Abstract
The interdiffusion behavior of ternary face-centered-cubic (fcc) Al-Co-Cr alloys has been studied by the diffusion-couple technique and CALculation of PHAse Diagram (CALPHAD) approach. The composition profiles of the fcc Al-Co-Cr diffusion couples at 1000 and 1200 °C were measured by using electron probe microanalysis (EPMA), followed by the extraction of interdiffusion coefficients through the Whittle-Green method. Based on the available thermodynamic description and diffusion coefficients in the literature as well as the experimental data obtained in the present work, the atomic mobilities of Al, Co, and Cr in fcc Al-Co-Cr alloys were assessed by means of the CALPHAD method. The diffusion coefficients, composition profiles and diffusion paths of fcc Al-Co-Cr alloys were calculated by adopting the atomic mobilities assessed in the present work, reaching a good agreement with the experimental data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Multicomponent cobalt-based superalloys and high entropy alloys (HEAs) have attracted much attention due to their excellent mechanical properties, high-temperature stability and corrosion resistance.[1,2,3,4,5,6] To efficiently design alloys that meet performance requirements, both phase diagram and diffusion kinetics are indispensable tools. Comprehensive understanding of bulk diffusion processes in the multicomponent alloys is essential for various applications, such as solidification, homogenization, recrystallization, precipitation, and creep. Although many versions of thermodynamic description of the cobalt-based superalloys and HEAs have been reported so far, there are fewer studies on atomic mobilities to describe diffusion coefficients. In 2004, Gómez-Acebo et al.[7] assessed the mobilities in the fcc phase for the binary and ternary sub-systems of the multicomponent Al-Co-Cr-Ni-Ti system but did not consider sufficient experimental data. Among them, the mobilities of the binary systems such as Al-Co, Co-Cr, Co-Ti and Cr-Ti were obtained by approximation and eight ternary systems including the Al-Co-Cr system were regarded as ideal without interaction parameters. Minamino et al.[8] conducted interdiffusion experiments on the binary, ternary, and quaternary alloys in Co-based solid solutions (Al-Co-Cr-Ni) at 1150 °C to quantitatively evaluate the effect of the addition of elements on the interdiffusion. More recently, Chen et al.[9] studied the compositional profiles of Ni-rich fcc Ni-Co-Cr and Al-Ni-Co-Cr diffusion couples. Based on these compositional profiles, atomic mobilities of the Al-Ni-Co-Cr system were obtained directly by HitDIC software. For the ternary Al-Co-Cr system, one of the basic sub-systems of cobalt-based superalloys and the common HEAs,[10,11,12,13] there is currently no systematic assessment of its atomic mobilities available. Therefore, the purpose of the present work is to study the diffusion kinetics of the ternary Al-Co-Cr system and provide a reference for design of high-performance alloys.
In 1998, Ishikawa et al.[14] investigated the phase equilibrium relationship of the Al-Co-Cr alloys from 1000 to 1350 °C using multi-phase alloys and diffusion couple method. Later, Gómez-Acebo et al.[7] assessed the thermodynamics of the Al-Co-Cr ternary system, which was extrapolated from the three binary sub-systems[15,16,17] without any ternary interaction parameter. However, there is a deviation compared to the experimental phase equilibrium data by Ishikawa et al.[14] Recently, Liu et al.[18,19] provided new phase equilibrium experiments and first-principles data and reassessed the Al-Co-Cr system. The thermodynamic description of the Al-Co-Cr ternary system by Liu et al.[19] reproduces all experimental results from Ishikawa et al.[14] and their own data. Therefore, the thermodynamic parameters for the Al-Co-Cr ternary system were taken from Liu et al.[19] in the present work. Its binary sub-systems of Al-Co, Al-Cr and Co-Cr were adopted from the work by Dupin et al.,[15] Ansara[20] and Kusoffsky et al.,[17] respectively.
