Abstract
The phase equilibria in the SnTe-Sb2Te3 system were re-investigated by a combination of Differential Thermal Analysis (DTA), Powder x-ray Diffraction (PXRD), and Scanning Electron Microscope equipped with Energy Dispersive x-ray Spectrometer (SEM-EDS) techniques. In an earlier reported version, this phase diagram was described as quasi-binary with only one intermediate compound, namely SnSb2Te4. Here we report the existence of the new van der Waals type compound SnSb4Te7 for the first time. Considerable homogeneity ranges for both ternary compounds are also revealed. According to DTA results, peritectic melting was found for both compounds. The corresponding temperatures were found to be 595 and 593 °C for SnSb2Te4 and SnSb4Te7, respectively. The PXRD pattern of the newly found phase was analyzed through the Rietveld method and the crystal structure was solved. In the considered quasi-binary system, two wide solid-solubility areas based on the constitutive compounds were also revealed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
During the last forty years, chalcogenide-based layered materials have been widely studied due to their promising electro-physical properties. Particularly, tetradymite-type (mixed) layered ternary phases of the quasi-binary AIVTe-AV2Te3 (AIV = Ge, Sn; AV = Sb, Bi) systems exhibit good electrical and significant low thermal conductivity which are key aspects for promising thermoelectric materials [1,2,3]. Thus, these materials are expected to have potential applications in the fabrication of high-performance solid-state coolers and waste heat recovery from different energy sources [4,5,6,7]. Since 2010, the nAIVTe∙mAV2Te3 homologous series of phases have been confirmed experimentally and theoretically as 3D topological insulator phases of quantum matter [8,9,10,11]. Thus, the unique properties of these ternaries make them tunable materials that exhibit both topological insulating and good thermoelectric properties [12,13,14]. Given the growing interest in this class of tunable materials, the re-investigation of one of the above-mentioned quasi-binary systems—the SnTe-Sb2Te3 phase diagram, is of particular importance for the chemical design of its intermediate phases.
In a previous report, the phase diagram of the SnTe-Sb2Te3 was found to host only one intermediate compound, namely SnSb2Te4, which has a wide homogeneity range [15, 16] and melts incongruently at 603 °C [16]. The tetradymite-type hexagonal crystal structure and lattice parameters of SnSb2Te4 (R-3m, a = 4.298(1) Å, c = 41.57(1) Å) were determined by using resonant single-crystal diffraction later [17]. A so-called 21R-type structure consisting of septuple rocksalt-type blocks are weakly connected by van der Waals (vdW) interactions. Each septuple block consists of four anion and three cation layers. Although tin atoms are distributed over all cation positions, most of them were found clustered in the central layers of the septuple blocks.
The Gibbs free energy and enthalpy, as well as the electronic structure and dynamics at the surface of the SnSb2Te4, were given in our previous works [18, 19].
The existence of the SnSb4Te7 is mentioned in Refs. [20, 21] where its crystal structure was determined by electron diffraction from thin-films: P-3m1, a = 4.37 Å; c = 23.78 Å [20]. An electronic and spin structure of this compound is theoretically calculated in [21]. However, polycrystalline SnSb4Te7 was successfully synthesized in our recent work [22] for the first time.
Here we provide new results, which require a revision of the SnTe-Sb2Te3 phase diagram, including the new compound SnSb4Te7 and its crystal structure.
2 Experimental Part
Both the starting compounds of the title system were synthesized by melting of high purity elements (99.999 wt.% Alfa Aesar) in evacuated (~10-3 Pa) silica ampoules at temperatures ~50 °C higher than their melting points. The constitution of the synthesized compounds was checked by means of DTA and PXRD techniques.
Totally seventeen alloys from the examined system (alloy compositions are shown as black circles in Fig. 5) were prepared by melting of the pre-synthesized starting compounds in various ratios approximately at 800 °C followed by water-quenching. The alloys were further annealed at 450 °C for 720 h to achieve equilibrium state.
Bulk single-crystalline ingots of the various alloys of the system with 3.5 cm in length and 0.8 cm in diameter were grown by the vertical Bridgman-Stockbarger method. In the growth process, the charged ampoules were held in the “hot” zone (~650 °C) of the furnace for 6h and then were moved to the “cold” zone of the furnace with a rate of 1.0 mm/h.
PXRD data collection was performed at room temperature with Bruker D2 Phaser diffractometer and with an Empyrean diffractometer (PANalytical) equipped with a curved Ge(111) monochromator. In both measurements, the CuKα1 radiation was used as x-ray source. Rietveld refinement using the fundamental parameter approach was performed with TOPAS 4.2 academic software. Morphological characterizations of the samples were done with SU8020 Scanning Electron Microscope (Hitachi) equipped with a triple detector system (Ua = 5 kV). DTA of the annealed alloys was carried out using SETARAM Instrumentation system (temperature accuracy ±2 °C) from room temperature up to 1000 °C with a heating and cooling rate of 5 °C⋅min-1. Temperatures of thermal effects were taken mainly from the heating curves.
