. Mater. Energy, 2018, 14, p 8–13.
J. Liang, H. Yu, A. Barry, E.C. Corcoran, L. Balogh, and M.R. Daymond, Re-investigation of Phase Transformations in the Zr-Excel Alloy, J. Alloys Compd., 2017, 716, p 7–12.
F. Stein, G. Sauthoff, and M. Palm, Experimental Determination of Intermetallic Phases, Phase Equilibria, and Invariant Reaction Temperatures in the Fe-Zr System, J. Phase Equilib., 2002, 23(6), p 480–494.
R.J. Perez, and B. Sundman, Thermodynamic Assessment of the Mo-Zr Binary Phase Diagram, Calphad, 2003, 27(3), p 253–262.
A.F.A. Guillermet, The Fe-Mo (Iron-Molybdenum) System, Bull. Alloy Phase Diagr., 1982, 3(3), p 359–367.
N.M. Gruzdeva, T.A. Tregubov, ЖEЛEЗO--MOЛИБДEH-ЦИPКOHИЙ (Iron-Molybdenum-Zirconium), Диaгpaммы cocтoяния мeтaлличecкиx cиcтeм (State Diagrams of Metal Systems), Aлиcoвa C.П., Бyдбepг П.Б., Aгeeв H.B. (peд) oпyбликoвaнныe в 1968 гoдy, Bыпycк 14, p 188. (In Russia) Alisova S.P., Budberg P.B., Ageev N.V. (ed), published in 1968, Issue 14, p. 188.
P. Rogl, H. Nowotny, and F. Benesovsky, New K-Borides and Related Phases (Filled up Re3B-Phases), Mon. Chem., 1973, 104(1), p 182–193.
M. Zinkevich, and N. Mattern, Thermodynamic Modeling of the Fe-Mo-Zr System, Acta Mater., 2002, 50(13), p 3373–3383.
Z. Du, L. Zou, C. Guo, X. Ren, and C. Li, Experimental Investigation and Thermodynamic Description of the Fe-Mo-Zr System, Calphad, 2021, 74, p 102314.
A. Boultif, and D.J. Louër, Indexing of Powder Diffraction Patterns for Low-Symmetry Lattices by the Successive Dichotomy Method, J. Appl. Crystallogr., 1991, 24, p 987–993.
A. Boultif, and D. Louër, Powder Pattern Indexing with the Dichotomy Method, J. Appl. Crystallogr., 2004, 37, p 724–731.
D. Louër, and A. Boultif, Indexing with the Successive Dichotomy Method, DICVOL04, Z. Kristallogr. Suppl., 2006, 23, p 225–230.
J. Schindelin, I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, and A. Cardona, Fiji: An Open-Source Platform for Biological-Image Analysis, Nat. Methods, 2012, 9(7), p 676–682.
International Centre for Diffraction Data, 12 Campus Boulevard, Newtown Square, PA 19073--3273, USA.
A.A. Lavrentyeve, B.V. Gabrelian, P.N. Shkumat, I.Y. Nikiforov, IYu. Zavaliy, and OYu. Khyzhun, Electronic Structure of Zr4Fe2O: Ab Initio APW+LO Calculations and x-ray Spectroscopy Studies, J. Phys. Chem. Solid., 2013, 74, p 590–594.
H. Holleck, and F. Thummler, Ternäre Komplex-Carbide, -Nitride und -Oxide Mit Teilweise Aufgefüllter Ti2Ni-Struktur, Monatsch. Chem., 1967, 98, p 133–134.
R. Mackay, G.J. Miller, and H.F. Franzen, New Oxides of the Filled-Ti2Ni Type Structure, J. Alloys Compd., 1994, 204(1–2), p 109–118.
S. Gupta, D.J. Sordelet, and J.D. Corbett, Structural and Compositional Investigations of Zr4Pt2O: A Filled-Cubic Ti2Ni-Type Phase, J. Solid State Chem., 2009, 182, p 1708–1712.
S. Wang, C. Zhang, C. Lin, Y. Peng, and Y. Du, Measurement of 900 °C Isothermal Section in the Mo-Ni-Zr System, J. Phase Equilib. Diffus., 2016, 37(5), p 672–679.
C. Lin, C. Zhang, S. Wang, P. Zhou, and Y. Du, Phase Equilibria of the Co-Mo-Zr Ternary System at 1000 °C, J. Phase Equilib. Diffus., 2018, 39(5), p 510–518.
J. Sayers, S. Lozano-Perez, and S.R. Ortner, The Progress of SPP Oxidation in Zircaloy-4 and Its Relation to Corrosion and Hydrogen Pickup, Corros. Sci., 2019, 158, p 108972.
C. Proff, S. Abolhassani, and C. Lemaignan, Oxidation Behaviour of Zirconium Alloys and Their Precipitates—A Mechanistic Study, J. Nucl. Mater., 2013, 432(1–3), p 222–238.
Acknowledgment
This project is supported by Guangxi Natural Science Foundation (Grant No. 2018GXNSFAA138043), Open Foundation of Guangxi Key Laboratory of Processing for Non-ferrous Metals and Featured Materials, Guangxi University (Grant No. GXYSOF1803), Guangxi Provincial Science and Technology (Grant No. 2020KY04026, 2022KY0150), Natural Science Foundation of Guangdong Province (2022A1515010919, 2023A1515012089), and the Special Projects of Universities in Guangdong Province in Key Areas (2022ZDZX3014).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, Y., Zhu, J., Liang, J. et al. Phase Relationship in the Zr-Mo-Fe(O) System (Zr > 30 at.%) at 1000 °C. J. Phase Equilib. Diffus. 44, 483–495 (2023). https://doi.org/10.1007/s11669-023-01052-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-023-01052-0