To study the diffusion behavior of the Co-rich fcc Al-Co-Cr alloys, Roper and Whittle[21] and Minamino et al.[8] combined electron probe microanalysis (EPMA) with the diffusion couple method to obtain interdiffusion coefficients at 1100 and 1150 °C, respectively. Gómez-Acebo et al.[7] presented the atomic mobility parameters of the fcc Al-Co-Cr system based on their own thermodynamic parameters. However, not all of the experimental data for the fcc Al-Co-Cr alloys were used in the assessment process. Therefore, it is necessary to systematically evaluate the diffusion behavior of the fcc Al-Co-Cr alloys to obtain accurate atomic mobilities.
The main objectives of this work are: (1) to experimentally determine the interdiffusion coefficients for fcc Al-Co-Cr alloys at 1000 and 1200 °C; (2) to evaluate the atomic mobilities of the fcc Al-Co, Al-Cr and Co-Cr binary systems based on the reliable experimental data in the literature; (3) to assess the atomic mobilities of the fcc Al-Co-Cr ternary system; (4) to validate the accuracy of atomic mobilities obtained in this work via comparisons of the experimental diffusion coefficients, composition profiles and diffusion paths; and (5) to predict the variation trend of interdiffusion coefficients with composition based on the present determined atomic mobilities.
2 Experimental Procedure
Alloys and pure Co button ingots were prepared in a non-consumable arc furnace under an argon atmosphere. Raw materials were high-purity Co (99.99 wt.%), Cr (99.95 wt.%) blocks and Al (99.95 wt.%) thin filaments. The ingots were turned over and re-melted five times to ensure chemical homogeneity. Then, all the ingots were annealed at 1200 °C for 72 h in sealed quartz tubes with highly pure argon atmosphere before water-quenching. This produced fcc alloy samples with homogeneous composition and coarse grains, which reduced the influence of grain boundaries and segregation on diffusion. Alloy blocks with a size of 4 \(\times\) 4 \(\times\) 4 mm3 were cut from the middle of the homogenized alloy. After grinding and polishing, the two polished surfaces were clamped and fixed by a special Mo clamp to form a diffusion couple assembly with good contact. The annealing treatments for diffusion couples were carried out at 1000 and 1200 °C respectively. Detailed heat-treatment conditions are listed in Table 1. After annealing, all diffusion couples were quickly quenched in cold water. The composition profiles of the diffusion couples were then measured by EPMA (JXA-8230, JEOL, Japan) operated at 20 kV voltage with a beam current of 20 mA. The EDS line scans along the diffusion direction were first used to roughly determine the effective diffusion zone in samples, according to which, the measurements were then performed with step sizes of 5 and 10 μm for the samples annealed at 1000 and 1200 °C, respectively. The variation of compositions for each component was determined to be within 0.5 at.%.
3 Methodology
3.1 Determination of Interdiffusion Coefficients
The experimental composition profile of a diffusion couple can be analyzed by the error function expansion (ERFEX) method as shown in the following equation,
where \(a_{i}\), \(b_{i}\), \(c_{i}\) and \(d_{i}\) are fitting parameters, and \(z\) is the diffusion distance. Thus, \(X(z)\) represents the fitted composition for Al, Co, and Cr with notations such as \(x_{{{\text{Cr}}}}\) for mole fraction of Cr in the following expressions. The parameters obtained from the ERFEX in the present work fitted the experimental data satisfactorily. The fitting can improve the accuracy of the resulting interdiffusion coefficients by reducing noise and smoothing local fluctuations of the measured data.