3 Results and Discussion
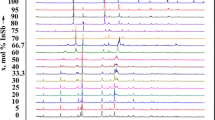
In order to re-study the phase equilibria in the SnTe-Sb2Te3 system, seventeen samples were synthesized with different SnTe:Sb2Te3 starting ratios. The PXRD patterns of five of these samples are given in Fig 1. As can be seen from Fig. 1a and e, the PXRD results of the SnTe- and Sb2Te3-rich samples have qualitatively identical diffraction pattern showing pure tin and antimony tellurides, respectively. The intermediate samples having 10 mol.% (Fig. 1a) and 85 mol.% Sb2Te3 (Fig. 1e) exhibit similar diffraction lines with a negligible shifting compared to pure SnTe and Sb2Te3, respectively. The results obviously confirm the existence of a solid solubility field based on SnTe (α phase, ~11–12 mol.%) and based on Sb2Te3 (β phase, ~20 mol.%). The sample containing 40 mol.% Sb2Te3 was found to be a biphasic mixture of α-SnTe and SnSb2Te4 (Fig. 1b), while the sample having 50 mol.% Sb2Te3 which is corresponding to ternary compound SnSb2Te4, is phase-pure (Fig. 1c). The sample having 66.7 mol.% Sb2Te3 (nominally SnSb4Te7) was a mixture of several phases. These results indicate, that the samples did not reach the thermodynamic equilibrium despite long-time thermal annealing. However, both ternary compounds can easily be distinguished in PXRD analysis by their typical diffraction peaks, particularly at small angles. The 75 mol.% Sb2Te3 sample shows reflections of Sb2Te3 and SnSb4Te7 which do not match those of other possible phases (Fig. 1d). Hence, these PXRD results confirm the formation of the new SnSb4Te7 compound in the SnTe-Sb2Te3 system.
The analysis of the samples obtained through the Bridgman-Stockbarger method confirms the existence of the newly revealed SnSb4Te7 as well. PXRD analysis of the single crystalline sample taken from the upper part of the as-grown ingot having 60 mol.% Sb2Te3 shows that it exhibits diffraction lines according to both SnSb4Te7 and SnSb2Te4 (Fig. 2). We did not detect typical diffraction intensities for higher members of the n(SnTe)∙m(Sb2Te3) (n=1, m>2) homologous series which are formed in the analogous system SnTe-Bi2Te3. The existence of large homogeneity fields based on both starting binary tellurides indicates that tin and antimony can replace each other to a certain extend on the cation sites of the crystal lattice. Such a mutual replacement of Sn and Sb atoms may also occur in the septuple and septuple-quintuple blocks of the SnSb2Te4 and SnSb4Te7 ternary compounds, respectively. This kind of cation intermixing and similar atomic sizes of the Sn and Sb atoms are probably the main reasons that prevent the formation of the higher homologues and, thus, more complex layered structures than SnSb4Te7.
To reveal the crystal structure of the newly synthesized SnSb4Te7, the PXRD pattern of the sample with 60 mol.% Sb2Te3 was refined by the Rietveld method (Fig. 2). The Rietveld refinement was performed on this alloy since no phase-pure sample of this compound could be obtained under the applied experimental conditions. Details of the crystal structure refinement and atomic parameters are summarized in Tables 1 and 2. An analysis of the XRD pattern shows that along with SnSb2Te4 (~29.3%), the sample contains SnSb4Te7 as second phase which is indexed in the trigonal crystal system with the unit cell parameters a=4.2951(2) Å and c=23.973(8) Å. From the reflection conditions, space group P-3m1 was assumed for SnSb4Te7. The Rietveld fitted pattern (Fig. 2) shows that the dominant phase in this sample is the SnSb4Te7 (~70%). SEM-EDS measurements further confirm the existence of the SnSb4Te7 phase in this sample. Fig. 3 summarizes the SEM micrograph, EDS spectrum, and EDS elemental microanalysis results for the selected area of the 60 mol.% Sb2Te3 sample where SnSb4Te7 is the majority phase.
The crystal structure of SnSb4Te7 is shown in Fig 4. As can be seen, the structure is an alternation of quintuple (five-layer) Te-Bi-Te-Bi-Te and septuple (seven-layer) Te-Bi-Te-Sn-Te-Bi-Te blocks along the c-axes. The first building block resembles the crystal structure of Sb2Te3, whereas the latter is the repeat unit of the crystal structure of SnSb2Te4 (17). Interatomic bonds within both building blocks can be considered as covalent, whereas the interaction between the two neighbor blocks is of a weak van der Waals type.