Then the interdiffusion coefficients for a ternary system are obtained by the Whittle-Green method,[22] which introduces a normalized composition variable \(Y_{i}\) as shown in Eq 2.
where \(x_{i}^{ - }\) and \(x_{i}^{ + }\) represent the mole fraction of \(i\) at the far left and far right ends of the diffusion couples, respectively. Based on Fick’s second law of diffusion, the interdiffusion coefficients can be obtained using the following expressions:
where \(t\) is the diffusion time, \(\tilde{D}_{{{\text{CrCr}}}}^{{{\text{Co}}}}\) and \(\tilde{D}_{{{\text{AlAl}}}}^{{{\text{Co}}}}\) are the main interdiffusion coefficients, \(\tilde{D}_{{{\text{CrAl}}}}^{{{\text{Co}}}}\) and \(\tilde{D}_{{{\text{AlCr}}}}^{{{\text{Co}}}}\) are the cross interdiffusion coefficients with Co as the dependent component. Therefore, two pairs of diffusion couples, with their diffusion paths having an intersecting point, establish four equations to determine interdiffusion coefficients for the ternary Al-Co-Cr system. In the present work, it is verified that all ternary interdiffusion coefficients satisfy the thermodynamic constraints.
3.2 Modeling of Atomic Mobility
According to Andersson and Ågren,[23] when there is no magnetic influence, the atomic mobility \(M_{i}\) of component \(i\) (i.e. Al, Co or Cr) can be expressed as a function of temperature.
where \(M_{i}^{0}\) is the frequency factor, \(Q_{i}^{s}\) is the activation energy, and R is the gas constant. The parameters, \(- Q_{i}^{s}\) and \({\text{RTLnM}}_{{\text{i}}}^{{0}}\), can be combined into a single parameter, \(Q_{i} = - Q_{i}^{s} {\text{ + RTLnM}}_{i}^{{0}}\). The parameter \(Q_{i}\) which is usually treated as composition-dependent can be expressed by the Redlich–Kister polynomial:
where \(x_{p}\) is the mole fraction of component \(p\), and \(r\) is the order of the Redlich–Kister polynomial. \(Q_{i}^{p}\) represents the mobility parameter of component \(i\) diffusing in pure component \(p\), \({}_{{}}^{{\text{r}}} {\text{Q}}_{{\text{i}}}^{{\text{p,q}}}\) is the binary interaction parameter. The trace diffusion coefficient is related to the atomic mobility by the Einstein relation \(D_{i}^{*} = {\text{RTM}}_{i}\). The interdiffusion coefficient, comprising a mobility term and a thermodynamic factor, can be expressed as:
where \(\delta_{ik}\) is the Kronecker delta (\(\delta_{ik} = 1\) if \(i = k\); otherwise \(\delta_{ik} = 0\)); \(\mu_{k}\) is the chemical potential of element \(k \); \(n\) represents the dependent component.
4 Results and Discussion
4.1 Experimental Results
Table 2 presents the experimentally measured interdiffusion coefficients for fcc Al-Co-Cr alloys at 1000 and 1200 °C. It can be found that all of the the main interdiffusion coefficients (\(\tilde{D}_{{{\text{AlAl}}}}^{{{\text{Co}}}}\) and \(\tilde{D}_{{{\text{CrCr}}}}^{{{\text{Co}}}}\)) are positive, while some of the cross interdiffusion coefficients (\(\tilde{D}_{{{\text{AlCr}}}}^{{{\text{Co}}}}\) and \(\tilde{D}_{{{\text{CrAl}}}}^{{{\text{Co}}}}\)) are negative. At the same temperature, for the main interdiffusion coefficients, \(\tilde{D}_{{{\text{AlAl}}}}^{{{\text{Co}}}} > \tilde{D}_{{{\text{CrCr}}}}^{{{\text{Co}}}}\) in the Co-rich region for all intersecting compositions except for the diffusion pair A3–A5. This indicates that the diffusion rate of Al is faster than that of Cr. Therefore, under the same condition, the diffusion distance of Al is longer than that of Cr, as shown in Fig. 4 and 5. In addition, it is obvious that all the main interdiffusion coefficients increase with increasing temperature.