Thereby, the newly synthesized SnSb4Te7 is the structural analogous of tetradymite-derivative mixed-layered AIVAV4Te7 compounds, which were earlier reported in most of the AIVTe-AV2Te3 systems, where AIV = Ge, Sn, Pb and AV = Sb, Bi.
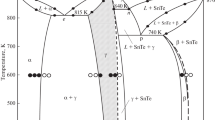
Because the novel SnSb4Te7 compound has been found in the SnTe-Sb2Te3 system, the phase diagram was re-plotted based on experimental data from DTA and XRD analysis of the equilibrated alloys (Fig. 5). The system is found to be a quasi-binary one and features the formation of the two intermediate compounds by the following peritectic reactions:
Along the system, the coordinates of the peritectic p1 and p2 points were found to be at 53 mol.% and 59 mol.% Sb2Te3, respectively. The temperature of eutectic transformation was measured as 587 °C at 58 mol.% Sb2Te3. As can be seen from Fig. 5, the SnSb4Te7 has a very narrow primary crystallization area meaning that it is extremely difficult to obtain a phase-pure crystalline sample via direct solidification. The primary experimental data show that SnSb4Te7 has a significant homogeneity area (δ phase) approximately from 66.5 to 72.7 mol.% Sb2Te3. Similar size of the homogeneity field for SnSb2Te4 (γ phase) has also been detected approximately from 50 to 54 mol.% Sb2Te3. Besides, the system has two more homogeneity areas based on both initial SnTe (α phase, up to ~11-13 mol.% at room temperature) and Sb2Te3 (β phase, up to ~18-19 mol.% at room temperature) compounds. It is worth mentioning that the updated phase diagram for the SnTe-Sb2Te3 systems differs from the earlier known version (14, 15) in terms of the number of ternary compounds, as well as for peritectic and eutectic reaction temperatures. This might have been overlooked previously as the two peritectic temperatures are very close to each other and the reflections, which allow for an unambiguous identification of the new compound SnSb4Te7 are of comparatively low intensity.
4 Conclusion
New experimental data on the phase equilibria of the SnTe-Sb2Te3 system were obtained by means of DTA, PXRD, and SEM-EDS analyses which are significantly different from earlier reported ones. An updated phase diagram is featured by the formation of the two ternary SnSb2Te4 and SnSb4Te7 compounds that melt peritectically at 595 °C and 593 °C, respectively. The primary crystallization fields for both ternary phases as well as types and coordinates of nonvariant equilibria were determined. The existence of homogeneity areas based on both ternary compounds and the initial phases SnTe and Sb2Te3 was detected. The crystal structure of the newly synthesized SnSb4Te7 was solved by Rietveld refinement. An updated phase diagram of the SnTe-Sb2Te3 system provides very valuable information for designing the synthesis and single crystal growth experiments of multifunctional ternary materials.
References
L.E. Shelimova, O.G. Karpinskii, P.P. Konstantinov, E.S. Avilov, M.A. Kretova, and V.A. Zemskov, Crystal Structures and Thermoelectric Properties of Layered Compounds in the ATe-Bi2Te3 (A=Ge, Sn, Pb) Systems, Inorg. Mater., 2004, 40(5), p 530–540
F. von Rohr, A. Schilling, and R.J. Cava, Single-crystal Growth, and Thermoelectric Properties of Ge(Bi, Sb)4Te7, J. Phys. Condens. Matter., 2013, 25(7), p 075804
D. Wu, L. Xie, X. Xu, and J.Q. He, High Thermoelectric Performance Achieved in GeTe—Bi2Te3 Pseudo-Binary via Van der Waals Gap-Induced Hierarchical Ferroelectric Domain Structure, Adv. Funct. Mater., 2019, 29(18), p 1806613
G.K. Ahluwalia, Applications of Chalcogenides: S, Se, and Te. Springer, Cham, 2016.
D.L. Duong, S.J. Yun, and Y.H. Lee, van der Waals Layered Materials: Opportunities and Challenges, ACS Nano, 2017, 11(12), p 11803–11830
J. He, and T.M. Tritt, Advances in Thermoelectric Materials Research: Looking Back and Moving Forward, Science, 2017, 357(6358), p 9997
Z. Tiejun, Y. Liu, C. Fu, J.P. Heremans, J.G. Snyder, and X. Zhao, Compromise and Synergy in High-efficiency Thermoelectric Materials, Adv. Mater., 2017, 29, p 1605884
L.L. Wang, and D.D. Johnson, Ternary Tetradymite Compounds as Topological Insulators, Phys. Rev. B, 2011, 83(24), p 241309
M.B. Babanly, E.V. Chulkov, Z.S. Aliev, A.V. Shevelkov, and I.R. Amiraslanov, Phase Diagrams in the Materials Science of Topological Insulators Based on Metal Chalcogenides, Russ. J. Inorg. Chem., 2017, 62(13), p 1703–1729
M. Nurmamat, K. Okamoto, S. Zhu, T.V. Menshchikova, I.P. Rusinov, V.O. Korostelev, K. Miyamoto, T. Okuda, T. Miyashita, X. Wang, Y. Ishida, K. Sumida, E.F. Schwier, M. Ye, Z.S. Aliev, M.B. Babanly, I.R. Amiraslanov, E.V. Chulkov, K.A. Kokh, O.E. Tereshchenko, K. Shimada, S. Shin, and A. Kimura, Topologically Non-trivial Phase-change Compound GeSb2Te4, ACS Nano, 2020, 14(7), p 9059–9065
D. Pacile, S.V. Eremeev, M. Caputo, M. Pisarra, O. De Luca, I. Grimaldi, J. Fujii, Z.S. Aliev, M.B. Babanly, I. Vobornik, R.G. Agostino, A. Goldoni, E.V. Chulkov, and M. Papagno, Deep Insight into the Electronic Structure of Ternary Topological Insulators: A Comparative Study of PbBi4Te7 and PbBi6Te10, Phys. Status Solidi, 2018, 12(12), p 1800341–1800348
J.P. Heremans, R.J. Cava, and N. Samarth, Tetradymites as Thermoelectrics and Topological Insulators, Nat. Rev. Mater., 2017, 2(10), p 17049
N. Xu, Y. Xu, and J. Zhu, Topological Insulators for Thermoelectrics, npj Quant. Mater., 2017, 2, p 51
D. Baldomir, and D. Faílde, On Behind the Physics of the Thermoelectricity of Topological Insulators, Sci. Rep., 2019, 9, p 6324
A. Stegherr, Das dreistoffsystem zinn-antimon-tellur, Philips Res. Rep., 1969, 6, p 1–71, in German
E.I. Elagina, and N.K. Abrikosov, The Investigation of the Systems PbTe–Bi2Te3 and SnTe–Sb2Te3, Russ. J. Inorg. Chem., 1959, 4, p 1638–1642
O. Oeckler, M.N. Schneider, F. Fahrnbauer, and G. Vaughan, Atom Distribution in SnSb2Te4 by Resonant x-ray Diffraction, Solid State Sci., 2011, 13, p 1057–1161
F.N. Guseinov, A.E. Seidzade, Y.A. Yusibov, and M.B. Babanly, Thermodynamic Properties of the SnSb2Te4 Compound, Inorg. Mater., 2017, 53(4), p 354–357
D. Niesner, S. Otto, V. Hermann, T. Fauster, T.V. Menshchikova, S.V. Eremeev, Z.S. Aliev, I.R. Amiraslanov, M.B. Babanly, P.M. Echenique, and E.V. Chulkov, Bulk and Surface Electron Dynamics in a p-type Topological Insulator SnSb2Te4, Phys. Rev. B, 2014, 89, p 081404
R.M. Imamov, S.A. Semiletov, and Z.G. Pinsker, The Crystal Chemistry of Semiconductors with Octradral and with Mixed Atomic Coordination, Kristallografiya, 1970, 15, p 239–243
M.G. Vergniory, T.V. Menshchikova, I.V. Silkin, Y.M. Koroteev, S.V. Eremeev, and E.V. Chulkov, Electronic and Spin Structure of a Family of Sn-based Ternary Topological Insulators, Phys. Rev. B, 2015, 92(4), p 045134
A.E. Seidzade, Phase Diagram of the SnSb4Te7-SnBi4Te7 System, New Mater. Compd. Appl., 2019, 3(3), p 193–197
Acknowledgment
The work was partially supported by the Science Development Foundation under the President of the Republic of Azerbaijan, a grant EİF/MQM/Elm-Tehsil-1-2016-1(26)-71/01/4-M-33 and by the DAAD program (Deutscher Akademischer Austauschdienst) as part of the Research Grants: Bi-nationally Supervised Doctoral Degree". Special thanks to the Markus Schmidt, Max Planck Institute of Chemical Physics of Solids for cooperation in crystal growth by the Bridgman technique.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seidzade, A.E., Orujlu, E.N., Doert, T. et al. An Updated Phase Diagram of the SnTe-Sb2Te3 System and the Crystal Structure of the New Compound SnSb4Te7. J. Phase Equilib. Diffus. 42, 373–378 (2021). https://doi.org/10.1007/s11669-021-00888-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-021-00888-8