4.2 Assessments of Atomic Mobilities
The self-diffusion mobilities for Al, Co and Cr were directly taken from Cui et al.,[24] Zhang et al.[25] and Engström and Ågren,[26] respectively. For the Al-Co and Co-Cr binary systems, the atomic mobility parameters need to be reassessed in accordance with the thermodynamic parameters provided by Liu et al.[17] and related work. Then, the atomic mobilities for the fcc Al-Co-Cr system were assessed. All assessed atomic mobilities for fcc Al-Co-Cr alloys are listed in Table 3.
4.2.1 Al-Co System
In 2011, Cui et al.[27] used the diffusion couple method to measure the interdiffusion coefficient of fcc Al-Co binary system in the temperature range of 900-1300 °C and the impurity diffusion coefficient of Al in fcc Co. Combined with the thermodynamic parameters by Ohnuma et al.[28] and the experimental impurity diffusion coefficients of Co in fcc Al in the literature,[29,30,31,32,33] the atomic mobilities of the Al-Co binary system were comprehensively evaluated. In the present work, the impurity mobilities \({\Phi }_{{{\text{Al}}}}^{{{\text{Co}}}}\) and \({\Phi }_{{{\text{Co}}}}^{{{\text{Al}}}}\) were adopted from Cui et al.’s assessment.[27] Since the self-diffusion mobility for fcc-Co and thermodynamic parameters of the fcc phase were adjusted, the interaction parameters of the mobilities were reassessed, resulting in a satisfactory agreement of interdiffusion coefficients between the calculated and experimental data as presented in Fig. 1.
Calculated interdiffusion coefficients of the fcc phase in the Al-Co system compared with the experimental data[27]
4.2.2 Co-Cr System
Zhang et al.[34] evaluated the impurity diffusion coefficients of Cr in fcc Co. Their calculation results were in good agreement with the experimental data measured by Weeton.[35] Therefore, the atomic mobilities, \(\Phi_{{{\text{Cr}}}}^{{{\text{Co}}}}\) evaluated by Zhang et al.[34] were adopted in the present work. Because fcc-Cr is not stable, there is no experimental data on the impurity diffusion coefficients of Co in fcc Cr. For the sake of simplicity, \(\Phi_{{{\text{Cr}}}}^{{{\text{Cr}}}}\) was assumed to be equal to \(\Phi_{{{\text{Co}}}}^{{{\text{Cr}}}}\).
Weeton[35] measured the interdiffusion coefficients of the fcc Co-Cr alloys in the temperature range of 1000-1369 °C by means of diffusion couple method. Davin et al.[36] determined the interdiffusion coefficients of the fcc Co-Cr alloys from 1000 to 1300 °C. Green et al.[37] published the interdiffusion coefficients of the Co-Cr alloys by means of the Boltzmann-Matano method coupled with EPMA. Most recently, Zhao et al.[38] supplemented the diffusion experiments at 1050, 1150 and 1300 °C, and reassessed the atomic mobilities of fcc Co-Cr based on their own thermodynamic parameters. Figure 2(a) shows the calculated interdiffusion coefficients of fcc Co-Cr alloys at the range of 1000-1369 °C along with the experimental data.[35,36,37,38] It is noticed that the calculated interdiffusion coefficients of the fcc Co-Cr alloys at 1000 and 1050 °C have a certain deviation from the experimental data. The reason may be that comparing to volume diffusion, the contribution of grain boundary diffusion at low temperatures is relatively large and not negligible. Additionally, the calculated interdiffusion coefficients at 1000 and 1050 °C both exhibit a sudden drop around the magnetic transition composition, which is caused by the magnetic contribution to the thermodynamic factor. Neumeier et al.[39] have also investigated the interdiffusion coefficients for the composition range 0 to 6 at.% (Cr) at 1100, 1200 and 1300 °C. However, the interdiffusion coefficients of the Co-Cr binary system measured by Neumeier et al.[39] were less than that of Weeton[35] and Zhao et al.[38] as shown in Fig. 2(b) and are not considered in the present optimization. In Fig. 2(c), the comparison between the calculated temperature dependence of interdiffusion coefficients in fcc Co-Cr alloys and the experimental data is presented. The agreement between the calculated results and experimental data is good.
4.2.3 Al-Cr System
The stable fcc phase region is very narrow in the Al-Cr binary system, so the experimental interdiffusion data are rather sparce. Therefore, no interaction parameters were assessed for this binary. In addition, the tracer diffusivity of Al in fcc Cr was assumed to be the same as the self-diffusivity of Cr, and the impurity diffusion of Cr in fcc Al evaluated by Engström and Ågren[40] was directly cited in the present work.
4.2.4 Al-Co-Cr System
Figure 3 presents the comparison between the calculated main interdiffusion coefficients and experimental data in the fcc Al-Co-Cr alloys at 1000, 1200, 1100[21] and 1150 °C.[8] The diagonal line in Fig. 3 indicates that the calculated logarithmic values of the main interdiffusion coefficients are equal to the experimental values. The dotted lines represent a factor of 2 or 0.5 deviation from the diagonal line, which is a generally accepted experimental error for the measurements of diffusivities. The results demonstrate that the majority of the main interdiffusion coefficients for Co-rich fcc Al-Co-Cr alloys fall within reasonable error limits, which suggests a good agreement between the calculation results and experimental data.
4.3 Diffusion Simulation
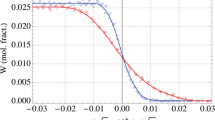
The assessed atomic mobilities combined with the thermodynamic parameters can be used to predict the diffusion behavior of the ternary diffusion couples, including composition profiles and diffusion paths. Figures 4 and 5 display the simulated composition profiles of fcc Al-Co-Cr diffusion couples compared with the experimental data at 1000 and 1200 °C, respectively. We can see that the present atomic mobility can satisfactorily reproduce the diffusion process of the diffusion couples. The calculated short diffusion distance of Cr at 1200 °C in Fig. 5 by Chen et al.[9] indicates incorrect low diffusion coefficients at high temperatures. Figure 6 presents the comparison between the calculated composition profiles and experimental data from Gómez-Acebo et al.[7] at 1100 °C. It can be found that the composition profile of Al is predicted well, but the calculated mobilities are slightly underestimated for Cr. The diffusion paths of diffusion couples at 1000 and 1200 °C are shown in Fig. 7. Generally, the calculated diffusion paths fit the experimental data well except for the diffusion couple B3 at 1200 °C.
Calculated composition profiles for the diffusion couples of (a) A1; (b) A2; (c) A3; (d) A4 and (e) A5 annealed at 1000 °C for 240 h compared with the experimental data from the present work. Solid line: this work; dashed line: Chen et al.[9]
Calculated composition profiles for the diffusion couples of (a) B1; (b) B2; (c) B3; (d) B4 and (e) B5 annealed at 1200 °C for 72 h compared with the experimental data from the present work. Solid line: this work; dashed line: Chen et al.[9]
The main interdiffusion coefficients for the fcc phase in a composition region of Al: 0-20 at.% and Cr:0-50 at.% calculated by the present atomic mobilities are plotted in Fig. 8. At 1000 °C, \(\tilde{D}_{{{\text{AlAl}}}}^{{{\text{Co}}}}\) increases as the Al composition increases but decreases with increasing Cr composition. The variation of the \(\tilde{D}_{{{\text{CrCr}}}}^{{{\text{Co}}}}\) with composition is more complicated than that of \(\tilde{D}_{{{\text{AlAl}}}}^{{{\text{Co}}}}\). \(\tilde{D}_{{{\text{CrCr}}}}^{{{\text{Co}}}}\) increases with the increase of Al composition when \(x\left( {Cr} \right) > 20\;{\text{at}}.\%\), while it decreases with the increasing Al composition when \(x\left( {Cr} \right) < 20\;{\text{at}}.\%\). The variation trend of the main interdiffusion coefficients with the compositions is almost the same at 1000 and 1200 °C.
Calculated main interdiffusion coefficient maps for the fcc phase in the Al-Co-Cr system at different temperatures. 1000 °C: (a) \({\text{Log}}_{10} \tilde{D}_{{{\text{AlAl}}}}^{{{\text{Co}}}}\), (b) \({\text{Log}}_{10} \tilde{D}_{{{\text{CrCr}}}}^{{{\text{Co}}}}\); 1200 °C: (c) \({\text{Log}}_{10} \tilde{D}_{{{\text{AlAl}}}}^{{{\text{Co}}}}\), (d) \({\text{Log}}_{10} \tilde{D}_{{{\text{CrCr}}}}^{{{\text{Co}}}}\)
5 Conclusions
By means of diffusion couple and EPMA technique, the present work investigated the composition profiles of diffusion couples at 1000 and 1200 °C for the fcc Al-Co-Cr alloys and deduced the interdiffusion coefficients. The atomic mobilities of Al, Co, and Cr in fcc Al-Co-Cr alloys were critically assessed by the CALPHAD approach based on available thermodynamic parameters and experimental interdiffusion coefficients. To further validate the atomic mobilities obtained in the present work, the calculated results were compared with the experimental data including diffusion coefficients, concentration distributions and diffusion paths, indicating that most of the experimental data were well reproduced. Besides, the interdiffusion coefficients of the fcc Al-Co-Cr ternary system in a wide range of composition were calculated to explore the influence of Al and Cr on the interdiffusion coefficients.
References
J. Sato, T. Omori, K. Oikawa, I. Ohnuma, R. Kainuma, and K. Ishida, Cobalt-Base High-Temperature Alloys, Science, 2006, 312(5770), p 90–91.
Y. Li, F. Pyczak, M. Oehring, L. Wang, J. Paul, U. Lorenz, and Z. Yao, Thermal Stability of γ′ Phase in Long-term Aged Co-Al-W Alloys, J. Alloys. Compd., 2017, 729, p 266–276.
Z.D. Liang, M. Göken, U. Lorenz, S. Neumeier, M. Oehring, F. Pyczak, A. Stark, and L. Wang, Influence of Small Amounts of Si and Cr on the High Temperature Oxidation Behavior of Novel Cobalt Base Superalloys, Corros. Sci., 2021, 184, p 109388.
X.D. Xu, P. Liu, A. Hirata, S.X. Song, T.G. Nieh, and M.W. Chen, Microstructural Origins for a Strong and Ductile Al0.1CoCrFeNi high-Entropy Alloy with Ultrafine Grains, Materialia., 2018, 4, p 395–405.
S.K. Varma, F. Sanchez, S. Moncayo, and C.V. Ramana, Static and Cyclic Oxidation of Nb-Cr-V-W-Ta High Entropy Alloy in Air From 600 to 1400 °C, J. Mater. Sci. Technol., 2020, 38, p 189–196.
N. Kumar, M. Fusco, M. Komarasamy, R.S. Mishra, M. Bourham, and K.L. Murty, Understanding Effect of 3.5 wt.% NaCl on the Corrosion of Al0.1CoCrFeNi High-Entropy Alloy, J. Nucl. Mater., 2017, 495, p 154–163.
T. Gómez-Acebo, B. Navarcorena, and F. Castro, Interdiffusion in Multiphase, Al-Co-Cr-Ni-Ti Diffusion Couples, J. Phase Equilib. Diff, 2004, 25, p 237–251.
Y. Minamino, Y. Koizumi, N. Tsuji, T. Yamada, and T. Takahashi, Interdiffusion in Co Solid Solutions of Co–Al–Cr–Ni System at 1423 K, Mater Trans, 2003, 44, p 63–71.
J. Chen, J.K. Xiao, Z. Lu, C.Y. Wang, and L.J. Zhang, Atomic Mobilities and Interdiffusivities in Ni-rich fcc Ni-Co-Cr and Ni-Al-Co-Cr Systems Evaluated Using Composition Profiles and HitDIC, J. Alloys. Compd., 2021, 865, p 158645.
M. Abhishek, and S. Yongho, Investigation of Sluggish Diffusion in FCC Al0.25CoCrFeNi High-Entropy Alloy, Mater. Res. Lett, 2021, 9(5), p 239–246.
D.B. Miracle, and O.N. Senkov, A Critical Review of High Entropy Alloys and Related Concepts, Acta Mater., 2017, 122, p 448–511.
T.S. Cao, J.L. Shang, J. Zhao, C.Q. Cheng, R. Wang, and H. Wang, The Influence of Al Elements on the Structure and the Creep Behavior of AlxCoCrFeNi High Entropy Alloys, Mater. Lett., 2016, 164, p 344–347.
J.C. Rao, H.Y. Diao, V. Ocelík, D. Vainchtein, C. Zhang, C. Kuo, Z. Tang, W. Guo, J.D. Poplawsky, Y. Zhou, P.K. Liaw, and JTh.M. De Hosson, Secondary Phases in AlxCoCrFeNi High-Entropy Alloys: An In-Situ TEM Heating Study and Thermodynamic Appraisal, Acta Mater., 2017, 131, p 206–220.
K. Ishikawa, M. Ise, I. Ohnuma, R. Kainuma, and K. Ishida, Phase Equilibria and Stability of the bcc Aluminide in the Co-Cr-Al System, Phys. Chem., 1998, 102(9), p 1206–1210.
N. Dupin, and I. Ansara, Thermodynamic Assessment of the System Al–Co, Rev. Métall, 1998, 95, p 1121–1129.
N Saunders. 1991 Unpublished Revision Based on N Saunders, V.G. Rivlin. Z Metallkde 1987, 78, p. 795–801.
A. Kusoffsky, and B. Jansson, A Thermodynamic Evaluation of the Co-Cr and the C-Co-Cr Systems, Calphad, 1997, 21(3), p 321–333.
X.L. Liu, T. Gheno, B.B. Lindahl, G. Lindwall, B. Gleeson, and Z.-K. Liu, First-Principles Calculations, Experimental Study, and Thermodynamic Modeling of the Al-Co-Cr System, PLoS One, 2015, 10(4), p e0121386.
X.L. Liu, G. Lindwall, T. Gheno, and Z.K. Liu, Thermodynamic Modeling of Al–Co–Cr, Al–Co Ni, Co–Cr–Ni Ternary Systems Towards a Description for Al–Co–Cr–Ni, Calphad, 2016, 52, p 125–142.
I. Ansara, A.T. Dinsdale, and M.H. Rand, COST 507: Definition of Thermochemical and Thermophysical Properties to Provide a Database for the Development of New Light Alloys-Thermochemical Database for Light Metal Alloys. Office for Official Publications of the European Communities, Luxembourg, 1998.
G.W. Roper, and D.P. Whittle, Interdiffusion in Ternary Co-Cr-Al Alloys, Metal Sci., 1980, 14(1), p 21–28.
D.P. Whittle, and A. Green, The Measurement of Diffusion Coefficients in Ternary Systems, Scr. Metal., 1974, 8(7), p 883–884.
J.O. Andersson, and J. Ågren, Models for Numerical Treatment of Multicomponent Diffusion in Simple Phases, J. Appl. Phys., 1992, 72(4), p 1350–1355.
Y.W. Cui, K. Oikawa, R. Kainuma, and K. Ishida, Study of Diffusion Mobility of Al−Zn Solid Solution, J. Phase Equilib. Diff, 2006, 27, p 333–342.
L.J. Zhang, Y. Du, Y.F. Ouyang, H.H. Xu, X.G. Lu, Y. Liu, K. Yi, and J. Wang, Atomic Mobilities, Diffusivities and Simulation of Diffusion Growth in the Co–Si System, Acta Mater., 2008, 56, p 3940–3950.
A. Engström, and J. Ågren, Assessment of Diffusional Mobilities in Face-centered Cubic Ni-Cr-Al Alloys, Z. Metallkd, 1996, 87, p 92–97.
Y.W. Cui, B. Tang, R. Kato, R. Kainuma, and K. Ishida, Interdiffusion and Atomic Mobility for Face-Centered-Cubic Co-Al Alloys, Metall. Mater. Trans. A, 2011, 42, p 2542–2546.
R, Ohnuma, R. Kainuma, K. Ishida: Tohoku University, Sendai, Japan, unpublished research (2008)
N.L. Peterson, and S.J. Rothman, Impurity Diffusion in Aluminum, Phys. Rev. B, 1970, 1, p 3264–3273.
M.S. Anand, and R.P. Agarwala, Diffusion of Cobalt in Aluminium, Philos. Mag., 1972, 26, p 297–309.
G. Erdelyi, D.L. Beke, F.J. Kedves, and I. Godeny, Determination of Diffusion Coefficients of Zn, Co and Ni in Aluminium by a Resistometric Method, Philos. Mag. B, 1978, 38, p 445–462.
G.M. Hood, R.J. Schultz, and J. Armstrong, Co Tracer Diffusion in Al, Philos. Mag. A, 1983, 47, p 775–779.
G. Rummel, T. Zumkley, M. Eggersmann, K. Freitag, and H. Mehrer, Diffusion of Implanted 3d-Transition Elements in Aluminium Part I: Temperature Dependence, Z. Metallkd, 1995, 85, p 122–130.
W.B. Zhang, D.D. Liu, L.J. Zhang, Y. Du, and B.Y. Huang, Experiment Investigation and Computational Study of Atomic Mobility in fcc Ternatry Co-Cr-W, Calphad, 2014, 45, p 118–126.
J.W. Weeton, Chromium Diffusivity in Alpha-Cobalt-Chromium Solid Solutions, Trans. A.S.M, 1952, 44, p 436–451.
A. Davin, V. Leroy, D. Coutsouradis, and L. Habraken, Comparison of the Diffusion of Some Substitution Elements in Nickel and Cobalt, Cobalt, 1963, 19, p 51–56.
A. Green, D.P. Whittle, J. Stringer, and N. Swindells, Interdiffusion in the Cobalt-Chromium System, Scr. Metall., 1963, 7, p 1079–1082.
N. Zhao, W. Liu, J.J. Wang, X.G. Lu, and L.J. Zhang, Thermodynamic Assessment of the Ni–Co–Cr System and Diffusion Study of its fcc Phase, Calphad, 2020, 71, p 101996.
S. Neumeier, H.U. Rehman, J. Neuner, C.H. Zenk, S. Michel, S. Schuwalow, J. Rogal, R. Drautz, and M. Goken, Diffusion of Solutes in fcc Cobalt Investigated by Diffusion Couples and First Principles Kinetic Monte Carlo, Acta Mater., 2016, 106, p 304–312.
A. Engström, and J. Ågren, Assessment of Diffusional Mobilities in Face-Centered-Cubic Ni-Cr-Al Alloys, Z. Metallkd, 1996, 87, p 92–97.
Acknowledgments
The authors would like to acknowledge the financial support from the National Key R&D Program of China (Grant Number: 2017YFB0701904).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shang, G., Lu, Y., Wang, J. et al. Experimental and Computational Studies of Atomic Mobilities for fcc Al-Co-Cr Alloys. J. Phase Equilib. Diffus. 43, 471–482 (2022). https://doi.org/10.1007/s11669-022-00986-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-022-00986-1